Abstract
There is a high prevalence of antisocial personality disorder (ASPD) in individuals affected by substance use disorders (SUD). However, there is limited information on the specific patterns of association of ASPD with SUD severity and specific SUD diagnostic criteria. We investigated the association of alcohol, cannabis, cocaine, opioid, and tobacco use disorders (AUD, CanUD, CocUD, OUD, and TUD, respectively) in 1660 individuals with ASPD and 6640 controls matched by sex (24% female), age, and racial/ethnic background in a sample ascertained for addiction-related traits. Generalized linear regressions were used to test ASPD with respect to the five DSM-5 SUD diagnoses, their severity (i.e., mild, moderate, severe), and their diagnostic criteria. We found that ASPD is associated with the diagnosis and severity of AUD (Odds Ratio, ORs = 1.89 and 1.25), CanUD (ORs = 2.13 and 1.32), and TUD (ORs = 1.50 and 1.21) (ps < 0.003). Of the specific diagnostic criteria, the “hazardous use” criterion showed the strongest association with ASPD across the five SUDs investigated (from ORTUD = 1.88 to ORCanUD = 1.37). However, when criteria of different SUDs were included in the same model, ASPD was independently associated only with TUD “hazardous use” and CocUD “attempts to quit”. Attempting to quit cocaine was inversely related to the presence of ASPD and remained significant (OR = 0.57, 95% confidence interval = 0.36–0.89) after controlling for interactive effects with sex. The current work provides novel insights into ASPD-SUD comorbidity, supporting the existence of different SUD patterns among individuals affected by ASPD.
Subject terms: Addiction, Psychiatric disorders
Introduction
Antisocial personality disorder (ASPD) is a psychiatric disorder characterized by a tendency to be manipulative, impulsive, irritable, aggressive, and show a lack of remorse [1]. ASPD is highly comorbid with substance use disorders (SUDs) [1–4]. While nationally representative cohorts report prevalence rates of ASPD around 3.6%, several clinical samples of individuals who misuse substances show a prevalence between 23.5% and 81.4% [5–7]. The higher prevalence of ASPD in the presence of substance misuse supports research on the nature of this relationship and its potential implications for our understanding of the onset, severity, and treatment of both ASPD and SUDs.
Research aimed at understanding the relationship between ASPD and various SUDs has used a variety of experimental designs and sampling techniques. Grant and colleagues (2004) investigated the co-occurrence of alcohol and other drug use disorders (10 different combined drug classes) with seven of the 10 DSM-IV personality disorders in the 2001–2002 wave of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC; N = 43,093). Participants in this sample with a current (i.e., past 12 months) alcohol use disorder or other SUD diagnosis were 4.8 and 11.8 times more likely to have an ASPD diagnosis, respectively. In line with this evidence, a study of participants seeking treatment from mental health or drug addiction centers in Spain found that ASPD was the only personality disorder measured that was more prevalent in participants with a lifetime diagnosis of any of the SUDs analyzed (alcohol, 76.5%; cocaine, 81.4%; opiates, 45.1%; cannabis, 67.6%; and sedatives, 23.5%) [6]. Lastly, a study of 200 participants from both inpatient and outpatient psychiatric treatment centers showed that an ASPD diagnosis was associated with a lifetime SUD (a variable that combined hallucinogens, opioids, sedatives, and polysubstance use) even after adjusting for other personality disorders [8].
Building on previous research on the comorbidity between ASPD and various SUD diagnoses, the current study expands our understanding of the complex ASPD-SUD relationship by analyzing the association of ASPD with individual and co-occurring SUDs, testing DSM-5 diagnoses, severity, and diagnostic criteria. Analyses were conducted in the Yale-Penn sample, which permitted us to investigate the comorbidity of ASPD across the SUD spectrum because of its large sample size, deep phenotypic assessment, and purposeful recruitment for SUDs and controls resulting in enrichment for SUD cases.
Methods
Yale-Penn cohort
The Yale-Penn cohort comprises participants recruited to investigate the molecular basis of substance use disorders and other comorbid conditions [9–13]. Yale-Penn participants completed the Semi-Structured Assessment for Drug Dependence and Alcoholism (i.e., SSADDA), a psychiatric research interview administered by trained lay interviewers [14, 15]. The SSADDA is composed of twenty-three sections that assess diagnostic criteria from which diagnoses of SUDs and ASPD can be made.
We investigated DSM-5 diagnoses and individual diagnostic criteria of alcohol use disorder (AUD), cannabis use disorder (CanUD), cocaine use disorder (CocUD), opioid use disorder (OUD), and tobacco use disorder (TUD). We chose to investigate DSM-5 SUDs because it enabled us to analyze the full spectrum of addiction-related behaviors without distinguishing between substance abuse and dependence as defined by DSM-IV. DSM-5 SUD criteria included: (a) hazardous use, (b) social problems due to use, (c) neglected roles due to use, (d) withdrawal, (e) tolerance, (f) using larger amounts, (g) attempts to quit, (h) much time spent using, (i) physical problems due to use, (j) activities given up due to use, and (k) craving [1]. The severity of each SUD was estimated by summing the respective diagnostic criteria (coded as 1 = present and 0 = not present) and classifying each participant based on the DSM-5 definition for mild (i.e., 2–3 criteria present), moderate (i.e., 4–5 criteria present), and severe (i.e., 6 or more criteria present) SUD. Because the SSADDA was originally designed to collect information related to DSM-IV criteria for substance abuse and dependence, which did not include tobacco abuse, we could not derive the DSM-5 TUD criteria b and c. In contrast, we analyzed DSM-IV diagnoses for ASPD (diagnosed independent of substance-induced behaviors), major depressive disorder (MDD), generalized anxiety disorder (GAD), and posttraumatic stress disorder (PTSD) because the SSADDA, which was designed to assess DSM-IV diagnoses, does not allow translation to DSM-5 for these disorders. There are only minor differences between DSM-IV and DSM-5 criteria for these mental illnesses. We also extracted data regarding sex, age, race/ethnicity, education, and household income.
