Abstract
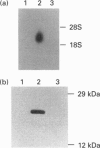
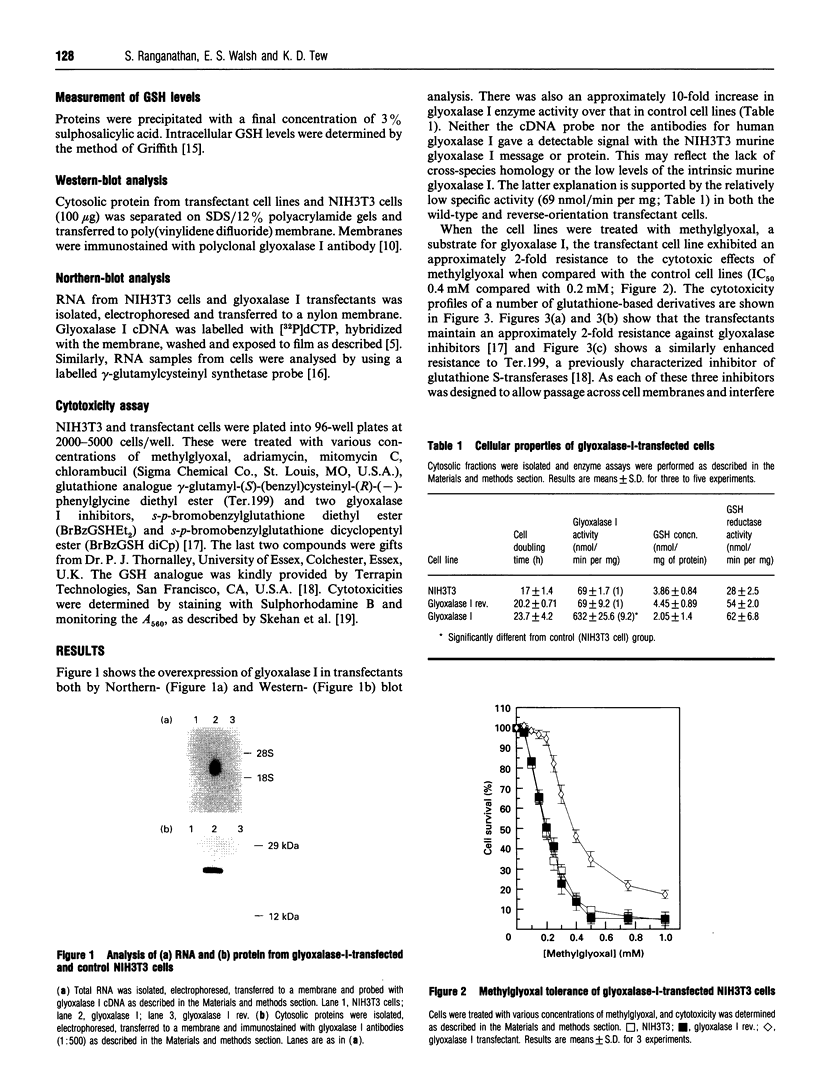
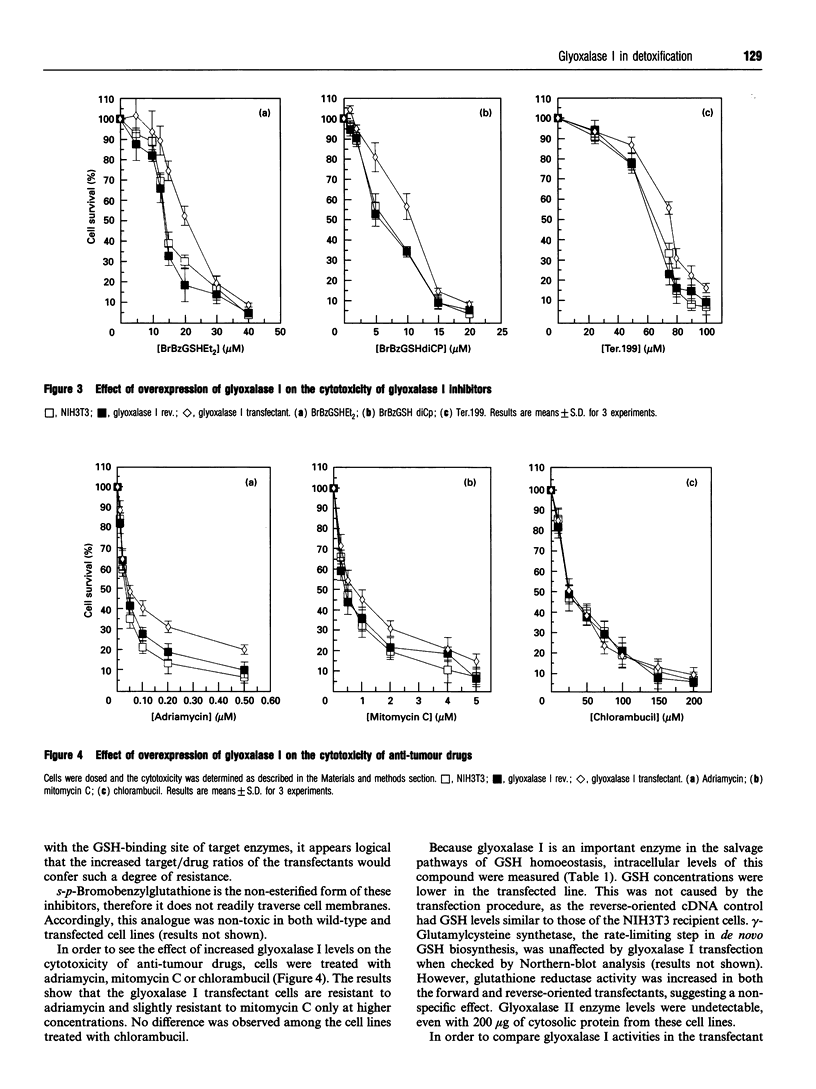
The glyoxalase system (glyoxalase I, glyoxalase II and GSH as cofactor) is involved in the detoxification of methylglyoxal (a byproduct of the glycolytic pathway) and other alpha-oxoaldehydes. We have transfected a 622 bp cDNA encoding human glyoxalase I into murine NIH3T3 cells. The recipient cells were shown to express elevated transcript and protein levels and a 10-fold increase in glyoxalase I enzyme activity. This was accompanied by an increased tolerance for exogenous methylglyoxal and enhanced resistance to the cytotoxic effects of two glyoxalase I inhibitors (s-p-bromobenzylglutathione diethyl ester and s-p-bromobenzylglutathione dicyclopentyl ester), a glutathione analogue [gamma-glutamyl-(S)-(benzyl)cysteinyl-(R)-(-)-phenylglycine diethyl ester] and the anti-cancer drugs mitomycin C and adriamycin. Steady-state levels of GSH were significantly lower in the transfected cells, perhaps reflecting increased flux as a consequence of elevated glyoxalase activity. This decrease did not alter the sensitivity to the alkylating agent chlorambucil. Although transfection did not affect the growth or doubling time of the NIH3T3 cells, analysis of glyoxalase I activity showed a consistent increase in tumour tissue when compared with pair-matched controls. Thus increased glyoxalase I is associated with the malignant phenotype and may also contribute to protection against the cytotoxicity of certain anti-cancer drugs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayoub F., Zaman M., Thornalley P., Masters J. Glyoxalase activities in human tumour cell lines in vitro. Anticancer Res. 1993 Jan-Feb;13(1):151–155. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen C. A., Okayama H. Calcium phosphate-mediated gene transfer: a highly efficient transfection system for stably transforming cells with plasmid DNA. Biotechniques. 1988 Jul-Aug;6(7):632–638. [PubMed] [Google Scholar]
- Cooper R. A. Metabolism of methylglyoxal in microorganisms. Annu Rev Microbiol. 1984;38:49–68. doi: 10.1146/annurev.mi.38.100184.000405. [DOI] [PubMed] [Google Scholar]
