Abstract
Acute myeloid leukemia (AML) with t(8;21) (q22;q22), which forms RUNX1::RUNX1T1 fusion gene, is classified as a favorable-risk group. However, the presence of mutations in KIT exon 17 results in an adverse prognosis in this group. Avapritinib, a novel tyrosine kinase inhibitor, was designed to target KIT mutation. We report a retrospective study of four pediatric patients with AML with t(8:21) and KIT exon 17 mutation who were treated with avapritinib, three of them failed to demethylate drugs and donor lymphocyte infusion targeting RUNX1::RUNX1T1-positivity after allogeneic hematopoietic stem cell transplantation (allo-HSCT). So far, all patients with RUNX1::RUNX1T1 positivity had turned negative after 1, 9, 7, 2 months of avapritinib treatment. The common adverse effect of avapritinib is neutropenia, which is well-tolerated. This case series indicates that avapritinib may be effective and safe for preemptive treatment of children with AML with t(8;21) and KIT mutation after allo-HSCT, providing a treatment option for preventing relapse after allo-HSCT.
Keywords: Acute myeloid leukemia, Avapritinib, KIT mutation, Pediatric, t(8;21)
Introduction
Core binding factor acute myeloid leukemia (CBF-AML) includes AML cases harboring RUNX1::RUNX1T1 or CBFβ::MYH11 fusion genes, which are classified as a favorable-risk group [1, 2]. However, 20–40% of adult patients with de novo CBF-AML [3–7] and 23% of children with CBF-AML [8] have KIT mutation with varied impacts on prognosis. Several studies have proposed that patients with AML with RUNX1::RUNX1T1 as well as KIT mutation [9], particularly KIT exon 17 mutation, have worse clinical outcomes [10, 11]. Patients with high-risk features can benefit from allogeneic hematopoietic stem cell transplantation (allo-HSCT), but 20% of patients with AML with RUNX1::RUNX1T1 relapse after that and the dynamics of the transcript levels can predict relapse in these patients [12, 13] .
Avapritinib, a novel tyrosine kinase inhibitor targeting KIT/PDGFRA, has been approved for treating gastrointestinal stromal tumors and systemic mastocytosis. It effectively treats minimal residual disease (MRD) in AML cases harboring RUNX1::RUNX1T1 and KIT mutations after allo-HSCT [14]. However, clinical application in pediatric AML is lacking. Here, we report four children with AML with RUNX1::RUNX1T1 and KIT mutations, who mostly were refractory to demethylation drugs (HMAs) and donor lymphocyte infusion (DLI), who were treated with avapritinib as a preemptive intervention after allo-HSCT.
Methods
The patient characteristics and treatment are summarized in Table 1. They were treated using the CALSIII-AML18 protocol, registered as ChiCTRl800015883 (the registration date: April 26, 2018), and it is is comparable that the result of long-term survival between standard-dose chemotherapy (SDC) low-dose chemotherapy (LDC) [15]. All four patients underwent HSCT with myeloablative conditioning regimens after achieving complete remission in morphology and the transplantation circumstances were shown in the Table 2. They underwent painless puncture for monitoring disease status dynamically. MRD was monitored by flow cytometry (FCM), the RUNX1::RUNX1T1 (Fig. 1) transcript level was measured quantitatively by real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR) and KIT mutation status was monitored by direct sequencing. Four patients were treated with avapritinib for RUNX1::RUNX1T1-positive AML in spite of complete remission in morphology, MRD by FCM and KIT mutation after allo-HSCT and three of them failed to HMAs and DLI. The starting dose of avapritinib was 50 mg/day and the administration would be adjusted if the patients experienced ≥ grade 3 adverse events (Table 3). The content and standards of adverse events were based on the Common Adverse Event Evaluation Standards (CTCAE) Version 5.0 [16]. The study protocol was approved by the Ethics Committee of Children’s Hospital of Soochow University, and informed consent was obtained from the parents of the patients. The data cut-off date for the primary analysis was January 2024.
Table 1.
