Abstract
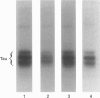
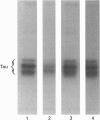
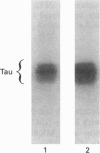
Alterations in situ in the phosphorylation state of the microtubule-associated protein tau were examined in response to increasing intracellular levels of Ca2+ through N-methyl-D-aspartate (NMDA)-receptor activation, or activating cyclic AMP (cAMP)-dependent protein kinase (cAMP-PK), in rat cerebral-cortical slices. Increasing intracellular concentrations of Ca2+ by treatment of the brain slices with the glutamate analogue NMDA in depolarizing conditions (55 mM KCl) resulted in dephosphorylation of tau. Addition of KCl+NMDA to the slices resulted in a 40% decrease in 32P incorporation into tau, whereas addition of KCl or NMDA alone had no effect on tau phosphorylation. The KCl+NMDA-induced dephosphorylation of tau was blocked by the non-competitive NMDA-receptor antagonist MK801. Determine the involvement of the Ca2+/calmodulin-dependent phosphatase, calcineurin, in the KCl+NMDA-induced dephosphorylation of tau, slices were pretreated with the calcineurin inhibitor Cyclosporin A. Pretreatment of the rat brain slices with Cyclosporin A completely abolished the dephosphorylation of tau induced by the addition of KCl+NMDA. The dephosphorylation of tau in situ was site-selective, as indicated by the loss of 32P label from only a few select peptides. Activation of cAMP-PK by stimulating adenylate cyclase in rat cerebral-cortical slices with forskolin resulted in a 73% increase over control levels in 32P incorporation into immunoprecipitated tau. Two-dimensional phosphopeptide mapping revealed that most of the sites on tau phosphorylated in brain slices in response to increased cAMP levels were the same as those phosphorylated on isolated tau by purified cAMP-PK. Although the state of tau phosphorylation is certainly regulated by many protein phosphatases and kinases in vivo, to our knowledge this study provides the first direct evidence of a specific protein phosphatase and kinase that modulate the phosphorylation state of tau in situ.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baudier J., Cole R. D. Phosphorylation of tau proteins to a state like that in Alzheimer's brain is catalyzed by a calcium/calmodulin-dependent kinase and modulated by phospholipids. J Biol Chem. 1987 Dec 25;262(36):17577–17583. [PubMed] [Google Scholar]
- Binder L. I., Frankfurter A., Rebhun L. I. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985 Oct;101(4):1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres A., Kosik K. S. Inhibition of neurite polarity by tau antisense oligonucleotides in primary cerebellar neurons. Nature. 1990 Feb 1;343(6257):461–463. doi: 10.1038/343461a0. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Cerebral hypoxia: some new approaches and unanswered questions. J Neurosci. 1990 Aug;10(8):2493–2501. doi: 10.1523/JNEUROSCI.10-08-02493.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline H. T., Debski E. A., Constantine-Paton M. N-methyl-D-aspartate receptor antagonist desegregates eye-specific stripes. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4342–4345. doi: 10.1073/pnas.84.12.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson T. M., Steiner J. P., Dawson V. L., Dinerman J. L., Uhl G. R., Snyder S. H. Immunosuppressant FK506 enhances phosphorylation of nitric oxide synthase and protects against glutamate neurotoxicity. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9808–9812. doi: 10.1073/pnas.90.21.9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond N. L., Levy W. B. Synaptic correlates of associative potentiation/depression: an ultrastructural study in the hippocampus. Brain Res. 1983 Apr 11;265(1):21–30. doi: 10.1016/0006-8993(83)91329-x. [DOI] [PubMed] [Google Scholar]
- Drewes G., Lichtenberg-Kraag B., Döring F., Mandelkow E. M., Biernat J., Goris J., Dorée M., Mandelkow E. Mitogen activated protein (MAP) kinase transforms tau protein into an Alzheimer-like state. EMBO J. 1992 Jun;11(6):2131–2138. doi: 10.1002/j.1460-2075.1992.tb05272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin D. G., Kirschner M. W. Tau protein function in living cells. J Cell Biol. 1986 Dec;103(6 Pt 2):2739–2746. doi: 10.1083/jcb.103.6.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Nido J., Montoro R. J., López-Barneo J., Avila J. High external potassium induces an increase in the phosphorylation of the cytoskeletal protein MAP2 in rat hippocampal slices. Eur J Neurosci. 1993 Jul 1;5(7):818–824. doi: 10.1111/j.1460-9568.1993.tb00933.x. [DOI] [PubMed] [Google Scholar]
