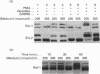
Abstract
Hepatic metabolism and gene expression are among the factors controlled by the cellular hydration state, which changes within minutes in response to aniso-osmotic environments, cumulative substrate uptake, oxidative stress and under the influence of hormones such as insulin. The signalling events coupling cell-volume changes to altered cell function were studied in H4IIE rat hepatoma cells. Hypo-osmotic cell swelling resulted within 1 min in a tyrosine kinase-mediated activation of the extracellular signal-regulated protein kinases Erk-1 and Erk-2, which was independent of protein kinase C and cytosolic calcium. Activation of mitogen-activated protein kinases was followed by an increased phosphorylation of c-Jun, which may explain our recently reported finding of an about 5-fold increase in c-jun mRNA level in response to cell swelling. Pretreatment of cells with pertussis or cholera toxin abolished the swelling-induced activation of Erk-1 and Erk-2, suggesting the involvement of G-proteins. Thus, a signal-transduction pathway resembling growth factor signalling is activated already by osmotic water shifts across the plasma membrane, thereby providing a new perspective for adaption of cell function to alterations of the environment.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alessi D. R., Smythe C., Keyse S. M. The human CL100 gene encodes a Tyr/Thr-protein phosphatase which potently and specifically inactivates MAP kinase and suppresses its activation by oncogenic ras in Xenopus oocyte extracts. Oncogene. 1993 Jul;8(7):2015–2020. [PubMed] [Google Scholar]
- Anderson N. G., Li P., Marsden L. A., Williams N., Roberts T. M., Sturgill T. W. Raf-1 is a potential substrate for mitogen-activated protein kinase in vivo. Biochem J. 1991 Jul 15;277(Pt 2):573–576. doi: 10.1042/bj2770573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Hattori K., Smeal T., Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988 Dec 2;55(5):875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- Bernstein L. R., Ferris D. K., Colburn N. H., Sobel M. E. A family of mitogen-activated protein kinase-related proteins interacts in vivo with activator protein-1 transcription factor. J Biol Chem. 1994 Apr 1;269(13):9401–9404. [PubMed] [Google Scholar]
- Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton T. G., Nye S. H., Robbins D. J., Ip N. Y., Radziejewska E., Morgenbesser S. D., DePinho R. A., Panayotatos N., Cobb M. H., Yancopoulos G. D. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991 May 17;65(4):663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Brewster J. L., de Valoir T., Dwyer N. D., Winter E., Gustin M. C. An osmosensing signal transduction pathway in yeast. Science. 1993 Mar 19;259(5102):1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- Busch G. L., Schreiber R., Dartsch P. C., Völkl H., Vom Dahl S., Häussinger D., Lang F. Involvement of microtubules in the link between cell volume and pH of acidic cellular compartments in rat and human hepatocytes. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):9165–9169. doi: 10.1073/pnas.91.19.9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao T. S., Byron K. L., Lee K. M., Villereal M., Rosner M. R. Activation of MAP kinases by calcium-dependent and calcium-independent pathways. Stimulation by thapsigargin and epidermal growth factor. J Biol Chem. 1992 Oct 5;267(28):19876–19883. [PubMed] [Google Scholar]
- Chao T. S., Foster D. A., Rapp U. R., Rosner M. R. Differential Raf requirement for activation of mitogen-activated protein kinase by growth factors, phorbol esters, and calcium. J Biol Chem. 1994 Mar 11;269(10):7337–7341. [PubMed] [Google Scholar]
- D'Onofrio F., Le M. Q., Chiasson J. L., Srivastava A. K. Activation of mitogen activated protein (MAP) kinases by vanadate is independent of insulin receptor autophosphorylation. FEBS Lett. 1994 Mar 7;340(3):269–275. doi: 10.1016/0014-5793(94)80152-5. [DOI] [PubMed] [Google Scholar]