Baseline characteristics of KIT-mutant AML patients
| NO | Age/sex | KIT mutation | Other mutations | Chemotherapya | Time from diagnosis to first relapse (month) | BM status before HSCT (Morphology / FCM-MRD /Fusion gene (relative value) /KIT) | Pretreatment schemeb |
|---|---|---|---|---|---|---|---|
| 1 | 12/F | KIT: D816V | KRAS、ASXL1 | CALSIII-AML18 protocol (SDC c) | none | 2%/<0.1% / 0.0007/negative | CCNU + CLAG + DEX + BuCy |
| 2 | 4/M | KIT: N822K、KIT: V560D | NRAS、FLT3 | CALSIII-AML18 protocol (LDC d) | none | 1%/<0.1%/0.0004/negative | CCNU + CLAG + DEX + BuCy + ATG |
| 3 | 12/M | KIT: D816Y | KDM6A | CALSIII-AML18 protocol (SDC) | none | 1%/<0.1%/0.0071/negative | CCNU + CLAG + DEX + BuCy + ATG |
| 4 | 11/M | KIT: N822K | none | CALSIII-AML18 protocol (SDC) | 2 | 1%/6.01%/0.0150/NA | CCNU + CLAG + DEX + BuCy + ATG |
BM, bone marrow; HSCT, hematopoietic stem cell transplantation; FCM-MRD, measurable residual disease monitored by flow cytometry; NA, not available. a All patients were treated by the protocol registered as ChiCTRl800015883. b All patients in this study received myeloablative conditioning regimens. c Standard-dose chemotherapy. d Low-dose cytarabine and mitoxantrone or omacetaxine mepesuccinate with concurrent granulocyte colony-stimulating factor (low-dose chemotherapy); CCNU, cyclonexyl-chloroethyl-nitrosourea; CLAG, Cladribine, Cytarabine, granulocyte colony-stimulating factor; DEX, Dexamethasone; BuCy Busulfan, Cyclophosphamide; ATG antithymocyte globulin
Table 2.
Transplantation circumstances of KIT-mutant AML patients
| NO | HLA-matched site | Cell doses of MNC (10^8 /kg) | Cell doses of CD34+ (10^6 /kg) | ANC engraftment (day) | PLT engraftment (day) | From HSCT to Molecular relapse (month) | DLIa (times) | DACb |
|---|---|---|---|---|---|---|---|---|
| 1 | 10/10 | 9.85 | 5.50 | 12 | 11 | 1 | 9 | 10 mg/m2*3d /course *7 courses |
| 2 | 10/10 | 6.74 | 11.91 | 11 | 11 | 1 | 4 | 10 mg/m2*5d /course *8 courses |
| 3 | 5/10 | 6.03 | 8.30 | 13 | 13 | 2 | 1 | 10 mg/m2*5d /course*3 courses |
| 4 | 5/10 | 5.80 | 10.17 | 12 | 13 | 2 | none | none |
AML, acute myeloid leukemia; HLA, human leukocyte antigen; MNC, mononuclear cell; ANC, absolute neutrophil count; PLT, platelet; DLI, donor lymphocyte infusions; DAC, decitabine; a CD3 + T cells were at least 0.5-1 × 107 /kg once a month. b10 mg/m2 for 3–5 days continuously in a month as a course
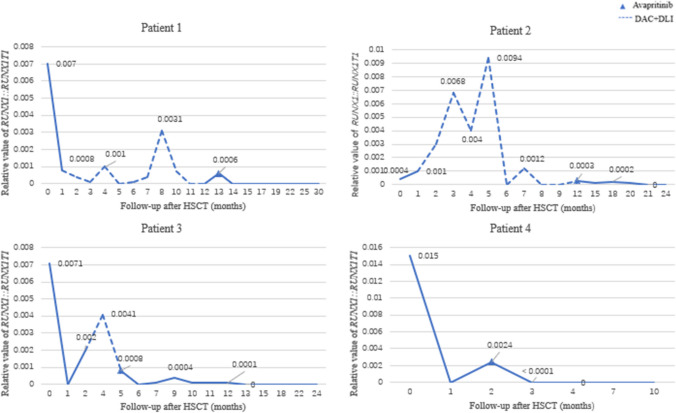
Fig. 1.