Ethics approval and consent to participate
The present study was conducted under protocols #9809010515 and #0102012183 approved by the institutional review board of the Yale School of Medicine, New Haven, CT, USA. Written informed consent was obtained from each participant enrolled in the Yale-Penn cohort. The study was performed in agreement with the Helsinki Declaration.
Case-Control matching
A total of 1660 Yale-Penn participants received a lifetime ASPD diagnosis. To maximize the statistical power of the analysis and to control for the effect of demographic characteristics on ASPD-SUD associations, we matched ASPD cases with controls with no ASPD diagnosis on sex, age, and race/ethnicity. ASPD case-control matching was performed in R Statistical Software (v4.2.2) using the MatchIt package (v4.5.2) [16]. We estimated only a 2%-increase in the effective sample size after reaching a 1:7 case-control ratio (Supplementary Table 1). Chi-square and t-test analyses were performed where appropriate on the matching criteria to confirm acceptable matching in the sample. Appropriate matching was observed at the 1:4 case-control ratio, which corresponded to 1660 ASPD cases and 6640 controls (Table 1).
Table 1.
Characteristics of ASPD cases and controls from Yale-Penn cohort.
| Characteristics | Cases (N = 1660) | Controls (N = 6640) | p |
|---|---|---|---|
| Sex, N (%) | |||
| Male | 1256 (76) | 5044 (76) | 0.822 |
| Female | 404 (24) | 1596 (24) | |
| Age | |||
| Years (SD) | 38.23 (11) | 38.30 (11) | 0.822 |
| Race-Ethnicity, N (%) | |||
| Native American | 11 (1) | 40 (1) | 0.609 |
| Asian | 3 (<1) | 10 (<1) | |
| Pacific Islander | 4 (<1) | 11 (<1) | |
| African American, no Hispanic descent | 681 (41) | 2859 (43) | |
| African American, Hispanic descent | 54 (3) | 185 (3) | |
| European-American, no Hispanic | 657 (40) | 2641 (40) | |
| European-American, Hispanic descent | 118 (7) | 413 (6) | |
| Other | 132 (8) | 481 (7) | |
| Education Level, N (%) | |||
| Less than High School | 130 (8) | 198 (3) | 1.33 × 10−68 |
| High School | 1090 (66) | 3615 (54) | |
| Some College | 357 (22) | 1782 (27) | |
| Bachelor’s or Higher | 83 (4) | 1045 (16) | |
| Annual Household Income, N (%) | |||
| $0–$9999 | 890 (54) | 2974 (45) | 4.06 × 10−16 |
| $10,000–$19,999 | 320 (19) | 1163 (18) | |
| $20,000–$29,999 | 169 (10) | 776 (12) | |
| $30,000–$39,999 | 109 (7) | 497 (7) | |
| $40,000–$49,999 | 67 (4) | 326 (5) | |
| $50,000–$74,999 | 59 (4) | 436 (6) | |
| $75,000–$99,999 | 20 (1) | 208 (3) | |
| $100,000–$149,999 | 18 (1) | 178 (3) | |
| $150,000+ | 8 (<1) | 82 (1) | |
Controls were matched to cases for sex, age, and race-ethnicity.
Statistical analysis
SUD associations with ASPD were tested using generalized linear regression, in which AUD, CanUD, CocUD, OUD, and TUD were considered in terms of diagnosis (case-control status), severity (mild, moderate, and severe), and individual diagnostic criteria. We included education, household income, age, sex, and race-ethnicity as covariates to account for the residual effects of demographic characteristics. To control for the comorbidity of other common mental health disorders, we also included MDD, GAD, and PTSD in the regression model [1, 17–19]. Variables related to different SUDs (i.e., AUD, CanUD, CocUD, OUD, and TUD) were also tested in a single omnibus model to account for polysubstance addiction. Statistical differences between effect sizes observed in the association of SUD diagnoses and SUD severity with ASPD were estimated using a z test. Bonferroni correction was applied to account for the multiple association tests performed. To assess the robustness of the regression models, we tested for possible biases due to multi-collinearity, autocorrelation, and outliers.
Results
Characteristics of ASPD cases and controls
As expected, because of the matching of the demographic characteristics, there were no statistically significant differences between ASPD cases and controls for sex, age, or race-ethnicity (p > 0.6; Table 1). The overall sample (N = 8300) overrepresented males (>75%) to reflect the epidemiology of ASPD [20]. There were comparable proportions of African Americans (43%) and European Americans (40%), with Hispanics comprising <10% of the sample. ASPD cases reported lower household income and education level than controls (ps = 4.06 × 10−16 and 1.33 × 10−68, respectively; Table 1). Overall, most of the sample had an annual household income ≤$9999 (47%) and reported high school as the highest degree completed (57%).