- Davis R. J. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993 Jul 15;268(20):14553–14556. [PubMed] [Google Scholar]
- Dent P., Lavoinne A., Nakielny S., Caudwell F. B., Watt P., Cohen P. The molecular mechanism by which insulin stimulates glycogen synthesis in mammalian skeletal muscle. Nature. 1990 Nov 22;348(6299):302–308. doi: 10.1038/348302a0. [DOI] [PubMed] [Google Scholar]
- Faure M., Voyno-Yasenetskaya T. A., Bourne H. R. cAMP and beta gamma subunits of heterotrimeric G proteins stimulate the mitogen-activated protein kinase pathway in COS-7 cells. J Biol Chem. 1994 Mar 18;269(11):7851–7854. [PubMed] [Google Scholar]
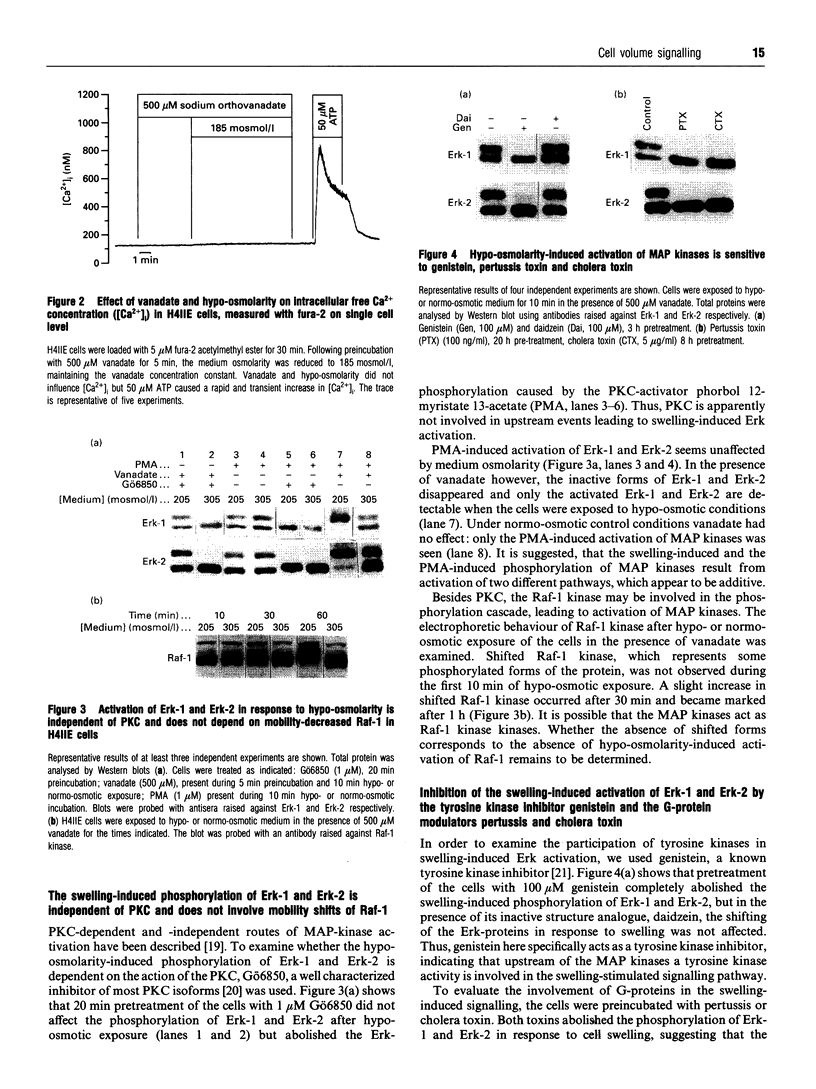
- Finkenzeller G., Newsome W., Lang F., Häussinger D. Increase of c-jun mRNA upon hypo-osmotic cell swelling of rat hepatoma cells. FEBS Lett. 1994 Mar 7;340(3):163–166. doi: 10.1016/0014-5793(94)80129-0. [DOI] [PubMed] [Google Scholar]
- Galcheva-Gargova Z., Dérijard B., Wu I. H., Davis R. J. An osmosensing signal transduction pathway in mammalian cells. Science. 1994 Aug 5;265(5173):806–808. doi: 10.1126/science.8047888. [DOI] [PubMed] [Google Scholar]
- Hallbrucker C., Ritter M., Lang F., Gerok W., Häussinger D. Hydroperoxide metabolism in rat liver. K+ channel activation, cell volume changes and eicosanoid formation. Eur J Biochem. 1993 Feb 1;211(3):449–458. doi: 10.1111/j.1432-1033.1993.tb17570.x. [DOI] [PubMed] [Google Scholar]
- Han J., Lee J. D., Bibbs L., Ulevitch R. J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994 Aug 5;265(5173):808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- Hepler J. R., Gilman A. G. G proteins. Trends Biochem Sci. 1992 Oct;17(10):383–387. doi: 10.1016/0968-0004(92)90005-t. [DOI] [PubMed] [Google Scholar]
- Hill T. D., Dean N. M., Mordan L. J., Lau A. F., Kanemitsu M. Y., Boynton A. L. PDGF-induced activation of phospholipase C is not required for induction of DNA synthesis. Science. 1990 Jun 29;248(4963):1660–1663. doi: 10.1126/science.2163545. [DOI] [PubMed] [Google Scholar]
- Howe L. R., Leevers S. J., Gómez N., Nakielny S., Cohen P., Marshall C. J. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992 Oct 16;71(2):335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Lang F., Bauers K., Gerok W. Interactions between glutamine metabolism and cell-volume regulation in perfused rat liver. Eur J Biochem. 1990 Mar 30;188(3):689–695. doi: 10.1111/j.1432-1033.1990.tb15451.x. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Lang F. Cell volume and hormone action. Trends Pharmacol Sci. 1992 Oct;13(10):371–373. doi: 10.1016/0165-6147(92)90114-l. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Lang F. Cell volume in the regulation of hepatic function: a mechanism for metabolic control. Biochim Biophys Acta. 1991 Dec 12;1071(4):331–350. doi: 10.1016/0304-4157(91)90001-d. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Lang F., Gerok W. Regulation of cell function by the cellular hydration state. Am J Physiol. 1994 Sep;267(3 Pt 1):E343–E355. doi: 10.1152/ajpendo.1994.267.3.E343. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Roth E., Lang F., Gerok W. Cellular hydration state: an important determinant of protein catabolism in health and disease. Lancet. 1993 May 22;341(8856):1330–1332. doi: 10.1016/0140-6736(93)90828-5. [DOI] [PubMed] [Google Scholar]
- Kizaka-Kondoh S., Okayama H. Raf-1 is not a major upstream regulator of MAP kinases in rat fibroblasts. FEBS Lett. 1993 Dec 27;336(2):255–258. doi: 10.1016/0014-5793(93)80814-b. [DOI] [PubMed] [Google Scholar]
- Lee R. M., Cobb M. H., Blackshear P. J. Evidence that extracellular signal-regulated kinases are the insulin-activated Raf-1 kinase kinases. J Biol Chem. 1992 Jan 15;267(2):1088–1092. [PubMed] [Google Scholar]