- Gipp J. J., Chang C., Mulcahy R. T. Cloning and nucleotide sequence of a full-length cDNA for human liver gamma-glutamylcysteine synthetase. Biochem Biophys Res Commun. 1992 May 29;185(1):29–35. doi: 10.1016/s0006-291x(05)80950-7. [DOI] [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Hooper N. I., Tisdale M. J., Thornalley P. J. Modification of the glyoxalase system in human HL60 promyelocytic leukaemia cells during differentiation to neutrophils in vitro. Biochim Biophys Acta. 1988 Sep 8;966(3):362–369. doi: 10.1016/0304-4165(88)90086-4. [DOI] [PubMed] [Google Scholar]
- Kalapos M. P. Methylglyoxal toxicity in mammals. Toxicol Lett. 1994 Jul;73(1):3–24. doi: 10.1016/0378-4274(94)90184-8. [DOI] [PubMed] [Google Scholar]
- Kar N. C., Pearson C. M. Glyoxalase enzyme system in human muscular dystrophy. Clin Chim Acta. 1975 Nov 15;65(1):153–155. doi: 10.1016/0009-8981(75)90348-4. [DOI] [PubMed] [Google Scholar]
- Lo T. W., Thornalley P. J. Inhibition of proliferation of human leukaemia 60 cells by diethyl esters of glyoxalase inhibitors in vitro. Biochem Pharmacol. 1992 Dec 15;44(12):2357–2363. doi: 10.1016/0006-2952(92)90680-h. [DOI] [PubMed] [Google Scholar]
- Lyttle M. H., Hocker M. D., Hui H. C., Caldwell C. G., Aaron D. T., Engqvist-Goldstein A., Flatgaard J. E., Bauer K. E. Isozyme-specific glutathione-S-transferase inhibitors: design and synthesis. J Med Chem. 1994 Jan 7;37(1):189–194. doi: 10.1021/jm00027a024. [DOI] [PubMed] [Google Scholar]
- MIZE C. E., LANGDON R. G. Hepatic glutathione reductase. I. Purification and general kinetic properties. J Biol Chem. 1962 May;237:1589–1595. [PubMed] [Google Scholar]
- McLellan A. C., Thornalley P. J., Benn J., Sonksen P. H. Modification of the glyoxalase system in clinical diabetes mellitus. Biochem Soc Trans. 1993 May;21(2):158S–158S. doi: 10.1042/bst021158s. [DOI] [PubMed] [Google Scholar]
- Oray B., Norton S. J. Glyoxalase I from mouse liver. Methods Enzymol. 1982;90(Pt E):542–546. doi: 10.1016/s0076-6879(82)90182-3. [DOI] [PubMed] [Google Scholar]
