Abstract
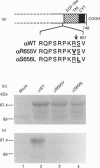
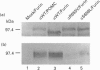
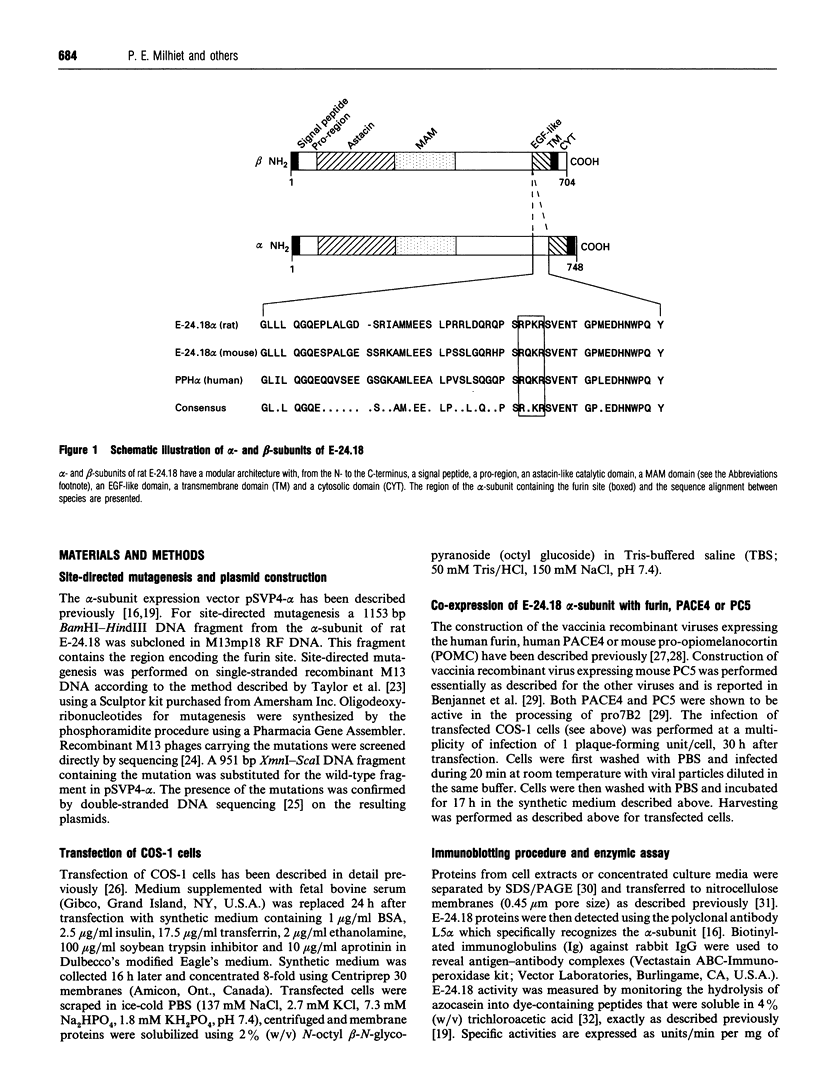
Endopeptidase-24.18 (EC 3.4.24.18; meprin) is a multisubunit metallopeptidase of the astacin family. It is found in brush-border membranes of rodent kidney and human intestine. The membrane-bound enzyme is composed of alpha/beta dimers. Molecular cloning has shown that both subunits have a similar structural domain organization. Soluble alpha 2 dimers have also been observed in vivo and in transfected cells. The structures of all known alpha-subunits contain, upstream from the transmembrane domain, the sequence RXKR, which corresponds to the RXK/RR consensus sequence for specific cleavage by furin. In order to investigate the involvement of this putative cleavage site in the secretion process of endopeptidase-24,.18 alpha-subunit, we expressed in COS-1 cells rat alpha-subunits in which residues R655 or S656 (within the sequence R652PKRS656) were mutated to valine or leucine respectively. In contrast to the wild-type protein, the alpha R655V and alphaS656L mutants were not secreted in the culture medium. Moreover, when cells expressing the alpha-subunit were infected with a furin-encoding vaccinia virus, immunoblotting showed a shift of the major cell-associated form of endopeptidase-24.18 alpha-subunit from 98 kDa to 85 kDa and an increase in the amounts of secreted alpha-subunit. This shift in molecular mass was not observed with the mutant alpha-subunits. As observed for the 98 kDa species, the 85 kDa cell-associated protein was sensitive to endoglycosidase H treatment, suggesting that the proteolytic cleavage occurred in the endoplasmic reticulum or in an early Golgi compartment. Similar experiments using PACE4 and PC5 instead of furin showed that these enzymes were not able to generate the 85 kDa species. We conclude that furin is most probably the cellular enzyme involved in the proteolysis resulting in secretion of rat endopeptidase-24.18 alpha-subunit.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubry M., Berteloot A., Beaumont A., Roques B. P., Crine P. The use of a monoclonal antibody for the rapid purification of kidney neutral endopeptidase ("enkephalinase") solubilized in octyl glucoside. Biochem Cell Biol. 1987 Apr;65(4):398–404. doi: 10.1139/o87-050. [DOI] [PubMed] [Google Scholar]
- Barnes K., Ingram J., Kenny A. J. Proteins of the kidney microvillar membrane. Structural and immunochemical properties of rat endopeptidase-2 and its immunohistochemical localization in tissues of rat and mouse. Biochem J. 1989 Dec 1;264(2):335–346. doi: 10.1042/bj2640335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr P. J., Mason O. B., Landsberg K. E., Wong P. A., Kiefer M. C., Brake A. J. cDNA and gene structure for a human subtilisin-like protease with cleavage specificity for paired basic amino acid residues. DNA Cell Biol. 1991 Jun;10(5):319–328. doi: 10.1089/dna.1991.10.319. [DOI] [PubMed] [Google Scholar]
- Beckmann G., Bork P. An adhesive domain detected in functionally diverse receptors. Trends Biochem Sci. 1993 Feb;18(2):40–41. doi: 10.1016/0968-0004(93)90049-s. [DOI] [PubMed] [Google Scholar]
- Benjannet S., Reudelhuber T., Mercure C., Rondeau N., Chrétien M., Seidah N. G. Proprotein conversion is determined by a multiplicity of factors including convertase processing, substrate specificity, and intracellular environment. Cell type-specific processing of human prorenin by the convertase PC1. J Biol Chem. 1992 Jun 5;267(16):11417–11423. [PubMed] [Google Scholar]