- Luiken J. J., Blommaart E. F., Boon L., van Woerkom G. M., Meijer A. J. Cell swelling and the control of autophagic proteolysis in hepatocytes: involvement of phosphorylation of ribosomal protein S6? Biochem Soc Trans. 1994 May;22(2):508–511. doi: 10.1042/bst0220508. [DOI] [PubMed] [Google Scholar]
- Lundgren D. W. Effect of hypotonic stress on ornithine decarboxylase mRNA expression in cultured cells. J Biol Chem. 1992 Apr 5;267(10):6841–6847. [PubMed] [Google Scholar]
- Martiny-Baron G., Kazanietz M. G., Mischak H., Blumberg P. M., Kochs G., Hug H., Marmé D., Schächtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö 6976. J Biol Chem. 1993 May 5;268(13):9194–9197. [PubMed] [Google Scholar]
- Meijer A. J., Baquet A., Gustafson L., van Woerkom G. M., Hue L. Mechanism of activation of liver glycogen synthase by swelling. J Biol Chem. 1992 Mar 25;267(9):5823–5828. [PubMed] [Google Scholar]
- Minton A. P., Colclasure G. C., Parker J. C. Model for the role of macromolecular crowding in regulation of cellular volume. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10504–10506. doi: 10.1073/pnas.89.21.10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome W. P., Warskulat U., Noe B., Wettstein M., Stoll B., Gerok W., Häussinger D. Modulation of phosphoenolpyruvate carboxykinase mRNA levels by the hepatocellular hydration state. Biochem J. 1994 Dec 1;304(Pt 2):555–560. doi: 10.1042/bj3040555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offensperger W. B., Offensperger S., Stoll B., Gerok W., Häussinger D. Effects of anisotonic exposure on duck hepatitis B virus replication. Hepatology. 1994 Jul;20(1 Pt 1):1–7. doi: 10.1016/0270-9139(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Pulverer B. J., Kyriakis J. M., Avruch J., Nikolakaki E., Woodgett J. R. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991 Oct 17;353(6345):670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- Rouse J., Cohen P., Trigon S., Morange M., Alonso-Llamazares A., Zamanillo D., Hunt T., Nebreda A. R. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994 Sep 23;78(6):1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Saha N., Schreiber R., vom Dahl S., Lang F., Gerok W., Häussinger D. Endogenous hydroperoxide formation, cell volume and cellular K+ balance in perfused rat liver. Biochem J. 1993 Dec 15;296(Pt 3):701–707. doi: 10.1042/bj2960701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels M. L., Weber M. J., Bishop J. M., McMahon M. Conditional transformation of cells and rapid activation of the mitogen-activated protein kinase cascade by an estradiol-dependent human raf-1 protein kinase. Mol Cell Biol. 1993 Oct;13(10):6241–6252. doi: 10.1128/mcb.13.10.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkadi B., Parker J. C. Activation of ion transport pathways by changes in cell volume. Biochim Biophys Acta. 1991 Dec 12;1071(4):407–427. doi: 10.1016/0304-4157(91)90005-h. [DOI] [PubMed] [Google Scholar]
- Schreiber R., Häussinger D. Characterization of the swelling-induced alkalinization of endocytotic vesicles in fluorescein isothiocyanate-dextran-loaded rat hepatocytes. Biochem J. 1995 Jul 1;309(Pt 1):19–24. doi: 10.1042/bj3090019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R., Stoll B., Lang F., Häussinger D. Effects of aniso-osmolarity and hydroperoxides on intracellular pH in isolated rat hepatocytes as assessed by (2',7')-bis(carboxyethyl)-5(6)-carboxyfluorescein and fluorescein isothiocyanate-dextran fluorescence. Biochem J. 1994 Oct 1;303(Pt 1):113–120. doi: 10.1042/bj3030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll B., Gerok W., Lang F., Häussinger D. Liver cell volume and protein synthesis. Biochem J. 1992 Oct 1;287(Pt 1):217–222. doi: 10.1042/bj2870217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sözeri O., Vollmer K., Liyanage M., Frith D., Kour G., Mark G. E., 3rd, Stabel S. Activation of the c-Raf protein kinase by protein kinase C phosphorylation. Oncogene. 1992 Nov;7(11):2259–2262. [PubMed] [Google Scholar]