Abstract
A 79-year-old woman presented with subacutely worsening headaches and right arm weakness. MRI showed diffuse pachymeningeal enhancement. Serologic workup revealed elevated erythrocyte sedimentation rate and C-reactive protein. CSF demonstrated elevated opening pressure, a lymphocytic pleocytosis, and elevated protein. We discuss our differential diagnosis and distinguish between 2 overlapping clinical entities.
Case Description
In August 2020, a 79-year-old German woman with a history of hypertension, recurrent sinusitis, and hypothyroidism experienced new-onset persistent pressure-like headaches. They were associated with photophobia and nausea and improved when lying flat. She had no significant trauma. Her primary care physician prescribed a short course of low-dose prednisone (10 mg daily for 5 days) and gabapentin, but these did not provide relief. After 2 months, she was referred to a neurologist who obtained noncontrast head CT, which showed a chronic right subdural hematoma. MRI with gadolinium demonstrated smooth, bilateral pachymeningeal thickening and enhancement. Initial erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were within normal limits. Based on these findings, her neurologist believed that the symptoms were secondary to intracranial hypotension and instructed the patient to increase fluid intake. One month later in November 2020, owing to persistent symptoms, she was admitted to the hospital for presumed CSF leak, with CT head again showing a chronic right subdural hematoma. CSF analysis was performed, with white blood cell (WBC) count 8, lymphocytes 56%, neutrophils 22%, monocytes 22%, protein 78, glucose 46, and bacterial cultures negative. No opening pressure was recorded. A blood patch trial provided no relief, and a follow-up CT myelogram showed no evidence of CSF leak. She was discharged from the hospital with butalbital-acetaminophen-caffeine. 3 days after discharge, she experienced an episode of word-finding difficulty and sudden-onset right arm weakness. She was urgently admitted to the hospital for presumed seizure, where EEG showed generalized slowing without epileptiform discharges. At that time, she was transferred to our tertiary care center for further workup.
When she initially presented to our hospital, her neurologic examination revealed psychomotor slowing of speech; word-finding difficulty during casual conversation; right arm weakness; and diffuse, symmetric hyperreflexia. As noted in the Table, blood studies revealed an elevated ESR of 128 and CRP of 7.3; normal total immunoglobulin G (IgG) of 1,267 though specific IgG4 subclass was elevated at 101.1. Her antineutrophil cytoplasmic antibodies (ANCA) along with protein-3 (PR-3) antibodies were also elevated. Her T-spot test for Mycobacterium tuberculosis (Tb) was positive. CSF analysis was notable for an elevated opening pressure (38 mmH2O); a lymphocytic pleocytosis (WBC 8/10) with lymphocytes 74%/61%, monocytes 25%/33%, and neutrophils 1%/6%; and elevated protein (77.3).
Table.
Summary of Patient Workup
| Serologic workup (reference values) | CSF analysis (reference values) | ||
| WBC | 8.15 (4.5–11.0) | Appearance | Clear, colorless |
| Hgb | 10.8 (12.0–15.0) | OP | 38 mmH20 (10–25) |
| Hct | 33.2 (36.0–46.0) | WBC | 8/10 (0–5), 61%/74% Lymph% |
| Plt | 422 (150–350) | RBC | 2/1 (0–5) |
| Na | 137 (135–148) | Glucose | 58 (50–80) |
| K | 3.7 (3.5–5.1) | Protein | 77.3 (15–60) |
| Cl | 92 (96–109) | Bacterial culture | Negative (Neg) |
| CO2 | 32 (21–31) | Viral studies (HSV, VZV, EBV, JCV) | Negative (Neg) |
| BUN | 7 (7.0–22.0) | ||
| Cr | 0.5 (0.5–1.2) | ||
| Glu | 111 (71–99) | ||
| ESR | 128 (0–20) | ||
| CRP | 7.3 (0–0.5) | ||
| ANA | Negative (Neg) | ||
| Anti-dsDNA | Negative (Neg) | ||
| RF | 41 (0.0–14.0) | ||
| Serum IgG | 1,267 (600–1700) | ||
| IgG4 subclass | 101.1 (2–96) | ||
| ANCA | Positive (Neg) | ||
| Anti-smooth muscle Ab | Positive (Neg) | ||
| Ro52 | Positive (Neg) | ||
| Anti-PR3 | 96.6 (0.0–0.4) | ||
| HIV 1/2 Ab | Negative (Neg) | ||
| Histoplasma Ab | Negative (Neg) | ||
| Lyme Ab | Negative (Neg) | ||
| T-spot Tb | Positive (Neg) | ||
Abbreviations: Ab = antibody; ANA = antinuclear antibody; ANCA = anti-neutrophil cytoplasmic antibodies; BUN = blood urea nitrogen; Cl = chloride; CO2 = carbon dioxide; Cr = creatinine; CRP = C-reactive protein; dsDNA = double-stranded DNA; EBV = Epstein-Barr virus; ESR = erythrocyte sedimentation rate; glu = glucose; H = high; Hct = hematocrit; Hgb = hemoglobin; HSV = herpes simplex virus; IgG = immunoglobulin G; JCV = John Cunningham virus; K = potassium; L = low; lymph = lymphocytes; Na = sodium; Neg = negative; OP = opening pressure; Plt = platelets; PR3 = proteinase 3; RBC = red blood cell; RF = rheumatoid factor; Tb = tuberculosis; VZV = varicella zoster virus; WBC = white blood cell.
