Abstract
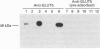
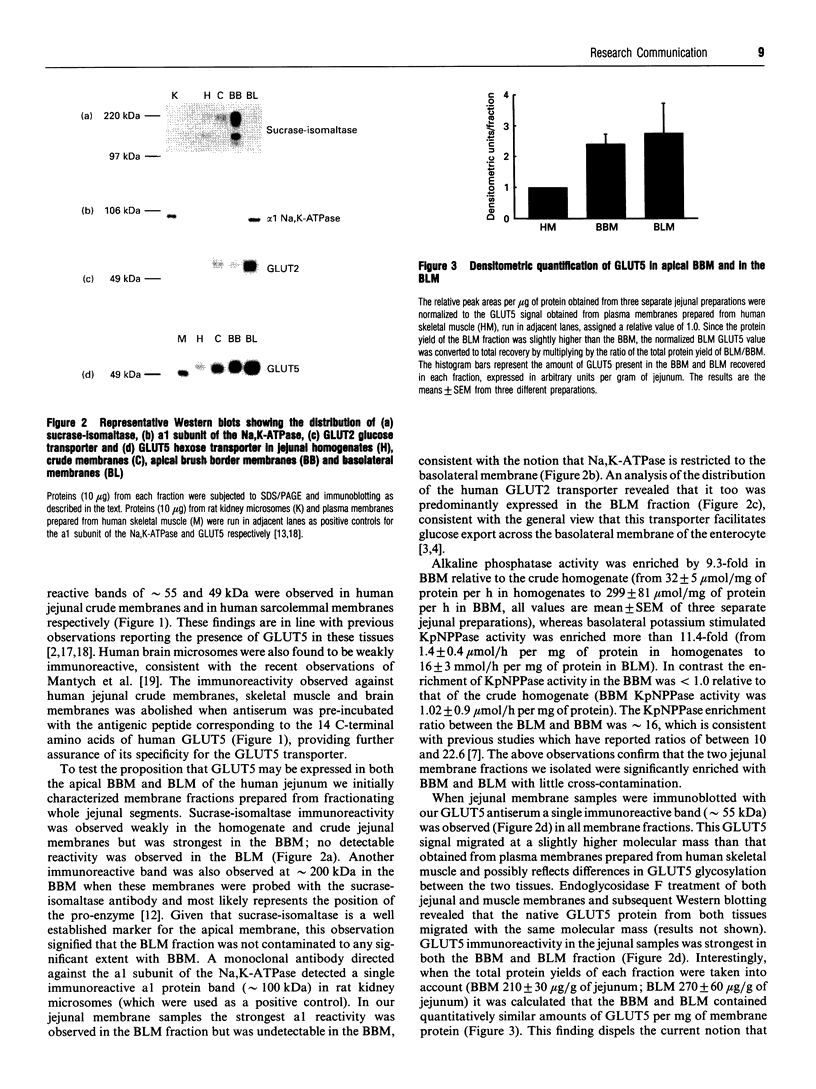
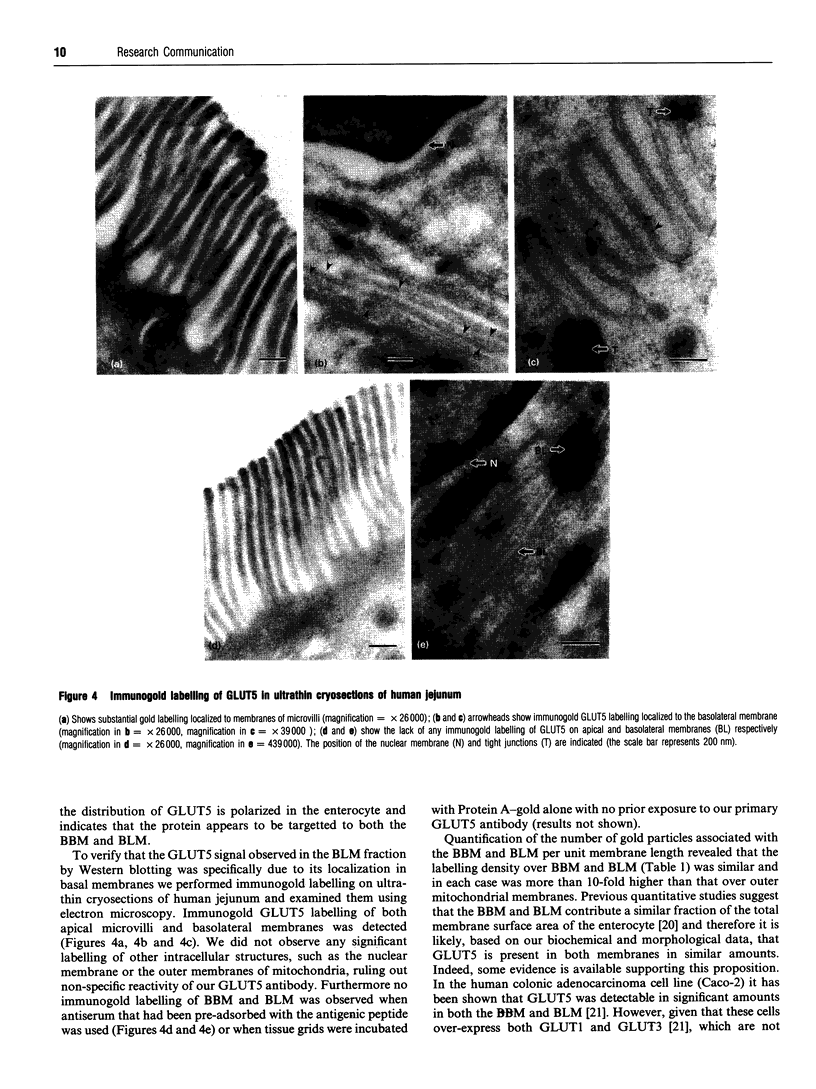
The intestine is a major site of expression of the human GLUT5 hexose transporter, which is thought to be localized exclusively to the brush border membrane (BBM) where its major role is likely to be in the absorption of fructose. In this study we present novel biochemical and morphological evidence showing that the GLUT5 transporter is also expressed in the basolateral membrane (BLM) of the human intestine. BBM and BLM were isolated by fractionation of human jejunum. BBM were enriched with alkaline phosphatase activity by over 9-fold relative to a crude jejunal homogenate and contained immunoreactive sucrase-isomaltase and GLUT5 proteins. By contrast the BBM fraction was substantially depleted of immunoreactive a1 subunits of the Na,K-ATPase and GLUT2 glucose transporters which were abundantly present in the BLM fraction. This BLM fraction was enriched by over 11-fold in potassium-stimulated phosphatase activity relative to the crude homogenate; BLM also reacted to immunological probes for GLUT5 but showed no observable reactivity with antibodies directed against sucrase-isomaltase. Quantitative immunoblotting revealed that the BBM and BLM contained near equal amounts of GLUT5 per mg of membrane protein. Immunogold localization of GLUT5 on ultrathin sections of human jejunum showed that GLUT5 was present in both apical BBM and BLM. This gold labelling was absent when antiserum was pre-incubated with the antigenic peptide corresponding to a specific C-terminal sequence of human GLUT5. Quantitative analyses of the number of gold particles per unit length of BBM and BLM indicated that the mean density of gold labelling was marginally greater in the BBM (0.399 gold particles/micrometer) than in the BLM (0.293 gold particle/micrometer). The localization of GLUT5 in the BLM of the human jejunum may suggest that it specifically participates in the transfer of fructose across the basal membrane of the enterocyte.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burant C. F., Takeda J., Brot-Laroche E., Bell G. I., Davidson N. O. Fructose transporter in human spermatozoa and small intestine is GLUT5. J Biol Chem. 1992 Jul 25;267(21):14523–14526. [PubMed] [Google Scholar]