Relative value of RUNX1::RUNX1T1 after allo-HSCT. 0 represents the last relative value of RUNX1::RUNX1T1 before allo-HSCT. DLI, donor lymphocyte infusion; DAC, decitabine
Evaluation of treatment with avapritinib
| NO | Usage | Response | Adverse events(grade) | Survival status | Follow-up since HSCT (months) |
|---|---|---|---|---|---|
| 1 | 50 mg QOD | Molecular Negative after 1 month | neutropenia (4), thrombocytopenia (2), puffiness (1) | Alive | 30 |
| 2 | 50 mg QD | Molecular Negative after 9 months | neutropenia (2), puffiness (1) | Alive | 24 |
| 3 | 50 mg QOD | Molecular Negative after 7 months | neutropenia (3) | Alive | 24 |
| 4 | 50 mg QD | Molecular Negative after 2 months | neutropenia (3) | Alive | 10 |
QOD, once every other day; QD, once a day
Case 1
A 12-year-old girl was admitted to our hospital with upper limb pain on March 19, 2021. A RUNX1::RUNX1T1-fusion gene and KIT D816V mutation were detected by RT-qPCR and next-generation sequencing, respectively. She was treated with the CALSIII-AML18 protocol (SDC), after which morphology normalized, MRD by FCM and KIT mutation had disappeared; however, RUNX1::RUNX1T1-positivity remained prior to allo-HSCT. To reduce relapse risk, she was transfused with human leukocyte antigen (HLA) 10/10 peripheral blood (PB) stem cells. RUNX1::RUNX1T1-positivity remained present in the first month after allo-HSCT. Decitabine (DAC) (10 mg/m2, 3 days/course, 7 courses) and DLI (9 cycles) were administered preemptively, and the fusion gene load was alleviated; however, negativity was not sustained and RUNX1::RUNX1T1 progressed once we tried stopping DAC and DLI trentment. Although no severe graft-versus-host disease (GVHD) during this period, adverse events such as abnormal liver function (grade 3) and neutropenia (grade 4) occurred. To prevent the progression of the disease, she was given avapritinib monotherapy, RUNX1::RUNX1T1-negativity was achieved after 1 month and has been 16 months. During avapritinib monotherapy, neutropenia (grade 4), thrombocytopenia (grade 2), and puffiness (grade 1) occurred, which improved after adjustment of medication.
Case 2
On August 23, 2021, a 4-year-old boy with t(8;21) and KIT N822K and KIT V560D mutations presented to the hospital with commissure distortion. The patient was treated with the CALSIII-AML18 protocol (LDC), with dasatinib during chemotherapy because of KIT mutation. He achieved a morphological complete response and KIT-negativity; however, RUNX1::RUNX1T1 remained positive before allo-HSCT. He was transfused with unrelated donor HLA 10/10 PB stem cells. RUNX1::RUNX1T1 also tested positive in the first month after allo-HSCT and showed a rising trend. DAC (10 mg/m2, 5 days/course, 8 courses) and DLI (4 cycles) did not maintain fusion gene-negativity and caused neutropenia (grade 4) in spite of without GVHD. The copy of transcript continued to decrease, turned negative after 9 months of avapritinib monotherapy and had stayed for 3 months. During the period, neutropenia (grade 2), puffiness (grade 1) occurred and those could improve after clinical observation.
Case 3
The third patient with CBF-AML was a 12-year-old boy with KIT D816Y-mutated who was hospitalized for ecchymosis of the skin on June 9, 2021. He was treated using the CALSIII-AML18 protocol (SDC) and achieved the same bone marrow (BM) remission status as Cases 1 and 2, before transfusion with his brother’s HLA 5/10 PB stem cells. RUNX1::RUNX1T1 fusion gene level was positive in the second month after allo-HSCT. DAC (10 mg/m2, 5 days/course, 3 courses) and DLI (once) failed to turn the fusion gene negative while caused grade II aGVHD and neutropenia (grade 3). After 7 months of avapritinib monotherapy, RUNX1::RUNX1T1 gene tests turned negative and has kept for 11 months. Although it happened to neutropenia (grade 3), which also improved by adjustment of medication.