ASPD association with SUD diagnoses and severity measures
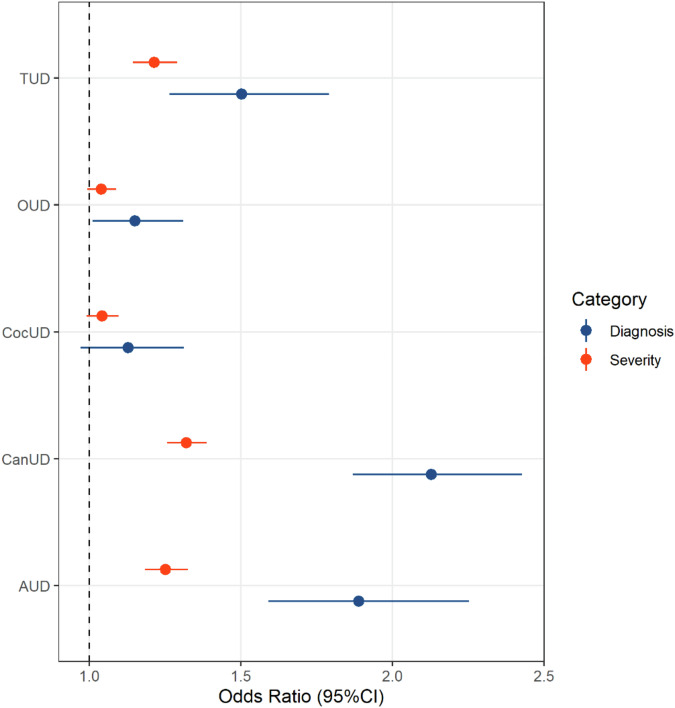
Most participants (N = 8300; Supplementary Table 2) had a lifetime diagnosis of AUD (72%), TUD (70%), CocUD (64%), CanUD (51%), or OUD (39%). Through a multivariable regression analysis also including sex, age, race-ethnicity, income, and education, we observed ASPD-SUD associations that survived Bonferroni correction (p < 0.003; Supplementary Table 3) for AUD (OR = 1.94, 95%CI = 1.63–2.31), CanUD (OR = 2.13, 95%CI = 1.94–2.52), and TUD (OR = 1.53, 95%CI = 1.28–1.82). Among the other variables included in the model, sex was associated with ASPD (p = 1.60 × 10−5; Supplementary Table 3) despite the ASPD cases and controls being well matched on sex (Table 1). This suggests that there are sex differences related to the co-occurrence of ASPD and SUD. Thus, we included GAD, MDD, and PTSD diagnoses as additional covariates, which although not changing the ASPD-SUD associations, attenuated the association with sex (Supplementary Table 3). We chose to include these internalizing disorders (i.e., conditions related to internal, cognition-based symptomology) as covariates in the subsequent analyses to control for possible residual confounding factors. Testing the effect of SUD severity on the association with ASPD demonstrated a pattern similar to that found for the diagnosis-based analysis (Fig. 1; Supplementary Table 4). However, SUD diagnoses were more strongly associated with ASPD than SUD severity measures (diagnosis vs. severity Δp ≤ 1.54 × 10−4). No presence of multicollinearity (statistics < 4), autocorrelation (Supplementary Table 5), or outliers (Supplementary Table 5) was observed in the diagnosis- and severity-based regression models.
Fig. 1. Association of antisocial personality disorder with alcohol use disorder (AUD), cannabis use disorder (CanUD), cocaine use disorder (CocUD), opioid use disorder (OUD), and tobacco use disorder (TUD).
Odds ratios and corresponding 95% confidence intervals (95%CI) are reported.
ASPD associations with SUD diagnostic criteria
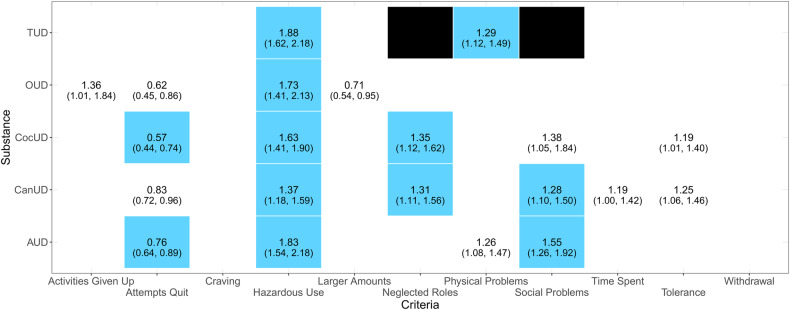
To test whether certain SUD features are associated with ASPD, we investigated individual diagnostic criteria for each of the five SUDs of interest. These models included SUD severity, demographic characteristics, socioeconomic factors, and internalizing disorders as covariates. Analyzing each SUD separately, we identified associations between ASPD and 12 substance-specific diagnostic criteria that survived Bonferroni correction (p < 0.002; Fig. 2; Supplementary Tables 6–10). The “Hazardous Use” criterion was statistically significant across all SUDs investigated (ORAUD = 1.83, ORCanUD = 1.37, ORCocUD = 1.63, OROUD = 1.73, and ORTUD = 1.88, ps < 0.002). Significant associations were seen for the “Social Problems” criterion in both AUD (OR = 1.55, p = 4.99 × 10−5) and CanUD (OR = 1.28, p = 1.67 × 10−3), the “Neglected Roles” criterion in both CanUD (OR = 1.31, p = 1.86 × 10−3) and CocUD (OR = 1.35, p = 1.62 × 10−3), the “Attempts to Quit” criterion in both AUD (OR = 0.76, p = 9.30 × 10−4) and CocUD (OR = 0.57, p = 1.48 × 10−5), and the “Physical Problems” criterion in TUD (OR = 1.29, p = 6.16 × 10−4). While there was no bias related to autocorrelation or outliers in the models investigating SUD diagnostic criteria (Supplementary Table 5), SUD severity variables showed multicollinearity statistics > 10 (Supplementary Tables 6–10). In the OUD model, the “Withdrawal” criterion showed evidence of multicollinearity (Supplementary Table 9). After removing variables with strong evidence of multicollinearity, we confirmed the robustness of the statistically significant associations observed (Supplementary Tables 6–10).