- Ferreira A., Kincaid R., Kosik K. S. Calcineurin is associated with the cytoskeleton of cultured neurons and has a role in the acquisition of polarity. Mol Biol Cell. 1993 Dec;4(12):1225–1238. doi: 10.1091/mbc.4.12.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster A. C., Wong E. H. The novel anticonvulsant MK-801 binds to the activated state of the N-methyl-D-aspartate receptor in rat brain. Br J Pharmacol. 1987 Jun;91(2):403–409. doi: 10.1111/j.1476-5381.1987.tb10295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruman D. A., Klee C. B., Bierer B. E., Burakoff S. J. Calcineurin phosphatase activity in T lymphocytes is inhibited by FK 506 and cyclosporin A. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3686–3690. doi: 10.1073/pnas.89.9.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Cohen E. S., Jakes R., Cohen P. p42 MAP kinase phosphorylation sites in microtubule-associated protein tau are dephosphorylated by protein phosphatase 2A1. Implications for Alzheimer's disease [corrected]. FEBS Lett. 1992 Nov 2;312(1):95–99. doi: 10.1016/0014-5793(92)81418-l. [DOI] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Jakes R., Rutherford D., Crowther R. A. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989 Oct;3(4):519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- Gong C. X., Singh T. J., Grundke-Iqbal I., Iqbal K. Alzheimer's disease abnormally phosphorylated tau is dephosphorylated by protein phosphatase-2B (calcineurin). J Neurochem. 1994 Feb;62(2):803–806. doi: 10.1046/j.1471-4159.1994.62020803.x. [DOI] [PubMed] [Google Scholar]
- Goto S., Yamamoto H., Fukunaga K., Iwasa T., Matsukado Y., Miyamoto E. Dephosphorylation of microtubule-associated protein 2, tau factor, and tubulin by calcineurin. J Neurochem. 1985 Jul;45(1):276–283. doi: 10.1111/j.1471-4159.1985.tb05504.x. [DOI] [PubMed] [Google Scholar]
- Greenwood J. A., Scott C. W., Spreen R. C., Caputo C. B., Johnson G. V. Casein kinase II preferentially phosphorylates human tau isoforms containing an amino-terminal insert. Identification of threonine 39 as the primary phosphate acceptor. J Biol Chem. 1994 Feb 11;269(6):4373–4380. [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Quinlan M., Tung Y. C., Zaidi M. S., Wisniewski H. M. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986 May 5;261(13):6084–6089. [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustke N., Steiner B., Mandelkow E. M., Biernat J., Meyer H. E., Goedert M., Mandelkow E. The Alzheimer-like phosphorylation of tau protein reduces microtubule binding and involves Ser-Pro and Thr-Pro motifs. FEBS Lett. 1992 Jul 28;307(2):199–205. doi: 10.1016/0014-5793(92)80767-b. [DOI] [PubMed] [Google Scholar]
- Hagestedt T., Lichtenberg B., Wille H., Mandelkow E. M., Mandelkow E. Tau protein becomes long and stiff upon phosphorylation: correlation between paracrystalline structure and degree of phosphorylation. J Cell Biol. 1989 Oct;109(4 Pt 1):1643–1651. doi: 10.1083/jcb.109.4.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpain S., Greengard P. Activation of NMDA receptors induces rapid dephosphorylation of the cytoskeletal protein MAP2. Neuron. 1990 Sep;5(3):237–246. doi: 10.1016/0896-6273(90)90161-8. [DOI] [PubMed] [Google Scholar]
- Harris E. W., Ganong A. H., Cotman C. W. Long-term potentiation in the hippocampus involves activation of N-methyl-D-aspartate receptors. Brain Res. 1984 Dec 3;323(1):132–137. doi: 10.1016/0006-8993(84)90275-0. [DOI] [PubMed] [Google Scholar]
- Hasegawa M., Morishima-Kawashima M., Takio K., Suzuki M., Titani K., Ihara Y. Protein sequence and mass spectrometric analyses of tau in the Alzheimer's disease brain. J Biol Chem. 1992 Aug 25;267(24):17047–17054. [PubMed] [Google Scholar]
- Johnson G. V. Differential phosphorylation of tau by cyclic AMP-dependent protein kinase and Ca2+/calmodulin-dependent protein kinase II: metabolic and functional consequences. J Neurochem. 1992 Dec;59(6):2056–2062. doi: 10.1111/j.1471-4159.1992.tb10094.x. [DOI] [PubMed] [Google Scholar]
- Johnson G. V., Jope R. S., Binder L. I. Proteolysis of tau by calpain. Biochem Biophys Res Commun. 1989 Sep 29;163(3):1505–1511. doi: 10.1016/0006-291x(89)91150-9. [DOI] [PubMed] [Google Scholar]