- Buschmann R. J., Manke D. J. Morphometric analysis of the membranes and organelles of small intestinal enterocytes. I. Fasted hamster. J Ultrastruct Res. 1981 Jul;76(1):1–14. doi: 10.1016/s0022-5320(81)80046-9. [DOI] [PubMed] [Google Scholar]
- Cheeseman C. I. GLUT2 is the transporter for fructose across the rat intestinal basolateral membrane. Gastroenterology. 1993 Oct;105(4):1050–1056. doi: 10.1016/0016-5085(93)90948-c. [DOI] [PubMed] [Google Scholar]
- Colville C. A., Seatter M. J., Jess T. J., Gould G. W., Thomas H. M. Kinetic analysis of the liver-type (GLUT2) and brain-type (GLUT3) glucose transporters in Xenopus oocytes: substrate specificities and effects of transport inhibitors. Biochem J. 1993 Mar 15;290(Pt 3):701–706. doi: 10.1042/bj2900701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson N. O., Hausman A. M., Ifkovits C. A., Buse J. B., Gould G. W., Burant C. F., Bell G. I. Human intestinal glucose transporter expression and localization of GLUT5. Am J Physiol. 1992 Mar;262(3 Pt 1):C795–C800. doi: 10.1152/ajpcell.1992.262.3.C795. [DOI] [PubMed] [Google Scholar]
- Felsenfeld D. P., Sweadner K. J. Fine specificity mapping and topography of an isozyme-specific epitope of the Na,K-ATPase catalytic subunit. J Biol Chem. 1988 Aug 5;263(22):10932–10942. [PubMed] [Google Scholar]
- Gould G. W., Holman G. D. The glucose transporter family: structure, function and tissue-specific expression. Biochem J. 1993 Oct 15;295(Pt 2):329–341. doi: 10.1042/bj2950329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., McDowall A., Back R., Dubochet J. On the preparation of cryosections for immunocytochemistry. J Ultrastruct Res. 1984 Oct;89(1):65–78. doi: 10.1016/s0022-5320(84)80024-6. [DOI] [PubMed] [Google Scholar]
- Harris D. S., Slot J. W., Geuze H. J., James D. E. Polarized distribution of glucose transporter isoforms in Caco-2 cells. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7556–7560. doi: 10.1073/pnas.89.16.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri H. P., Bucher K. Immunoblotting with monoclonal antibodies: importance of the blocking solution. Anal Biochem. 1986 Dec;159(2):386–389. doi: 10.1016/0003-2697(86)90357-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lucocq J. Quantitation of gold labelling and antigens in immunolabelled ultrathin sections. J Anat. 1994 Feb;184(Pt 1):1–13. [PMC free article] [PubMed] [Google Scholar]