- Benjannet S., Rondeau N., Day R., Chrétien M., Seidah N. G. PC1 and PC2 are proprotein convertases capable of cleaving proopiomelanocortin at distinct pairs of basic residues. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3564–3568. doi: 10.1073/pnas.88.9.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjannet S., Rondeau N., Paquet L., Boudreault A., Lazure C., Chrétien M., Seidah N. G. Comparative biosynthesis, covalent post-translational modifications and efficiency of prosegment cleavage of the prohormone convertases PC1 and PC2: glycosylation, sulphation and identification of the intracellular site of prosegment cleavage of PC1 and PC2. Biochem J. 1993 Sep 15;294(Pt 3):735–743. doi: 10.1042/bj2940735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjannet S., Savaria D., Chrétien M., Seidah N. G. 7B2 is a specific intracellular binding protein of the prohormone convertase PC2. J Neurochem. 1995 May;64(5):2303–2311. doi: 10.1046/j.1471-4159.1995.64052303.x. [DOI] [PubMed] [Google Scholar]
- Beynon R. J., Kay J. The inactivation of native enzymes by a neutral proteinase from rat intestinal muscle. Biochem J. 1978 Jul 1;173(1):291–298. doi: 10.1042/bj1730291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beynon R. J., Shannon J. D., Bond J. S. Purification and characterization of a metallo-endoproteinase from mouse kidney. Biochem J. 1981 Dec 1;199(3):591–598. doi: 10.1042/bj1990591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chaudhuri B., Latham S. E., Helliwell S. B., Seeboth P. A novel Kex2 enzyme can process the proregion of the yeast alpha-factor leader in the endoplasmic reticulum instead of in the Golgi. Biochem Biophys Res Commun. 1992 Feb 28;183(1):212–219. doi: 10.1016/0006-291x(92)91630-9. [DOI] [PubMed] [Google Scholar]
- Corbeil D., Gaudoux F., Wainwright S., Ingram J., Kenny A. J., Boileau G., Crine P. Molecular cloning of the alpha-subunit of rat endopeptidase-24.18 (endopeptidase-2) and co-localization with endopeptidase-24.11 in rat kidney by in situ hybridization. FEBS Lett. 1992 Sep 7;309(2):203–208. doi: 10.1016/0014-5793(92)81095-4. [DOI] [PubMed] [Google Scholar]
- Corbeil D., Milhiet P. E., Simon V., Ingram J., Kenny A. J., Boileau G., Crine P. Rat endopeptidase-24.18 alpha subunit is secreted into the culture medium as a zymogen when expressed by COS-1 cells. FEBS Lett. 1993 Dec 13;335(3):361–366. doi: 10.1016/0014-5793(93)80420-y. [DOI] [PubMed] [Google Scholar]
- Dumermuth E., Eldering J. A., Grünberg J., Jiang W., Sterchi E. E. Cloning of the PABA peptide hydrolase alpha subunit (PPH alpha) from human small intestine and its expression in COS-1 cells. FEBS Lett. 1993 Dec 13;335(3):367–375. doi: 10.1016/0014-5793(93)80421-p. [DOI] [PubMed] [Google Scholar]
- Flannery A. V., Dalzell G. N., Stephen A. G., Beynon R. J. Metallo-endopeptidase activity in mouse and rat urine. Biochem Soc Trans. 1990 Oct;18(5):1023–1024. doi: 10.1042/bst0181023. [DOI] [PubMed] [Google Scholar]
- Hosaka M., Nagahama M., Kim W. S., Watanabe T., Hatsuzawa K., Ikemizu J., Murakami K., Nakayama K. Arg-X-Lys/Arg-Arg motif as a signal for precursor cleavage catalyzed by furin within the constitutive secretory pathway. J Biol Chem. 1991 Jul 5;266(19):12127–12130. [PubMed] [Google Scholar]
- Johnson G. D., Hersh L. B. Expression of meprin subunit precursors. Membrane anchoring through the beta subunit and mechanism of zymogen activation. J Biol Chem. 1994 Mar 11;269(10):7682–7688. [PubMed] [Google Scholar]