- Kincaid R. L., Balaban C. D., Billingsley M. L. Differential localization of calmodulin-dependent enzymes in rat brain: evidence for selective expression of cyclic nucleotide phosphodiesterase in specific neurons. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1118–1122. doi: 10.1073/pnas.84.4.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Krinks M. H. Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6270–6273. doi: 10.1073/pnas.76.12.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knops J., Kosik K. S., Lee G., Pardee J. D., Cohen-Gould L., McConlogue L. Overexpression of tau in a nonneuronal cell induces long cellular processes. J Cell Biol. 1991 Aug;114(4):725–733. doi: 10.1083/jcb.114.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik K. S., Joachim C. L., Selkoe D. J. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Binder L., Trojanowski J. Q., Lee V. M., Lee G. Epitopes that span the tau molecule are shared with paired helical filaments. Neuron. 1988 Nov;1(9):817–825. doi: 10.1016/0896-6273(88)90129-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee V. M., Balin B. J., Otvos L., Jr, Trojanowski J. Q. A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science. 1991 Feb 8;251(4994):675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- Lindwall G., Cole R. D. Phosphorylation affects the ability of tau protein to promote microtubule assembly. J Biol Chem. 1984 Apr 25;259(8):5301–5305. [PubMed] [Google Scholar]
- Litersky J. M., Johnson G. V. Phosphorylation by cAMP-dependent protein kinase inhibits the degradation of tau by calpain. J Biol Chem. 1992 Jan 25;267(3):1563–1568. [PubMed] [Google Scholar]
- Litersky J. M., Scott C. W., Johnson G. V. Phosphorylation, calpain proteolysis and tubulin binding of recombinant human tau isoforms. Brain Res. 1993 Feb 26;604(1-2):32–40. doi: 10.1016/0006-8993(93)90349-r. [DOI] [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Malenka R. C., Nicoll R. A. Mechanisms underlying long-term potentiation of synaptic transmission. Annu Rev Neurosci. 1991;14:379–397. doi: 10.1146/annurev.ne.14.030191.002115. [DOI] [PubMed] [Google Scholar]
- Murthy A. S., Bramblett G. T., Flavin M. The sites at which brain microtubule-associated protein 2 is phosphorylated in vivo differ from those accessible to cAMP-dependent kinase in vitro. J Biol Chem. 1985 Apr 10;260(7):4364–4370. [PubMed] [Google Scholar]
- Nicoll R. A., Kauer J. A., Malenka R. C. The current excitement in long-term potentiation. Neuron. 1988 Apr;1(2):97–103. doi: 10.1016/0896-6273(88)90193-6. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Polli J. W., Billingsley M. L., Kincaid R. L. Expression of the calmodulin-dependent protein phosphatase, calcineurin, in rat brain: developmental patterns and the role of nigrostriatal innervation. Brain Res Dev Brain Res. 1991 Nov 19;63(1-2):105–119. doi: 10.1016/0165-3806(91)90071-p. [DOI] [PubMed] [Google Scholar]
- Reimann E. M., Beham R. A. Catalytic subunit of cAMP-dependent protein kinase. Methods Enzymol. 1983;99:51–55. doi: 10.1016/0076-6879(83)99039-0. [DOI] [PubMed] [Google Scholar]
- Schneider H. H., Schmiechen R., Brezinski M., Seidler J. Stereospecific binding of the antidepressant rolipram to brain protein structures. Eur J Pharmacol. 1986 Aug 7;127(1-2):105–115. doi: 10.1016/0014-2999(86)90210-4. [DOI] [PubMed] [Google Scholar]
- Scott C. W., Spreen R. C., Herman J. L., Chow F. P., Davison M. D., Young J., Caputo C. B. Phosphorylation of recombinant tau by cAMP-dependent protein kinase. Identification of phosphorylation sites and effect on microtubule assembly. J Biol Chem. 1993 Jan 15;268(2):1166–1173. [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihag R. K., Nixon R. A. Phosphorylation of the amino-terminal head domain of the middle molecular mass 145-kDa subunit of neurofilaments. Evidence for regulation by second messenger-dependent protein kinases. J Biol Chem. 1990 Mar 5;265(7):4166–4171. [PubMed] [Google Scholar]
- Swanson S. K., Born T., Zydowsky L. D., Cho H., Chang H. Y., Walsh C. T., Rusnak F. Cyclosporin-mediated inhibition of bovine calcineurin by cyclophilins A and B. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3741–3745. doi: 10.1073/pnas.89.9.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E. H., Kemp J. A., Priestley T., Knight A. R., Woodruff G. N., Iversen L. L. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., Saitoh Y., Fukunaga K., Nishimura H., Miyamoto E. Dephosphorylation of microtubule proteins by brain protein phosphatases 1 and 2A, and its effect on microtubule assembly. J Neurochem. 1988 May;50(5):1614–1623. doi: 10.1111/j.1471-4159.1988.tb03051.x. [DOI] [PubMed] [Google Scholar]