This table summarizes the serologic and CSF workup our patient underwent on admission to the tertiary care center. Reference values are provided.
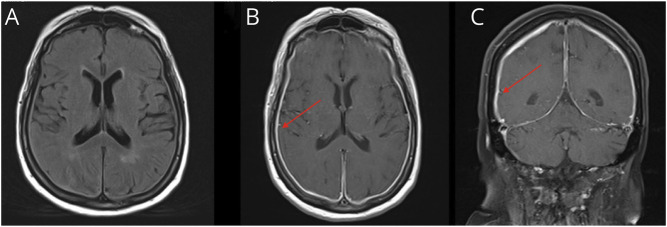
Brain MRI with gadolinium (Figure 1) was notable for diffuse smooth pachymeningeal enhancement along with chronic known subdural hematoma (not shown). A CT of the chest, abdomen, and pelvis with contrast revealed a mass-like opacity at both lung apices along with some mild paraseptal emphysema. Subsequent PET scan showed no hypometabolic or hypermetabolic areas in the brain, but intensely fluorodeoxyglucose (FDG)-avid biapical lung opacities.
Figure 1. Brain MRI From November 2020.
This figure highlights our patient's brain MRI in November 2020. (A) A T2/FLAIR axial sequence of our patient, which notes no significant abnormality. However, on T1 postcontrast sequences, both axial cut (B) and coronal cut (C) note diffuse and smooth pachymeningeal thickening and enhancement (red arrows).
Differential Diagnosis
Our patient presented with new-onset persistent, diffuse, and positional headaches with her examination showing psychomotor slowing and right arm weakness. After review of her serum, CSF, and radiographic studies, we considered the following differential diagnosis.
Inflammatory Considerations
Given the numerous elevated inflammatory markers (that is, ESR, CRP, ANCA, anti-smooth muscle Ab, Ro52, anti-PR3, and IgG4) and the diffuse pachymeningeal enhancement, inflammatory considerations including hypertrophic pachymeningitis (HP), granulomatosis with polyangiitis (GPA), IgG4-related disease (RD), neurosarcoidosis, and giant cell arteritis (GCA) were high on our differential. HP is described in the literature to have a variable clinical presentation, with most common symptoms including headaches and cranial nerve (predominantly optic and facial nerve) involvement.1 In differentiating whether HP is idiopathic or secondary to another process, extraneurologic or systemic manifestations may help to define the etiology. The first of these secondary diagnoses includes GPA. GPA is an anti-ANCA-associated vasculitis with involvement of the nasopharyngeal, pulmonary, or renal systems; neurologic involvement includes pachymeningeal inflammation and cranial neuropathies due to nerve compression, with the most common presenting neurologic symptom being new-onset headaches.1-3 Extraneurologic manifestations most commonly include granulomatous disease (for example, ear, nose, and throat disease; lung nodules or masses; and retro-orbital tumors) or vasculitic disease (glomerulonephritis, alveolar hemorrhage, or scleritis).4 Thus, given our patient's presenting symptoms, history of sinusitis, MRI findings, CT scan with pulmonary involvement, and ANCA positivity, GPA was high on our differential. Yet, IgG4 subclass was elevated in our patient, so IgG4-RD was also a possible diagnosis. However, she lacked systemic features of this disorder. IgG4-RD has been described as a systemic fibroinflammatory disease that can affect any organ (most commonly lacrimal and salivary glands, pancreas, and retroperitoneum, and rarely the meninges and pituitary gland).1,5 In particular, neurologic manifestations include pachymeningitis (presenting as headaches).5 Next, sarcoidosis frequently affects the lung in 80%–90% of patients, with neurologic involvement including facial nerve neuropathy, optic neuritis, meningitis, and peripheral neuropathy.1 Finally, GCA is always important to maintain on the differential for a patient older than 50 years with new-onset headaches; if left untreated, it may result in severe neurologic and ophthalmologic manifestations.6 While there are no specific serologic markers to definitively diagnose GCA, ESR and CRP are commonly elevated; temporal artery biopsy is the gold standard for diagnosis.6
Infectious Considerations