Fig. 2. Associations of antisocial personality disorder with individual diagnostic criteria of alcohol use disorder (AUD), cannabis use disorder (CanUD), cocaine use disorder (CocUD), opioid use disorder (OUD), and tobacco use disorder (TUD).
Blue cells represent associations surviving Bonferroni correction (p < 0.002). White cells with odds ratio statistics represent nominally significant associations (p < 0.05). Empty cells represent null associations (p > 0.05). Black cells represent TUD diagnostic criteria not available.
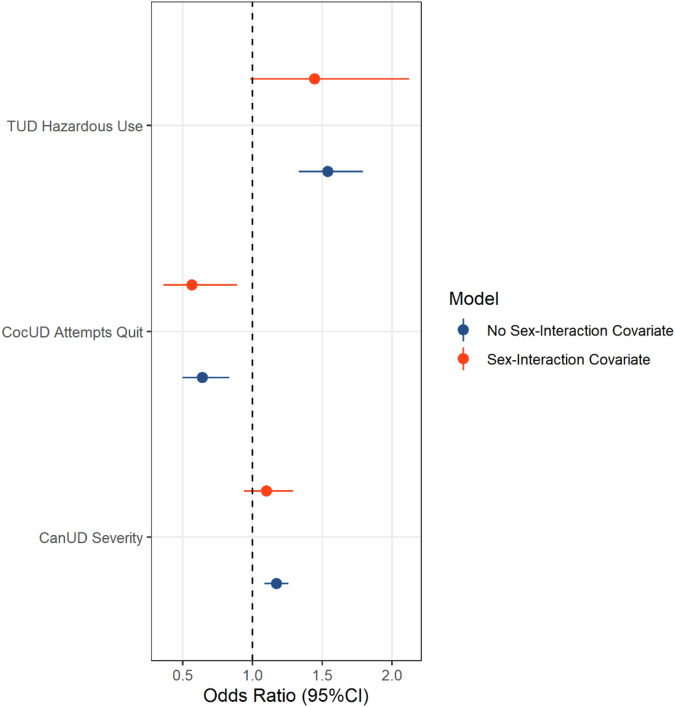
To account for comorbidity across SUDs, we reanalyzed the significant diagnostic criteria in each SUD-specific model, entering them into a regression model together with the previously listed covariates (Supplementary Table 11). In this multi-SUD analysis, three SUD-related phenotypes survived Bonferroni correction (p < 1.61 × 10−3; Fig. 3): CanUD severity (OR = 1.17, p = 3.66 × 10−5), CocUD “Attempts to Quit” (OR = 0.64, p = 8 × 10−4), and TUD “Hazardous Use” (OR = 1.54, p = 7.38 × 10−9). However, among the covariates included in the model, sex was associated with ASPD (p = 1.15 × 10−4; Supplementary Table 11) despite being matched between ASPD cases and controls (Table 1). To account for this residual effect of sex, we entered sex interaction terms in the model for the three SUD-related phenotypes (Supplementary Table 12). After accounting for sex interactions, only CocUD “Attempts to Quit” was still negatively associated with ASPD (OR = 0.57, 95%CI = 0.36–0.89, p = 0.013; Fig. 3). No bias due to autocorrelation or outliers was observed in these omnibus models (Supplementary Table 5). While no evidence of multicollinearity was observed in the primary omnibus model (Supplementary Table 11), the sex interaction terms showed collinearity with the corresponding main terms (Supplementary Table 12).
Fig. 3. Associations of antisocial personality disorder with individual diagnostic criteria across multiple substance use disorders.
Criteria surviving Bonferroni multiple testing correction are reported before and after including sex-interaction covariates. The results of the full models are reported in Supplementary Tables 11 and 12.
Discussion
We investigated associations of ASPD with a broad spectrum of SUD-related outcomes. We did this by modeling ASPD in relation to SUD diagnostic status, both independently and simultaneously (i.e., all SUDs included in the same model), SUD severity thresholds, and individual substance-specific diagnostic criteria. Previous studies extensively characterized SUD comorbidity with ASPD, also comparing differences with other personality disorders [21–24]. However, there is limited knowledge regarding ASPD patterns across the SUD spectrum. The present investigations expanded our understanding of ASPD-SUD comorbidity in the context of variation among SUD diagnostic criteria and types of psychoactive substances.
We found that diagnoses and severity measures of AUD, CanUD, CocUD, OUD, and TUD were positively associated with an ASPD diagnosis, although only the associations with AUD, CanUD, and TUD survived multiple testing correction. This may be due to the higher population prevalence of those three diagnoses than for CocUD and OUD [25]. The diagnosis-based associations are consistent with previous findings of SUDs being positively associated with ASPD [26–28]. This association may arise from shared etiologies and predispositions between ASPD and SUDs, as well as other externalizing disorders (i.e., disorders with outward, behavior-based symptomology) [29]. One common feature linked to both ASPD and SUDs is behavioral disinhibition [29–31], which is loosely composed of sensation seeking, impulsivity, and aggressivity [32, 33]. AUD specifically has been linked to low harm avoidance, high novelty seeking, and low reward dependence, which are characteristics shared with ASPD [34–36]. It is reasonable to infer that these behaviors may extend beyond alcohol-related traits to include other substance-related traits consistent with our findings that ASPD is associated with drug use disorder diagnoses and measures of severity. Additionally, because diagnosis-based effect sizes were statistically stronger than severity-based associations, we hypothesize that ASPD-SUD comorbidity may be more related to the shared mechanisms between these pathologic conditions rather than ASPD association with increased risk symptoms across the substance misuse spectrum.