Case 4
The fourth AML patient was an 11-year-old boy with KIT N822K and t(8;21) who visited the hospital with anemia and bleeding on April 6, 2022. He was primarily treatment refractory/resistant, and salvage therapy before allo-HSCT was suggested after a BM smear showed 6.01% MRD by FCM though CR in morphology. He was transfused with HLA 5/10 BM and PB stem cells. RUNX1::RUNX1T1 tests were positive in the second month after allo-HSCT. Due to the first three patients did not respond quickly to DAC and DLI, the fourth patient was directly administered avapritinib monotherapy for preemptive intervention to observe the efficacy and exclude the intervention of DAC and DLI. After 2 months of preemptive treatment, the fusion gene turned negative and disease-free state has been maintained for 7 months. He also suffered neutropenia (grade 3) and improved after clinical observation.
Discussion
KIT is composed of 21 exons and is located on chromosomal segment 4q11 [17]. Exon 8 and 17 mutations commonly occur in AML, particularly in exon 17, which are most strongly related to inferior prognosis in patients with de novo AML with t(8;21) [3, 7, 18], and allo-HSCT may improve prognosis in these patients [19]. Our four cases had AML with RUNX1::RUNX1T1 and KIT exon 17 mutations and all patients achieved complete morphological remission before allo-HSCT; however, RUNX1::RUNX1T1-positivity was found within 60 days after allo-HSCT. Preemptive HMAs and DLI following allo-HSCT can prevent relapse in high-risk AML cases [20–22]. Three of our patients received DAC and DLI because of RUNX1::RUNX1T1-positivity after allo-HSCT, but fusion gene-negativity could not be sustained and case 3 occurred to grade II aGVHD. A previous study demonstrated that AML patients with RUNX1::RUNX1T1 and KIT mutation in relapse after allo-HSCT show a rapid response to avapritinib [14, 23], 80% of such patients achieved the decrease of RUNX1::RUNX1T1-positivity transcript levels after 1 month of avapritinib treatment for MRD [14]. Our four patients achieved sustained fusion gene-negativity after 1, 9, 7, 2 months of avapritinib treatment and stayed complete remission until now. Because the establishment of graft-versus-leukemia effect of allo-HSCT and DAC + DLI can take some time, the complete remission in our patients cannot be attributed solely to avapritinib. However, none of the three patients maintained negative transcript more than a month during DAC and DLI while case 4 had an effective and quick performance to avapritinib preemptive monotherapy, which supported they could bebefit from avapritinib as preemptive therapy after HSCT.
The most common adverse event associated with avapritinib treatment is myelosuppression. In a study of patients with gastrointestinal stromal tumors [24], decreased hemoglobin, while in AML patients [14], thrombocytopenia was the most common hematological adverse event after avapritinib. This could be related to the heterogeneity of different diseases, avapritinib doses, and sample sizes. In our study, the most common adverse event was neutropenia, of which three of the four patients had grade 3–4. One patient developed grade 2 thrombocytopenia. Besides, two patients had grade 1 puffiness. All adverse events were tolerated and improved after clinical observation and medication adjustments compared to DAC and DLI. All the patients have not stopped avapritinib and survived with disease-free to the present date.
In previous studies [14, 23, 25], avapritinib doses mostly ranged from 50 to 200 mg/day and 50 mg/day as an initial dose was recommended to 2 children. In our study, 50 mg/day was also used as the initial dose, adjusted according to children’s tolerance, which could be a clinical reference for children’s medication. However, no study indicates the recommended duration of avapritinib as preemptive treatment for patients with AML after allo-HSCT. In addition, our sample size was too small to evaluate the clinical efficacy and safety of avapritinib. As a retrospective study, some bias may have existed in patient selection. Further studies and an observation period are required to address these issues. We have started a clinical trial (GMCAIII, registered as NCT06221683) in which the dose was based on these four patients used.
In conclusion, in our patients, avapritinib was well-tolerated and can be effective as a preemptive treatment of pediatric AML with KIT mutation after allo-HSCT.
Abbreviations
- AML
acute myeloid leukemia
- MRD
measurable residual disease
- allo-HSCT
allogeneic hematopoietic stem cell transplantation
- CBF-AML
core binding factor acute myeloid leukemia
- HMAs
demethylation drugs
- DLI
donor lymphocyte infusion
- PB
peripheral blood
- BM
bone marrow
- RT-qPCR
Real-time quantitative reverse transcription-polymerase chain reaction
- DAC
decitabine
Author contributions
Q. W. W, Y. X H, L. G, S. L Z: Data and original draft. J. L, B. H L, J. L: Conceptualization and methodology; Y. H. Y, S. Q. C: Investigation and project administration; P. F X and S. Y. H: Supervision and writing – review & editing. All authors reviewed the manuscript.