- Mantych G. J., James D. E., Devaskar S. U. Jejunal/kidney glucose transporter isoform (Glut-5) is expressed in the human blood-brain barrier. Endocrinology. 1993 Jan;132(1):35–40. doi: 10.1210/endo.132.1.8419132. [DOI] [PubMed] [Google Scholar]
- Mesonero J., Mahraoui L., Matosin M., Rodolosse A., Rousset M., Brot-Laroche E. Expression of the hexose transporters GLUT1-GLUT5 and SGLT1 in clones of Caco-2 cells. Biochem Soc Trans. 1994 Aug;22(3):681–684. doi: 10.1042/bst0220681. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Takagi T., Fujii T., Matsubara T., Hase K., Taketani Y., Oka T., Minami H., Nakabou Y. Role of liver-type glucose transporter (GLUT2) in transport across the basolateral membrane in rat jejunum. FEBS Lett. 1992 Dec 21;314(3):466–470. doi: 10.1016/0014-5793(92)81528-t. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Tatsumi S., Morimoto A., Minami H., Yamamoto H., Sone K., Taketani Y., Nakabou Y., Oka T., Takeda E. Characterization of the rabbit intestinal fructose transporter (GLUT5). Biochem J. 1994 Nov 1;303(Pt 3):877–883. doi: 10.1042/bj3030877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsenigo M. N., Tosco M., Esposito G., Faelli A. The basolateral membrane of rat enterocyte: its purification from brush border contamination. Anal Biochem. 1985 Feb 1;144(2):577–583. doi: 10.1016/0003-2697(85)90156-3. [DOI] [PubMed] [Google Scholar]
- Penny J. I., Campbell F. C. Active transport of benzo[a]pyrene in apical membrane vesicles from normal human intestinal epithelium. Biochim Biophys Acta. 1994 May 25;1226(2):232–236. doi: 10.1016/0925-4439(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Rand E. B., Depaoli A. M., Davidson N. O., Bell G. I., Burant C. F. Sequence, tissue distribution, and functional characterization of the rat fructose transporter GLUT5. Am J Physiol. 1993 Jun;264(6 Pt 1):G1169–G1176. doi: 10.1152/ajpgi.1993.264.6.G1169. [DOI] [PubMed] [Google Scholar]
- Thorens B., Cheng Z. Q., Brown D., Lodish H. F. Liver glucose transporter: a basolateral protein in hepatocytes and intestine and kidney cells. Am J Physiol. 1990 Dec;259(6 Pt 1):C279–C285. doi: 10.1152/ajpcell.1990.259.2.C279. [DOI] [PubMed] [Google Scholar]
- Wilde S. W., Kilberg M. S. Glutamine transport by basolateral plasma-membrane vesicles prepared from rabbit intestine. Biochem J. 1991 Aug 1;277(Pt 3):687–691. doi: 10.1042/bj2770687. [DOI] [PMC free article] [PubMed] [Google Scholar]