Previous studies highlighted how distinct personality characteristics can influence the use and abuse of a drug of choice and the extent of polysubstance comorbidities [37–40]. Building on these earlier analyses, we reported the first evidence regarding specific associations of ASPD with the individual diagnostic criteria used to diagnose SUDs. The criteria that survived multiple testing correction in the SUD-specific analyses (“Hazardous Use”, “Social Problems”, “Neglected Roles”, “Physical Problems”, and “Attempts to Quit”; Fig. 2) align well with the impulsive, reckless, and irresponsible characteristics of ASPD. “Hazardous Use” has been linked to measures of disinhibition and antisocial behaviors [41, 42] and is the only criterion to associate across all the SUDs, indicating that individuals with co-occurring ASPD and SUD use substances in hazardous situations, regardless of the substance. This is consistent with the conceptualization of the “Hazardous Use” criterion as measuring general externalizing behavior independent of the substance itself and varying between demographic groups [43, 44]. Notably, the “Attempts to Quit” criterion was the only one that was negatively associated with ASPD, suggesting that the absence of ASPD, or decreased ASPD symptomology, allows individuals to make more attempts to terminate their substance use. However, there are different views on whether the presence of ASPD supports or hinders SUD treatment-seeking behavior and abstinence [45–47]. Further, the different associations of ASPD with criteria for individual SUDs, as well as the presence of distinct personality characteristics associated with the drug of choice in previous research [37–40], supports our decision to analyze each separately rather than creating a composite SUD variable for analysis.
While significant when tested individually, most associations related to SUD criteria were null when combined into a multi-SUD analysis of diagnostic criteria. This is likely due to the intercorrelation among diagnostic criteria across SUDs [48]. Sex was significantly associated with ASPD/SUD associations despite having controlled for sex differences in ASPD prevalence by matching cases and controls. The association with sex was attenuated when internalizing disorders were included as covariates in the regression analysis. ASPD [20, 49], SUDs [50, 51], and internalizing disorders (i.e., MDD, GAD, and PTSD) [52–54], and their respective comorbidities [18, 19] all have distinct sex distributions. Despite controlling for sex across ASPD cases and controls, the re-emergence of sex as a significant covariate in the regression analysis of multi-SUD, diagnostic criteria regression likely arose from symptom-level sex differences in SUD criteria that exceed those at the SUD diagnostic or severity level. The inclusion of sex-interaction terms in the analysis of significant SUD criteria (e.g., Sex-TUD Hazardous Use, Sex-CocUD Attempts to Quit, and Sex-CanUD Severity) controlled for these sex effects.
This study has several limitations. Despite providing a large, racially diverse sample of participants, recruitment of the Yale-Penn cohort targeted individuals with addiction phenotypes (and controls without these phenotypes). Thus, results from these analyses cannot be generalized to the general population or other cohorts with differing characteristics. However, they bring to light specific ASPD associations across the SUD spectrum and underscore the potential utility of conducting similar analyses in a nationally representative cohort for comparison. Secondly, although the goal of the present study was to identify ASPD associations across the SUD spectrum, the current design does not permit us to investigate the cause-effect relationships that may contribute to the associations observed. Thirdly, only DSM-IV criteria were available for ASPD, MDD, GAD, and PTSD. We do not anticipate any significant impact on the analyses from this as there were only minor differences between DSM-IV and DSM-5 for these disorders. Lastly, two DSM-5 criteria for TUD (i.e., “Social Problems” and “Neglected Roles”) are not available in the SSADDA data. Excluding these criteria could have led to an under-identification of individuals with TUD and misclassification of TUD severity. Additionally, it may directly impact the TUD-specific and SUD-combined criteria analyses as “Social Problems” and “Neglected Roles” were seen to be significantly associated with ASPD in multiple SUDs.
Future studies are needed to replicate these findings in cohorts selected for characteristics other than substance use phenotypes, which would show how generalizable the current findings are. It may also be helpful to assess whether similar associations exist between SUDs and other externalizing disorders that comprise ASPD: namely conduct disorder [55] and adult antisocial behavior [56, 57]. These analyses could enhance our understanding of the extent to which these associations reflect overall deviance from cultural norms and mental health comorbidities of the population at large.
In conclusion, the comorbidity of ASPD and SUD is of significant theoretical and clinical interest. This study builds on previous work in this area by showing consistent associations between ASPD and SUD diagnoses and uncovering unique, substance-specific associations between ASPD and individual SUD diagnostic criteria. The present study expands our understanding of polysubstance addiction and co-occurring mental health disorders. Our findings support personalized interventions targeting mechanism-based subtyping in relation to ASPD-SUD comorbidities. Further work in other samples, particularly general population samples, is needed to confirm and extend these associations, exploring clinical and molecular implications.