Funding
This work was supported by following grants: the National Natural Science Foundation of China (No.82170218, 82100229, 82200177), Jiangsu key project (No. BE2021654), Suzhou project (No. SZS201615, GSWS2020039, SKY2022012, and SZS2023014), National Clinical Research Center for Hematologic Disease (No. 2020ZKPB02).
Declarations
Ethical approval
The study protocol was approved by the Ethics Committee of Children’s Hospital of Soochow University, and informed consent was obtained from the parents of the patients.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qingwei Wang, Yixin Hu, Li Gao and Senlin Zhang contributed equally to this study.
Contributor Information
Peifang Xiao, Email: xpfdr@163.com.
Shaoyan Hu, Email: hushaoyan@suda.edu.cn.
References
- 1.Grimwade D, Walker H, Oliver F et al (1998) The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood 92(7):2322–33 10.1182/blood.V92.7.2322 [DOI] [PubMed] [Google Scholar]
- 2.Döhner H, Estey E, Grimwade D et al (2017) Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129(4):424–447 10.1182/blood-2016-08-733196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krauth MT, Eder C, Alpermann T et al (2014) High number of additional genetic lesions in acute myeloid leukemia with t(8;21)/RUNX1-RUNX1T1: frequency and impact on clinical outcome. Leukemia 28(7):1449–1458 10.1038/leu.2014.4 [DOI] [PubMed] [Google Scholar]
- 4.Qin YZ, Zhu HH, Jiang Q et al (2014) Prevalence and prognostic significance of c-KIT mutations in core binding factor acute myeloid leukemia: a comprehensive large-scale study from a single Chinese center. Leuk Res 38(12):1435–1440 10.1016/j.leukres.2014.09.017 [DOI] [PubMed] [Google Scholar]
- 5.Allen C, Hills RK, Lamb K et al (2013) The importance of relative mutant level for evaluating impact on outcome of KIT, FLT3 and CBL mutations in core-binding factor acute myeloid leukemia. Leukemia 27(9):1891–1901 10.1038/leu.2013.186 [DOI] [PubMed] [Google Scholar]
- 6.Paschka P, Du J, Schlenk RF et al (2013) Secondary genetic lesions in acute myeloid leukemia with inv(16) or t(16;16): a study of the german-austrian AML Study Group (AMLSG). Blood 121(1):170–177 10.1182/blood-2012-05-431486 [DOI] [PubMed] [Google Scholar]
- 7.Kim HJ, Ahn HK, Jung CW et al (2013) KIT D816 mutation associates with adverse outcomes in core binding factor acute myeloid leukemia, especially in the subgroup with RUNX1/RUNX1T1 rearrangement. Ann Hematol 92(2):163–171 10.1007/s00277-012-1580-5 [DOI] [PubMed] [Google Scholar]
- 8.Fan J, Gao L, Chen J et al (2020) Influence of KIT mutations on prognosis of pediatric patients with core-binding factor acute myeloid leukemia: a systematic review and meta-analysis. Transl Pediatr 9(6):726–733 10.21037/tp-20-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wichmann C, Quagliano-lo Coco I, Yildiz Ö et al (2015) Activating c-KIT mutations confer oncogenic cooperativity and rescue RUNX1/ETO-induced DNA damage and apoptosis in human primary CD34 + hematopoietic progenitors. Leukemia 29(2):279–289 10.1038/leu.2014.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duan W, Liu X, Zhao X et al (2021) Both the subtypes of KIT mutation and minimal residual disease are associated with prognosis in core binding factor acute myeloid leukemia: a retrospective clinical cohort study in single center. Ann Hematol 100(5):1203–1212 10.1007/s00277-021-04432-z [DOI] [PubMed] [Google Scholar]