- Kaushal G. P., Walker P. D., Shah S. V. An old enzyme with a new function: purification and characterization of a distinct matrix-degrading metalloproteinase in rat kidney cortex and its identification as meprin. J Cell Biol. 1994 Sep;126(5):1319–1327. doi: 10.1083/jcb.126.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaoka Y., Webster R. G. Sequence requirements for cleavage activation of influenza virus hemagglutinin expressed in mammalian cells. Proc Natl Acad Sci U S A. 1988 Jan;85(2):324–328. doi: 10.1073/pnas.85.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny A. J., Ingram J. Proteins of the kidney microvillar membrane. Purification and properties of the phosphoramidon-insensitive endopeptidase ('endopeptidase-2') from rat kidney. Biochem J. 1987 Jul 15;245(2):515–524. doi: 10.1042/bj2450515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D. Sorting and traffic in the central vacuolar system. Cell. 1989 Jun 2;57(5):703–706. doi: 10.1016/0092-8674(89)90783-6. [DOI] [PubMed] [Google Scholar]
- LaVallie E. R., Rehemtulla A., Racie L. A., DiBlasio E. A., Ferenz C., Grant K. L., Light A., McCoy J. M. Cloning and functional expression of a cDNA encoding the catalytic subunit of bovine enterokinase. J Biol Chem. 1993 Nov 5;268(31):23311–23317. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leduc R., Molloy S. S., Thorne B. A., Thomas G. Activation of human furin precursor processing endoprotease occurs by an intramolecular autoproteolytic cleavage. J Biol Chem. 1992 Jul 15;267(20):14304–14308. [PubMed] [Google Scholar]
- Lenter M., Vestweber D. The integrin chains beta 1 and alpha 6 associate with the chaperone calnexin prior to integrin assembly. J Biol Chem. 1994 Apr 22;269(16):12263–12268. [PubMed] [Google Scholar]
- Liu Y. C., Kawagishi M., Mikayama T., Inagaki Y., Takeuchi T., Ohashi H. Processing of a fusion protein by endoprotease in COS-1 cells for secretion of mature peptide by using a chimeric expression vector. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8957–8961. doi: 10.1073/pnas.90.19.8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusson J., Vieau D., Hamelin J., Day R., Chrétien M., Seidah N. G. cDNA structure of the mouse and rat subtilisin/kexin-like PC5: a candidate proprotein convertase expressed in endocrine and nonendocrine cells. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6691–6695. doi: 10.1073/pnas.90.14.6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand P., Tang J., Bond J. S. Membrane association and oligomeric organization of the alpha and beta subunits of mouse meprin A. J Biol Chem. 1994 May 27;269(21):15388–15393. [PubMed] [Google Scholar]
- Matthews D. J., Goodman L. J., Gorman C. M., Wells J. A. A survey of furin substrate specificity using substrate phage display. Protein Sci. 1994 Aug;3(8):1197–1205. doi: 10.1002/pro.5560030805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milhiet P. E., Corbeil D., Simon V., Kenny A. J., Crine P., Boileau G. Expression of rat endopeptidase-24.18 in COS-1 cells: membrane topology and activity. Biochem J. 1994 May 15;300(Pt 1):37–43. doi: 10.1042/bj3000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy S. S., Thomas L., VanSlyke J. K., Stenberg P. E., Thomas G. Intracellular trafficking and activation of the furin proprotein convertase: localization to the TGN and recycling from the cell surface. EMBO J. 1994 Jan 1;13(1):18–33. doi: 10.1002/j.1460-2075.1994.tb06231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Murakami K., Nakayama K. Identification of an isoform with an extremely large Cys-rich region of PC6, a Kex2-like processing endoprotease. FEBS Lett. 1993 Jul 26;327(2):165–171. doi: 10.1016/0014-5793(93)80163-o. [DOI] [PubMed] [Google Scholar]
