Abstract
Cancer has emerged as a significant threat to human health. Nucleic acid therapeutics regulate the gene expression process by introducing exogenous nucleic acid fragments, offering new possibilities for tumor remission and even cure. Their mechanism of action and high specificity demonstrate great potential in cancer treatment. However, nucleic acid drugs face challenges such as low stability and limited ability to cross physiological barriers in vivo. To address these issues, various nucleic acid delivery vectors have been developed to enhance the stability and facilitate precise targeted delivery of nucleic acid drugs within the body. In this review article, we primarily introduce the structures and principles of nucleic acid drugs commonly used in cancer therapy, as well as their cellular uptake and intracellular transportation processes. We focus on the various vectors commonly employed in nucleic acid drug delivery, highlighting their research progress and applications in recent years. Furthermore, we propose potential trends and prospects of nucleic acid drugs and their carriers in the future.
Keywords: Nucleic acids, Genetic vectors, Cancer therapy, Drug delivery
Introduction
Cancer has become one of the primary causes of death worldwide, posing a grave threat to human health [1]. Traditional cancer treatment methods primarily include surgery, chemotherapy, radiotherapy, and molecular targeted drugs. However, due to the heterogeneity, metastasis, and recurrence of cancer cells, traditional cancer treatment methods have become ineffective for many malignant tumors. Taking chemotherapy as an example, many commonly used chemotherapeutic drugs have low efficacy and induce drug resistance, leading to incomplete tumor regression in most cases. Furthermore, numerous studies have reported that traditional chemotherapeutic drugs lack specificity and exhibit limitations in non-specific distribution. This not only results in poor bioavailability but also causes significant side effects when non-specifically delivered to healthy tissues, thereby reducing patient tolerance [2, 3]. Therefore, novel treatment methods are urgently needed to address these challenges.
With the advancements and progress in bioinformatics and genomics, nucleic acid therapeutics have provided a new avenue for cancer treatment [4, 5]. The nucleic acid therapeutics discussed in this article primarily refer to the use of nucleic acid drugs, including siRNA drugs, microRNA drugs, antisense oligonucleotide (ASO) drugs, mRNA drugs, for cancer treatment. These drugs are introduced exogenously to regulate the genetic system of cells in the body, thereby silencing or upregulating the expression of target proteins and intervening in the occurrence and development of diseases. This therapeutic approach primarily focuses on the RNA level and does not involve alterations in the underlying DNA sequence [6].
However, the inherent instability, high immunogenicity, and presence of various physiological barriers within the body limit the delivery efficiency and therapeutic efficacy of RNA-based therapies. Safe and effective gene delivery is the foundation and key factor for the success of nucleic acid therapeutics. Gene carriers can act as Trojan horses, protecting RNA from degradation by nucleases and facilitating targeted delivery to the desired site of action. Numerous gene carriers have been extensively studied, including viral vectors and non-viral vectors. Common non-viral vectors include lipid-based nanoparticles, polymer nanoparticles, inorganic nanoparticles, extracellular vesicles (such as exosomes), and nucleic acid conjugates.
We conducted a comprehensive search across multiple databases, including PubMed, Medline, and Web of Science, encompassing the literature published within the last decade (2014–2024). Our search strategy included medical terminology associated with “nucleic acids therapy” or “genetic therapy,” “neoplasm,” “genetic vectors,” “drug delivery,” and other appropriate terms and combinations. Additionally, we performed a search through the references cited in related studies to acquire further information. In this article, we present an overview of the structures and principles of commonly used nucleic acid drugs and provide a brief introduction to the processes of cellular uptake and intracellular transport of these drugs. We focus on the research advancements and applications of carriers in nucleic acid drug delivery, and discuss the potential future trends and prospects for nucleic acid drugs and their carriers.
Concept of nucleic acid therapeutics
Over the past two decades, nucleic acid-based therapeutic approaches have shown tremendous potential in the treatment and prevention of various diseases, including cancer, diabetes, and neurodegenerative disorders [7, 8]. Nucleic acid drugs can be broadly categorized into two main types: small nucleic acid drugs and mRNA drugs. Small nucleic acid drugs, known as oligonucleotide drugs, encompass a range of molecules such as siRNA, microRNA, and ASOs. These small nucleic acid drugs are designed to target specific genes and modulate their expression or activity. mRNA products can be further divided into mRNA therapeutics and mRNA vaccines.