While most bacterial or viral infectious causes were less likely given the subacute time course of our patient's symptoms and clinical appearance, we considered syphilis, fungal meningitis, and Tb meningitis as causative agents of her presentation.7 Syphilis was least likely, given her negative venereal disease research laboratory testing and preserved posterior column examination findings.8 Fungal meningitis, which usually affects the immunocompromised population, tends to present with subacute-to-chronic clinical symptoms including headaches; CSF analysis shows normal-to-elevated opening pressure, moderate lymphocytic pleocytosis, elevated protein, and low glucose, with specific fungal cultures often rendering results.7 Tb meningitis is rare in Western countries and in HIV-negative patients but can present with worsening fever and headaches over 2–3 weeks; other frequently observed clinical features include lethargy, seizures, and cranial nerve palsies.8 While routine serologic studies are less helpful (and T-spot can be nonspecific), CSF analysis can show elevated protein, low glucose, and lymphocytic pleocytosis, similar to those CSF findings in fungal meningitis. Acid-fast bacilli (AFB) smears of CSF are positive in less than 25% of patients, so ultimately mycobacteria culture remains the gold standard for detection and diagnosis.7,8 MRI usually shows basilar leptomeningitis rather than a smooth pachymeningitis, as seen in our patient.9 Although our patient had a positive T-spot test, the CSF profile and imaging characteristics were not consistent with a diagnosis of Tb meningitis.
Malignant Infiltration
Finally, in an elderly patient with new-onset headaches, it is important to consider malignant processes, whether primary malignancy or metastatic disease. In our patient, there were undifferentiated masses noted in the lung apices on CT chest, with PET scan showing intensely FDG-avid lung opacities, which raised the suspicion of a malignant process. While there was no primary malignancy noted on her MRI brain, CNS lymphoma is a rare form of non-Hodgkin lymphoma that is in the differential diagnosis of patients presenting with progressive encephalopathy or focal neurologic deficits, although it is most commonly seen in immunocompromised patients.10 However, it can be seen in immunocompetent patients, although imaging tends to show uniformly solitary enhancing mass lesions bordering the subarachnoid space without necrosis,10 rather than pachymeningeal disease.
Final Diagnosis and Treatment Plan
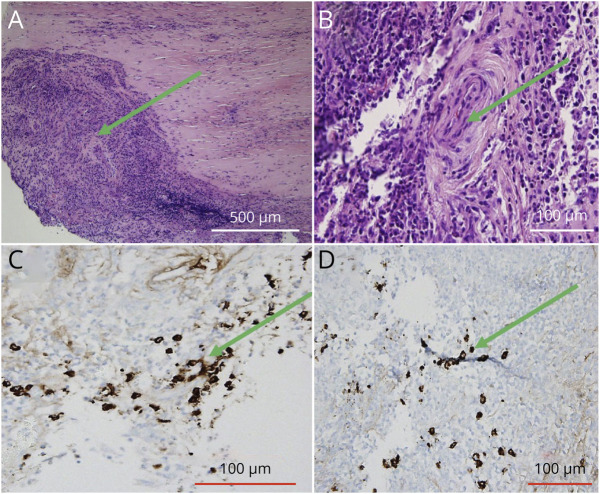
Our initial working diagnosis was IgG4-RD due to her elevated IgG4 in the serum and clinical findings. To confirm the diagnosis before treatment initiation, the patient underwent dural biopsy (Figure 2), which showed acute and chronic pachymeningitis without evidence of neoplasm, although there were significantly increased IgG4-positive plasma cells that did not clearly meet criteria for IgG4-RD. She underwent extensive evaluation for her positive T-spot testing, including blood, CSF, and sputum AFB cultures, which were negative. She was presumed to have latent Tb and was started on appropriate treatment. Finally, she underwent lung biopsy that showed evidence of small vessel vasculitis and organizing lung injury without evidence of granulomatous vasculitis or necrosis; immunostains for IgG4-RD were negative. She was discharged home with close follow-up, with working diagnosis of GPA; she was placed on a 60-mg prednisone taper and planned to start methotrexate as an outpatient. Within 1 week after discharge, she developed symptoms of sinusitis and was treated accordingly. Reconsideration of her diagnosis after clinical sinusitis with the presenting pachymeningitis on MRI, lung biopsy demonstrating small vessel vasculitis, and positive PR3 and ANCA ultimately supported the diagnosis of granulomatosis with polyangiitis with early systemic involvement. She was started on methotrexate and steroids for induction therapy, with plan for subsequent transition to rituximab, given remission rates of approximately 90% seen in the literature.11
Figure 2. Dural Biopsy From November 2020.