Supplementary information
Acknowledgements
The authors thank the research participants enrolled in the Yale-Penn cohort. This study was supported by the National Institutes of Health (R33 DA047527, R21 DC018098, RF1 MH132337, and T32 AA028259), One Mind, and the VISN 4 Mental Illness Research, Education and Clinical Center at the Crescenz VAMC. The Yale-Penn cohort was supported by multiple grants from the National Institutes of Health (RC2 DA028909, R01 DA12690, R01 DA12849, R01 DA18432, R01 AA11330, R01 AA017535). The funding sources had no role in the design of this study, its execution, analyses, interpretation of the data, and the decision to publish the results.
Author contributions
AL and RP designed the study. YZN, HRK, and JG coordinated the participants’ recruitment and assessment. AL, BS, KA, RHP, and RP contributed to the data analysis. AL, BS, YZN, KA, JDD, RHP, HRK, JG, and RP contributed to the interpretation of the results. AL and RP drafted the manuscript. AL, BS, YZN, KA, JDD, RHP, HRK, JG, and RP substantively revised the manuscript.
Data availability
The data presented are included in the article and its supplemental material. Yale-Penn individual-level information is available in dbGaP (https://www.ncbi.nlm.nih.gov/gap/) under accession numbers phs000952.v1.p1 and phs000425.v1.p1.
Competing interests
RP received a research grant from Alkermes outside the scope of the present study. RP and JG are paid for their editorial work on the journal Complex Psychiatry. JG and HRK are named as inventors on PCT patent application #15/878,640 entitled: “Genotype-guided dosing of opioid agonists,” filed January 24, 2018. HRK is a member of advisory boards for Dicerna Pharmaceuticals, Sophrosyne Pharmaceuticals, and Enthion Pharmaceuticals; a consultant to Sophrosyne Pharmaceuticals; the recipient of research funding and medication supplies for an investigator-initiated study from Alkermes; a member of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative, which for the past three years was supported by Alkermes, Ethypharm, Lundbeck, Mitsubishi, Otsuka, and Pear Therapeutics, and is paid for his editorial work on the journal Alcohol: Clinical and Experimental Research. The other authors have no competing interests to report.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-024-03054-z.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5 ed. Washington, D.C.: American Psychiatric Association; 2013.
- 2.Kelly TM, Daley DC. Integrated treatment of substance use and psychiatric disorders. Soc Work Public Health. 2013;28:388–406. 10.1080/19371918.2013.774673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross S, Peselow E. Co-occurring psychotic and addictive disorders: neurobiology and diagnosis. Clin Neuropharmacol. 2012;35:235–43. 10.1097/WNF.0b013e318261e193 [DOI] [PubMed] [Google Scholar]
- 4.Witkiewitz K, King K, McMahon RJ, Wu J, Luk J, Bierman KL, et al. Evidence for a multi-dimensional latent structural model of externalizing disorders. J Abnorm Child Psychol. 2013;41:223–37. 10.1007/s10802-012-9674-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant BF, Stinson FS, Dawson DA, Chou SP, Ruan WJ, Pickering RP. Co-occurrence of 12-Month Alcohol and drug use disorders and personality disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61:361–8. 10.1001/archpsyc.61.4.361 [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez E, Arias F, Szerman N, Vega P, Mesias B, Basurte I. Coexistence between personality disorders and substance use disorder. Madrid study about prevalence of dual pathology. Actas Esp Psiquiatr. 2019;47:218–28. [PubMed] [Google Scholar]
- 7.Rounsaville BJ, Kranzler HR, Ball S, Tennen H, Poling J, Triffleman E. Personality disorders in substance abusers: relation to substance use. J Nerv Ment Dis. 1998;186:87–95. 10.1097/00005053-199802000-00004 [DOI] [PubMed] [Google Scholar]
- 8.Skodol AE, Oldham JM, Gallaher PE. Axis II comorbidity of substance use disorders among patients referred for treatment of personality disorders. Am J Psychiatry. 1999;156:733–8. 10.1176/ajp.156.5.733 [DOI] [PubMed] [Google Scholar]
- 9.Gelernter J, Kranzler HR, Sherva R, Almasy L, Herman AI, Koesterer R, et al. Genome-wide association study of nicotine dependence in American populations: identification of novel risk loci in both African-Americans and European-Americans. Biol Psychiatry. 2015;77:493–503. 10.1016/j.biopsych.2014.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gelernter J, Kranzler HR, Sherva R, Almasy L, Koesterer R, Smith AH, et al. Genome-wide association study of alcohol dependence:significant findings in African- and European-Americans including novel risk loci. Mol Psychiatry. 2014;19:41–9. 10.1038/mp.2013.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gelernter J, Kranzler HR, Sherva R, Koesterer R, Almasy L, Zhao H, et al. Genome-wide association study of opioid dependence: multiple associations mapped to calcium and potassium pathways. Biol Psychiatry. 2014;76:66–74. 10.1016/j.biopsych.2013.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelernter J, Sherva R, Koesterer R, Almasy L, Zhao H, Kranzler HR, et al. Genome-wide association study of cocaine dependence and related traits: FAM53B identified as a risk gene. Mol Psychiatry. 2014;19:717–23. 10.1038/mp.2013.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kember RL, Hartwell EE, Xu H, Rotenberg J, Almasy L, Zhou H, et al. Phenome-wide association analysis of substance use disorders in a deeply phenotyped sample. Biol Psychiatry. 2023;93:536–45. 10.1016/j.biopsych.2022.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pierucci-Lagha A, Gelernter J, Feinn R, Cubells JF, Pearson D, Pollastri A, et al. Diagnostic reliability of the Semi-structured Assessment for Drug Dependence and Alcoholism (SSADDA). Drug Alcohol Depend. 2005;80:303–12. 10.1016/j.drugalcdep.2005.04.005 [DOI] [PubMed] [Google Scholar]