- Notice of retraction. J Biol Chem. 1993 Aug 15;268(23):17647–17647. [PubMed] [Google Scholar]
- Noël G., Zollinger L., Larivière N., Nault C., Crine P., Boileau G. Expression of porcine pro-opiomelanocortin cDNA in heterologous monkey kidney cells. Biosynthesis and secretion of the prohormone without processing. J Biol Chem. 1987 Feb 5;262(4):1876–1881. [PubMed] [Google Scholar]
- Rawlings N. D., Barrett A. J. Evolutionary families of peptidases. Biochem J. 1993 Feb 15;290(Pt 1):205–218. doi: 10.1042/bj2900205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehemtulla A., Dorner A. J., Kaufman R. J. Regulation of PACE propeptide-processing activity: requirement for a post-endoplasmic reticulum compartment and autoproteolytic activation. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8235–8239. doi: 10.1073/pnas.89.17.8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalken J. A., Roebroek A. J., Oomen P. P., Wagenaar S. S., Debruyne F. M., Bloemers H. P., Van de Ven W. J. fur gene expression as a discriminating marker for small cell and nonsmall cell lung carcinomas. J Clin Invest. 1987 Dec;80(6):1545–1549. doi: 10.1172/JCI113240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer-Dene B., Thorogood P., Nair S., Kenny A. J., Harris M., Henderson B. Distribution of, and a putative role for, the cell-surface neutral metallo-endopeptidases during mammalian craniofacial development. Development. 1994 Nov;120(11):3213–3226. doi: 10.1242/dev.120.11.3213. [DOI] [PubMed] [Google Scholar]
- Stephenson S. L., Kenny A. J. The metabolism of neuropeptides. Hydrolysis of peptides by the phosphoramidon-insensitive rat kidney enzyme 'endopeptidase-2' and by rat microvillar membranes. Biochem J. 1988 Oct 1;255(1):45–51. doi: 10.1042/bj2550045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterchi E. E., Naim H. Y., Lentze M. J. Biosynthesis of N-benzoyl-L-tyrosyl-p-aminobenzoic acid hydrolase: disulfide-linked dimers are formed at the site of synthesis in the rough endoplasmic reticulum. Arch Biochem Biophys. 1988 Aug 15;265(1):119–127. doi: 10.1016/0003-9861(88)90377-3. [DOI] [PubMed] [Google Scholar]
- Sterchi E. E., Naim H. Y., Lentze M. J., Hauri H. P., Fransen J. A. N-benzoyl-L-tyrosyl-p-aminobenzoic acid hydrolase: a metalloendopeptidase of the human intestinal microvillus membrane which degrades biologically active peptides. Arch Biochem Biophys. 1988 Aug 15;265(1):105–118. doi: 10.1016/0003-9861(88)90376-1. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Nakagawa T., Ikemizu J., Nagahama M., Murakami K., Nakayama K. Sequence requirements for precursor cleavage within the constitutive secretory pathway. J Biol Chem. 1992 Apr 25;267(12):8270–8274. [PubMed] [Google Scholar]
- Wolz R. L., Harris R. B., Bond J. S. Mapping the active site of meprin-A with peptide substrates and inhibitors. Biochemistry. 1991 Aug 27;30(34):8488–8493. doi: 10.1021/bi00098a029. [DOI] [PubMed] [Google Scholar]
- Yanagita M., Nakayama K., Takeuchi T. Processing of mutated proinsulin with tetrabasic cleavage sites to bioactive insulin in the non-endocrine cell line, COS-7. FEBS Lett. 1992 Oct 12;311(1):55–59. doi: 10.1016/0014-5793(92)81366-t. [DOI] [PubMed] [Google Scholar]
- Yurewicz E. C., Hibler D., Fontenot G. K., Sacco A. G., Harris J. Nucleotide sequence of cDNA encoding ZP3 alpha, a sperm-binding glycoprotein from zona pellucida of pig oocyte. Biochim Biophys Acta. 1993 Aug 19;1174(2):211–214. doi: 10.1016/0167-4781(93)90119-x. [DOI] [PubMed] [Google Scholar]