This figure showcases our patient's dural biopsy. Her hematoxylin and eosin stains (A and B) are indicative of a substantial number of plasma cells and inflammatory infiltrates, with multinucleated giant cell formation (green arrows). There is no overwhelming disruption of blood vessels. The IgG4 stains (C and D) identify plasma cells that produce IgG4, but this histological slide is not suggestive of IgG4-RD.
Discussion
As seen in our patient, IgG4-RD and GPA can be difficult to differentiate due to overlapping symptoms and syndromes and similar serologic markers. Initially described by the German pathologist Dr. Friedrich Wegener in 1936, GPA was termed Wegener granulomatosis until 2011 when evidence exposed Dr. Wegener's involvement with the Nazi party and participation in medical experiments on Jewish citizens in the Lodz Ghetto.12,13 The eponym has thus been rejected with preference for the mechanistic descriptor. GPA usually affects small and medium-sized vessels; it primarily involves the nose, sinuses, lungs, and kidneys, with neurologic involvement seen in 20–50% of patients.3,14 Peripheral nervous system involvement is more common than CNS involvement, with peripheral neuropathy being the most common disorder.14 CNS involvement, present in approximately 10% of patients, most commonly manifests as headaches, sensory or motor impairment, or vestibular syndrome.15 In some patients, endothelial cell dysfunction may induce a hypercoagulable state and lead to ischemic strokes. Meningeal inflammation of the dura leads to HP, as seen in our patient.1 Similarly, IgG4-RD is a multisystem inflammatory disorder with uncommon neurologic involvement, although in contrast to GPA, neurologic involvement is predominantly CNS-based and manifests as hypophysitis or HP and is seen in approximately 0.949 cases per 100,000 in ethnic populations.5 Patients present with panhypopituitarism (symptoms include general malaise, polyuria, polydipsia, amenorrhea, decreased libido, and weight loss).1,5,16
Epidemiology of GPA and IgG4-RD differs; while GPA is most prevalent between 64 and 75 years with equal prevalence between men and women, IgG4-RD has a peak onset at ages 61–70 and is more prevalent in men.3,5 While the overall incidence of GPA is low in the United States, it is significantly higher in European countries including Germany or Scandinavian countries, with an incidence of 34 cases per million per year in Germany.17,18 By contrast, there is no established ethnic predisposition for IgG4-RD, although there is speculation that there may be a greater incidence in the Asian (specifically, Japanese) populations, where IgG4-RD was first described.19,20
The American College of Rheumatology classification criteria for GPA have a sensitivity of 93% and specificity of 94% and use positive test results for cytoplasmic (c)-ANCA as part of the diagnosis.21 Ultimately, CSF analysis, MRI brain, PET scan, serologic workup (often demonstrating anemia, leukopenia, and thrombocytosis on basic blood work, in addition to positive c-ANCA and anti-PR3 antibodies), and biopsy are useful in establishing the diagnosis of GPA.3,14 In contrast to the lack of definitive diagnostic criteria for GPA, the diagnosis of IgG4-RD is based on strict criteria, requiring clinical and radiologic features, serologic diagnosis of serum IgG4 levels greater than 135 mg per deciliter, and positive biopsy findings (with a ratio of IgG4-positive plasma cells over IgG-positive cells greater than 40% and number of IgG4-positive plasma cells greater than 10 per high-powered field).22
Our case report is instructive because it emphasizes the critical point of meeting strict diagnostic criteria or classification guidelines, especially in cases where 2 diagnoses share overlapping features. While initially considered to be IgG4-RD, our patient ultimately did not meet these strict diagnostic criteria. However, as with many autoimmune processes, tissue diagnosis can be definitive. When 2 diagnoses overlap to a large degree, it is imperative to perform tissue biopsy of at least one site, although numerous sites may be helpful to confirm a diagnosis and exclude mimics. This case emphasizes the importance of being mindful of anchoring bias and integrating new diagnostic information to provide the most effective clinical care for our patients.