- 15.Pierucci-Lagha A, Gelernter J, Chan G, Arias A, Cubells JF, Farrer L, et al. Reliability of DSM-IV diagnostic criteria using the semi-structured assessment for drug dependence and alcoholism (SSADDA). Drug Alcohol Depend. 2007;91:85–90. 10.1016/j.drugalcdep.2007.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42:1–28. 10.18637/jss.v042.i08 [DOI] [Google Scholar]
- 17.O’Neil KA, Conner BT, Kendall PC. Internalizing disorders and substance use disorders in youth: comorbidity, risk, temporal order, and implications for intervention. Clin Psychol Rev. 2011;31:104–12. 10.1016/j.cpr.2010.08.002 [DOI] [PubMed] [Google Scholar]
- 18.Conway KP, Compton W, Stinson FS, Grant BF. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2006;67:247–57. 10.4088/JCP.v67n0211 [DOI] [PubMed] [Google Scholar]
- 19.Khan S, Okuda M, Hasin DS, Secades-Villa R, Keyes K, Lin KH, et al. Gender differences in lifetime alcohol dependence: results from the national epidemiologic survey on alcohol and related conditions. Alcohol Clin Exp Res. 2013;37:1696–705. 10.1111/acer.12158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulte Holthausen B, Habel U. Sex differences in personality disorders. Curr Psychiatry Rep. 2018;20:107. 10.1007/s11920-018-0975-y [DOI] [PubMed] [Google Scholar]
- 21.Brennan GM, Hyde LW, Baskin-Sommers AR. Antisocial pathways associated with substance use disorders: characterizing etiological underpinnings and implications for treatment. Curr Opin Behav Sci. 2017;13:124–9. 10.1016/j.cobeha.2016.11.014 [DOI] [Google Scholar]
- 22.Trull TJ, Solhan MB, Brown WC, Tomko RL, Schaefer L, McLaughlin KD, et al. Substance use disorders and personality disorders. In: Sher KJ, editor. The Oxford handbook of substance use and substance use disorders. Oxford University Press, Oxford; 2016. pp 116–48.
- 23.Korsgaard HO, Torgersen S, Wentzel-Larsen T, Ulberg R. Substance abuse and personality disorder comorbidity in adolescent outpatients: are girls more severely ill than boys? Child Adolesc Psychiatry Ment Health. 2016;10:8. 10.1186/s13034-016-0096-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alcorn JL 3rd, Gowin JL, Green CE, Swann AC, Moeller FG, et al. Aggression, impulsivity, and psychopathic traits in combined antisocial personality disorder and substance use disorder. J Neuropsychiatry Clin Neurosci. 2013;25:229–32. 10.1176/appi.neuropsych.12030060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shmulewitz D, Greene ER, Hasin D. Commonalities and differences across substance use disorders: phenomenological and epidemiological aspects. Alcohol Clin Exp Res. 2015;39:1878–900. 10.1111/acer.12838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Compton WM, Conway KP, Stinson FS, Colliver JD, Grant BF. Prevalence, correlates, and comorbidity of DSM-IV antisocial personality syndromes and alcohol and specific drug use disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry. 2005;66:677–85. 10.4088/JCP.v66n0602 [DOI] [PubMed] [Google Scholar]
- 27.Stiltner B, Pietrzak RH, Tylee DS, Nunez YZ, Adhikari K, Kranzler HR, et al. Polysubstance addiction patterns among 7,989 individuals with cocaine use disorder. iScience. 2023;26:107336. 10.1016/j.isci.2023.107336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flynn PM, Luckey JW, Brown BS, Hoffman JA, Dunteman GH, Theisen AC, et al. Relationship between Drug Preference and Indicators of Psychiatric Impairment. Am J Drug Alcohol Abuse. 1995;21:153–66. 10.3109/00952999509002685 [DOI] [PubMed] [Google Scholar]
- 29.Krueger RF. The structure of common mental disorders. Arch Gen Psychiatry. 1999;56:921–6. 10.1001/archpsyc.56.10.921 [DOI] [PubMed] [Google Scholar]
- 30.Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: an integrative quantitative model of the adult externalizing spectrum. J Abnorm Psychol. 2007;116:645–66. 10.1037/0021-843X.116.4.645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorenstein EE, Newman JP. Disinhibitory psychopathology: a new perspective and a model for research. Psychol Rev. 1980;87:301–15. 10.1037/0033-295X.87.3.301 [DOI] [PubMed] [Google Scholar]
- 32.Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, et al. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry. 2003;160:1078–85. 10.1176/appi.ajp.160.6.1078 [DOI] [PubMed] [Google Scholar]
- 33.Hawkins JD, Catalano RF, Miller JY. Risk and protective factors for alcohol and other drug problems in adolescence and early adulthood: implications for substance abuse prevention. Psychol Bull. 1992;112:64–105. 10.1037/0033-2909.112.1.64 [DOI] [PubMed] [Google Scholar]
- 34.Cloninger CR. A systematic method for clinical description and classification of personality variants. A proposal. Arch Gen Psychiatry. 1987;44:573–88. 10.1001/archpsyc.1987.01800180093014 [DOI] [PubMed] [Google Scholar]