- 11.Qin YZ, Zhu HH, Jiang Q et al (2018) Heterogeneous prognosis among KIT mutation types in adult acute myeloid leukemia patients with t(8;21). Blood Cancer J 8(8):76 10.1038/s41408-018-0116-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Wu DP, Liu Q et al (2014) In adults with t(8;21)AML, posttransplant RUNX1/RUNX1T1-based MRD monitoring, rather than c-KIT mutations, allows further risk stratification. Blood 124(12):1880–1886 10.1182/blood-2014-03-563403 [DOI] [PubMed] [Google Scholar]
- 13.Qin YZ, Wang Y, Xu LP et al (2017) The dynamics of RUNX1-RUNX1T1 transcript levels after allogeneic hematopoietic stem cell transplantation predict relapse in patients with t(8;21) acute myeloid leukemia. J Hematol Oncol 10(1):44 10.1186/s13045-017-0414-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong J, Zheng FM, Wang ZD et al (2023) Avapritinib is effective for treatment of minimal residual disease in acute myeloid leukemia with t (8;21) and kit mutation failing to immunotherapy after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 58(7):777–783 10.1038/s41409-023-01973-x [DOI] [PubMed] [Google Scholar]
- 15.Hu Y, Chen A, Gao L et al (2021) Minimally myelosuppressive regimen for remission induction in pediatric AML: long-term results of an observational study. Blood Adv 5(7):1837–1847 10.1182/bloodadvances.2020003453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Published: November 27, 2017. U.S. Department of Health and Human Services. National Institutes of Health; National Cancer Institute. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm
- 17.Lennartsson J, Rönnstrand L (2012) Stem cell factor receptor/c-Kit: from basic science to clinical implications. Physiol Rev 92(4):1619–1649 10.1152/physrev.00046.2011 [DOI] [PubMed] [Google Scholar]
- 18.Ishikawa Y, Kawashima N, Atsuta Y et al (2020) Prospective evaluation of prognostic impact of KIT mutations on acute myeloid leukemia with RUNX1-RUNX1T1 and CBFB-MYH11. Blood Adv 4(1):66–75 10.1182/bloodadvances.2019000709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu TM, Xue SL, Li Z et al (2021) [Prognostic value of KIT and other clonal genetic mutations in core-binding factor acute myeloid leukemia]. Zhonghua xue ye xue za zhi = Zhonghua xueyexue zazhi 42(8):646–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.GUILLAUME T, MALARD F, MAGRO L et al (2019) Prospective phase II study of prophylactic low-dose azacitidine and donor lymphocyte infusions following allogeneic hematopoietic stem cell transplantation for high-risk acute myeloid leukemia and myelodysplastic syndrome. Bone Marrow Transplant 54(11):1815–1826 10.1038/s41409-019-0536-y [DOI] [PubMed] [Google Scholar]
- 21.Karakulska-Prystupiuk E, Drozd-Sokołowska J, Waszczuk-Gajda A et al (2018) Azacitidine for relapse after allogeneic stem cell transplantation-single-center study. Transplant Proc 50(7):2212–7 10.1016/j.transproceed.2018.02.148 [DOI] [PubMed] [Google Scholar]
- 22.Schroeder T, Rautenberg C, Haas R et al (2018) Hypomethylating agents for treatment and prevention of relapse after allogeneic blood stem cell transplantation. Int J Hematol 107(2):138–150 10.1007/s12185-017-2364-4 [DOI] [PubMed] [Google Scholar]
- 23.Xue S, Huang W, Liu F et al (2022) Rapid response to avapritinib of acute myeloid leukemia with t(8;21) and KIT mutation relapse post allo-HSCT. Leuk Lymphoma 63(9):2247–2250 10.1080/10428194.2022.2064994 [DOI] [PubMed] [Google Scholar]
- 24.Liu Q, Luo JW (2023) Hypopharyngeal carcinoma complicated with Plummer-Vinson syndrome: a case report. Zhonghua zhong liu za zhi [Chin J Oncol] 45(2):188–190 [DOI] [PubMed] [Google Scholar]
- 25.Yin J, Zhu F, Zhang ZB et al (2022) Rapid and deep response to avapritinib in heavily treated acute myeloid leukemia with t (8;21) and KIT mutation. Ann Hematol 101(10):2347–2350 10.1007/s00277-022-04897-6 [DOI] [PubMed] [Google Scholar]