Glossary
- ANCA
antineutrophil cytoplasmic antibodies
- CRP
C-reactive protein
- ESR
erythrocyte sedimentation rate
- FDG
fluorodeoxyglucose
- GCA
giant cell arteritis
- GPA
granulomatosis with polyangiitis
- HP
hypertrophic pachymeningitis
- RD
related disease
- WBC
white blood cell
Appendix. Authors
| Name | Location | Contribution |
| Manali S. Sheth, MD | Harvard Medical School; Department of Neurology, Brigham and Women's Hospital, Boston, MA | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| David E. Hale | Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD | Drafting/revision of the manuscript for content, including medical writing for content |
| Justin C. Mcarthur | Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD | Drafting/revision of the manuscript for content, including medical writing for content |
| Scott S. Zamvil, MD, PhD | Department of Neurology and Program in Immunology, University of California at San Francisco | Drafting/revision of the manuscript for content, including medical writing for content |
| Myla D. Goldman | Department of Neurology, Virginia Commonwealth University, Richmond | Drafting/revision of the manuscript for content, including medical writing for content |
| Claire Riley, MD | Department of Neurology, Columbia University, New York | Drafting/revision of the manuscript for content, including medical writing for content |
| Tanuja Chitnis, MD | Harvard Medical School; Department of Neurology, Brigham and Women's Hospital, Boston, MA | Drafting/revision of the manuscript for content, including medical writing for content |
Study Funding
The authors report no targeted funding.
Disclosure
M.S. Sheth has received fellowship funding/research support from the National Multiple Sclerosis Society. Disclosure does not conflict with the work being present; D.E. Hale reports no disclosures relevant to the manuscript; J.C. McArthur reports no disclosures relevant to the manuscript; S.S. Zamvil is current Deputy Editor of Neurology: Neuroimmunology Neuroinflammation; M.D. Goldman is currently co-chair of National MS Society, the MS Clinical Fellows Complex Case Webinar where this case was first presented; C.S. Riley is currently co-chair of National MS Society, the MS Clinical Fellows Complex Case Webinar where this case was first presented; T. Chitnis has received compensation from the National MS Society for fellowship funding and research support. Go to Neurology.org/NN for full disclosures.
References
- 1.Abrantes FF, Moraes MPM, Rezende Filho FM, Pedroso JL, Barsottini OGP. A clinical approach to hypertrophic pachymeningitis. Arq Neuropsiquiatr. 2020;78(12):797-804. doi: 10.1590/0004-282x20200073 [DOI] [PubMed] [Google Scholar]
- 2.Smoleńska Ż, Masiak A, Zdrojewski Z. Hypertrophic pachymeningitis as an important neurological complication of granulomatosis with polyangiitis. Reumatologia. 2018;56(6):399-405. doi: 10.5114/reum.2018.80719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kubaisi B, Abu Samra K, Foster CS. Granulomatosis with polyangiitis (Wegener's disease): an updated review of ocular disease manifestations. Intractable Rare Dis Res. 2016;5(2):61-69. doi: 10.5582/irdr.2016.01014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guzman-Soto MI, Kimura Y, Romero-Sanchez G, et al. . From head to toe: granulomatosis with polyangiitis. RadioGraphics. 2021;41(7):1973-1991. doi: 10.1148/rg.2021210132 [DOI] [PubMed] [Google Scholar]