- Principato G. B., Bodo M., Biagioni M. G., Rosi G., Liotti F. S. Glyoxalases and glutathione reductase activity changes in chicken liver during embryo development and after hatching. Acta Embryol Morphol Exp. 1982 Dec;3(3):173–179. [PubMed] [Google Scholar]
- Principato G. B., Locci P., Rosi G., Talesa V., Giovannini E. Activity changes of glyoxalases I-II and glutathione reductase in regenerating rat liver. Biochem Int. 1983 Feb;6(2):249–255. [PubMed] [Google Scholar]
- RACKER E. The mechanism of action of glyoxalase. J Biol Chem. 1951 Jun;190(2):685–696. [PubMed] [Google Scholar]
- Ranganathan S., Tew K. D. Analysis of glyoxalase-I from normal and tumor tissue from human colon. Biochim Biophys Acta. 1993 Oct 20;1182(3):311–316. doi: 10.1016/0925-4439(93)90074-b. [DOI] [PubMed] [Google Scholar]
- Ranganathan S., Walsh E. S., Godwin A. K., Tew K. D. Cloning and characterization of human colon glyoxalase-I. J Biol Chem. 1993 Mar 15;268(8):5661–5667. [PubMed] [Google Scholar]
- Rhee H., Murata K., Kimura A. Molecular cloning of the Pseudomonas putida glyoxalase I gene in Escherichia coli. Biochem Biophys Res Commun. 1987 Sep 15;147(2):831–838. doi: 10.1016/0006-291x(87)91005-9. [DOI] [PubMed] [Google Scholar]
- Skehan P., Storeng R., Scudiero D., Monks A., McMahon J., Vistica D., Warren J. T., Bokesch H., Kenney S., Boyd M. R. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990 Jul 4;82(13):1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Thornalley P. J., Hooper N. I., Jennings P. E., Florkowski C. M., Jones A. F., Lunec J., Barnett A. H. The human red blood cell glyoxalase system in diabetes mellitus. Diabetes Res Clin Pract. 1989 Aug 1;7(2):115–120. doi: 10.1016/0168-8227(89)90101-0. [DOI] [PubMed] [Google Scholar]
- Thornalley P. J. The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem J. 1990 Jul 1;269(1):1–11. doi: 10.1042/bj2690001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Jagt D. L., Hunsaker L. A., Campos N. M., Baack B. R. D-lactate production in erythrocytes infected with Plasmodium falciparum. Mol Biochem Parasitol. 1990 Sep-Oct;42(2):277–284. doi: 10.1016/0166-6851(90)90171-h. [DOI] [PubMed] [Google Scholar]