- 35.Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–6. 10.1126/science.2882604 [DOI] [PubMed] [Google Scholar]
- 36.Cloninger CR, Sigvardsson S, Bohman M. Type I and Type II alcoholism: an update. Alcohol Health Res World. 1996;20:18–23. [PMC free article] [PubMed] [Google Scholar]
- 37.Conway KP, Kane RJ, Ball SA, Poling JC, Rounsaville BJ. Personality, substance of choice, and polysubstance involvement among substance dependent patients. Drug Alcohol Depend. 2003;71:65–75. 10.1016/S0376-8716(03)00068-1 [DOI] [PubMed] [Google Scholar]
- 38.Conway KP, Swendsen JD, Rounsaville BJ, Merikangas KR. Personality, drug of choice, and comorbid psychopathology among substance abusers. Drug Alcohol Depend. 2002;65:225–34. 10.1016/S0376-8716(01)00168-5 [DOI] [PubMed] [Google Scholar]
- 39.Hopwood CJ, Baker KL, Morey LC. Personality and drugs of choice. Personal Individ Differ. 2008;44:1413–21. 10.1016/j.paid.2007.12.009 [DOI] [Google Scholar]
- 40.Gerra G, Bertacca S, Zaimovic A, Pirani M, Branchi B, Ferri M. Relationship of personality traits and drug of choice by cocaine addicts and heroin addicts. Subst Use Misuse. 2008;43:317–30. 10.1080/10826080701202726 [DOI] [PubMed] [Google Scholar]
- 41.Mewton L, Teesson M, Slade T, Cottler L. Psychometric performance of DSM-IV alcohol use disorders in young adulthood: evidence from an Australian general population sample. J Stud Alcohol Drugs. 2011;72:811–22. 10.15288/jsad.2011.72.811 [DOI] [PubMed] [Google Scholar]
- 42.Martin CS, Chung T, Langenbucher JW. How should we revise diagnostic criteria for substance use disorders in the DSM-V? J Abnorm Psychol. 2008;117:561–75. 10.1037/0021-843X.117.3.561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin CS, Sher KJ, Chung T. Hazardous use should not be a diagnostic criterion for substance use disorders in DSM-5. J Stud Alcohol Drugs. 2011;72:685–6. 10.15288/jsad.2011.72.685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watts AL, Watson D, Heath AC, Sher KJ. Alcohol use disorder criteria exhibit different comorbidity patterns. Addiction. 2023;118:1457–68. 10.1111/add.16121 [DOI] [PubMed] [Google Scholar]
- 45.Sargeant MN, Bornovalova MA, Trotman AJ, Fishman S, Lejuez CW. Facets of impulsivity in the relationship between antisocial personality and abstinence. Addict Behav. 2012;37:293–8. 10.1016/j.addbeh.2011.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pineiro B, del Rio EF, Lopez-Duran A, Martinez U, Becona E. The association between probable personality disorders and smoking cessation and maintenance. Addic Behav. 2013;38:2369–73. 10.1016/j.addbeh.2013.03.017 [DOI] [PubMed] [Google Scholar]
- 47.Cooperman NA, Lu SE, Richter KP, Bernstein SL, Williams JM. Influence of psychiatric and personality disorders on smoking cessation among individuals in opiate dependence treatment. J Dual Diagn. 2016;12:118–28. 10.1080/15504263.2016.1172896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hasin DS, O’Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, et al. DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry. 2013;170:834–51. 10.1176/appi.ajp.2013.12060782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alegria AA, Blanco C, Petry NM, Skodol AE, Liu SM, Grant B, et al. Sex differences in antisocial personality disorder: results from the National Epidemiological Survey on Alcohol and Related Conditions. Personal Disord. 2013;4:214–22. 10.1037/a0031681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caris L, Wagner FA, Rios-Bedoya CF, Anthony JC. Opportunities to use drugs and stages of drug involvement outside the United States: evidence from the Republic of Chile. Drug Alcohol Depend. 2009;102:30–4. 10.1016/j.drugalcdep.2008.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delva J, Van Etten ML, Gonzalez GB, Cedeno MA, Penna M, Caris LH, et al. First opportunities to try drugs and the transition to first drug use: evidence from a national school survey in Panama. Subst Use Misuse. 1999;34:1451–67. 10.3109/10826089909029392 [DOI] [PubMed] [Google Scholar]
- 52.Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish national twin study of lifetime major depression. Am J Psychiatry. 2006;163:109–14. 10.1176/appi.ajp.163.1.109 [DOI] [PubMed] [Google Scholar]
- 53.Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jonsson B, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:655–79. 10.1016/j.euroneuro.2011.07.018 [DOI] [PubMed] [Google Scholar]
- 54.Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26:537–47. 10.1002/jts.21848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fairchild G, Hawes DJ, Frick PJ, Copeland WE, Odgers CL, Franke B, et al. Conduct disorder. Nat Rev Dis Primers. 2019;5:43. 10.1038/s41572-019-0095-y [DOI] [PubMed] [Google Scholar]
- 56.Mata AD, van Dulmen MH. Adult-onset antisocial behavior trajectories: associations with adolescent family processes and emerging adulthood functioning. J Interpers Violence. 2012;27:177–93. 10.1177/0886260511416467 [DOI] [PubMed] [Google Scholar]
- 57.Gomez-Smith Z, Piquero AR. An examination of adult onset offending. J Crimin Justice. 2005;33:515–25. 10.1016/j.jcrimjus.2005.08.001 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented are included in the article and its supplemental material. Yale-Penn individual-level information is available in dbGaP (https://www.ncbi.nlm.nih.gov/gap/) under accession numbers phs000952.v1.p1 and phs000425.v1.p1.