- 5.Baptista B, Casian A, Gunawardena H, D'Cruz D, Rice CM. Neurological manifestations of IgG4-related disease. Curr Treat Options Neurol. 2017;19(4):14. doi: 10.1007/s11940-017-0450-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bilton EJ, Mollan SP. Giant cell arteritis: reviewing the advancing diagnostics and management. Eye. 2023;37(12):2365-2373. doi: 10.1038/s41433-023-02433-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorsett M, Liang SY. Diagnosis and treatment of central nervous system infections in the emergency department. Emerg Med Clin North Am. 2016;34(4):917-942. doi: 10.1016/j.emc.2016.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Archibald LK, Quisling RG. Central nervous system infections. Textbook of Neurointensive Care. 2013;7:427-517. doi: 10.1007/978-1-4471-5226-2_22 [DOI] [Google Scholar]
- 9.Garg RK, Malhotra HS, Jain A. Neuroimaging in tuberculous meningitis. Neurol India. 2016;64(2):219-227. doi: 10.4103/0028-3886.177608 [DOI] [PubMed] [Google Scholar]
- 10.Scott BJ, Douglas VC, Tihan T, Rubenstein JL, Josephson SA. A Systematic approach to the diagnosis of suspected central nervous system lymphoma. JAMA Neurol. 2013;70(3):311-319. doi: 10.1001/jamaneurol.2013.606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geetha D, Kallenberg C, Stone JH, et al. . Current therapy of granulomatosis with polyangiitis and microscopic polyangiitis: the role of rituximab. J Nephrol. 2015;28(1):17-27. doi: 10.1007/s40620-014-0135-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woywodt A, Matteson EL. Wegener's granulomatosis—probing the untold past of the man behind the eponym. Rheumatology. 2006;45(10):1303-1306. doi: 10.1093/rheumatology/kel258 [DOI] [PubMed] [Google Scholar]
- 13.Falk RJ, Gross WL, Guillevin L, et al. . Granulomatosis with polyangiitis (Wegener's): an alternative name for Wegener's granulomatosis. Arthritis Rheum. 2011;63(4):863-864. doi: 10.1002/art.30286 [DOI] [PubMed] [Google Scholar]
- 14.Jazayeri SB, Rahimian A, Ahadi MS, Tavakolpour S, Alesaeidi S. Neurologic involvement in granulomatosis with polyangiitis: a comparative study. Biol Life Sci Forum. 2022;19(1):19. [Google Scholar]
- 15.Seror R, Mahr A, Ramanoelina J, Pagnoux C, Cohen P, Guillevin L. Central nervous system involvement in Wegener granulomatosis. Medicine (Baltimore). 2006;85(1):53-65. doi: 10.1097/01.md.0000200166.90373.41 [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Xu J. Imaging features, clinicopathological analysis and diagnostic strategy of IgG4-related hypertrophic pachymeningitis. Ann Palliat Med. 2020;9(5):2551-2558. doi: 10.21037/apm-19-452 [DOI] [PubMed] [Google Scholar]
- 17.Panupattanapong S, Stwalley DL, White AJ, Olsen MA, French AR, Hartman ME. Epidemiology and outcomes of granulomatosis with polyangiitis in pediatric and working-age adult populations in the United States: analysis of a large national claims database. Arthritis Rheumatol. 2018;70(12):2067-2076. doi: 10.1002/art.40577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellmich B, Lamprecht P, Spearpoint P, et al. . New insights into the epidemiology of ANCA-associated vasculitides in Germany: results from a claims data study. Rheumatology. 2021;60(10):4868-4873. doi: 10.1093/rheumatology/keaa924 [DOI] [PubMed] [Google Scholar]
- 19.Floreani A, Okazaki K, Uchida K, Gershwin ME. IgG4-related disease: Changing epidemiology and new thoughts on a multisystem disease. J Transl Autoimmun. 2021;4:100074. doi: 10.1016/j.jtauto.2020.100074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallace ZS, Miles G, Smolkina E, et al. . Incidence, prevalence and mortality of IgG4-related disease in the USA: a claims-based analysis of commercially insured adults. Ann Rheum Dis. 2023;82(7):957-962. doi: 10.1136/ard-2023-223950 [DOI] [PubMed] [Google Scholar]
- 21.Robson JC, Grayson PC, Ponte C, et al. . 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for granulomatosis with polyangiitis. Ann Rheum Dis. 2022;81(3):315-320. doi: 10.1136/annrheumdis-2021-221795 [DOI] [PubMed] [Google Scholar]
- 22.Umehara H, Okazaki K, Kawa S, et al. . The 2020 revised comprehensive diagnostic (RCD) criteria for IgG4-RD. Mod Rheumatol. 2021;31(3):529-533. doi: 10.1080/14397595.2020.1859710 [DOI] [PubMed] [Google Scholar]