In living organisms, the transfer of genetic information follows the central dogma. In eukaryotic cells, during the process of protein synthesis and function, the DNA is first transcribed into mRNA precursors. The mRNA precursors undergo splicing to form mature mRNA, which is then translated to synthesize proteins. Throughout this process, there are various levels of gene modifications and regulations, including transcriptional, posttranscriptional, and translational levels, which tightly and precisely control the production of functional proteins to maintain them at a constant level and preserve cellular homeostasis. Due to genetic mutations or defects, patients may experience critical deficiencies in essential functional proteins, imbalances in protein levels, or the production of pathogenic proteins. These conditions can disrupt the internal homeostasis of tissues, impair metabolism, and in severe cases, even pose a threat to life. Addressing these conditions, the restoration or adjustment of the desired protein levels becomes a viable strategy.
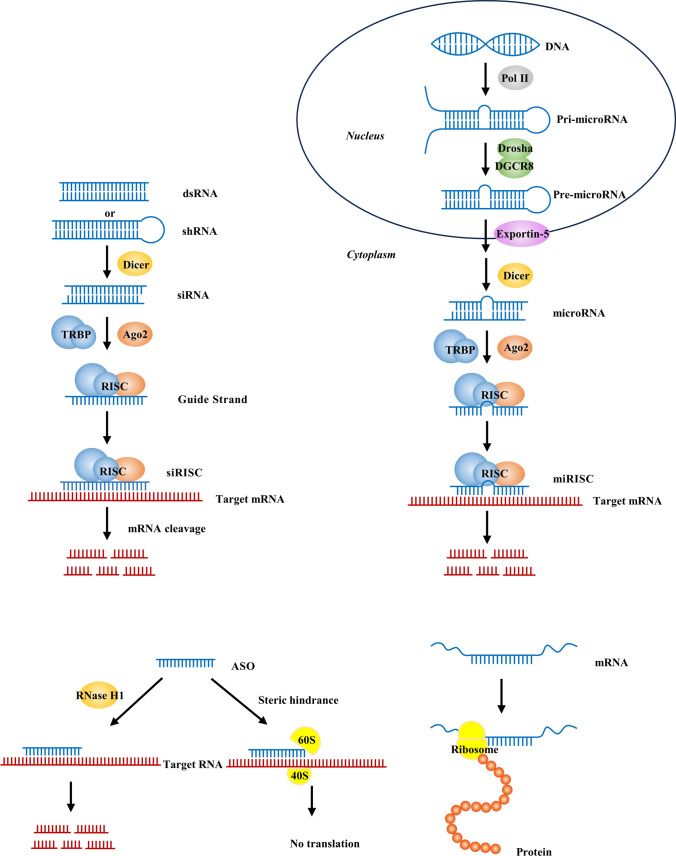
Nucleic acid therapeutics involve the exogenous introduction of nucleic acid fragments into cells for the treatment of diseases. Certain nucleic acid drugs, such as short-chain nucleotide sequences (siRNA or ASOs), efficiently regulate gene expression by targeting specific genes based on the principle of complementary base pairing. Additionally, if a gene product is known to have therapeutic effects for a specific disease, the mRNA of the corresponding target gene can be directly introduced into cells. Through the processes of transcription and translation, the mRNA is utilized to synthesize the gene product, thereby enabling precise treatment of the disease. Ultimately, nucleic acid therapeutics aim to modulate the level of DNA transcription product mRNA or regulate the process of mRNA translation into proteins. This leads to a decrease in pathogenic protein levels or supplementation of essential functional proteins (Fig. 1). As a result, cellular and tissue metabolism and physiological functions are restored, ultimately achieving the therapeutic goal of treating the disease [9, 10].
Fig. 1.
Mechanisms of siRNA, microRNA, ASO, and mRNA in nucleic acid therapeutics
RNAi (siRNA and microRNA)
RNA interference (RNAi) is a fundamental cellular regulatory mechanism that can specifically block the expression of target genes at the transcriptional and translational levels through various pathways [11].
Tumor cells employ various strategies to promote immune evasion and drive immune-suppressive microenvironments. These strategies include reducing antigen expression, inhibiting antigen presentation capacity of dendritic cells (DCs), suppressing infiltration of cytotoxic T cells, and upregulating the expression of immune checkpoint proteins or “don’t eat me” signals [12–14]. Based on these mechanisms, RNAi can effectively induce antitumor immune responses by silencing key factors of tumor progression in cancer cells or immunosuppressive genes in tumor-associated immune cells [15].
The core of the RNAi mechanism is the RNA-induced silencing complex (RISC), which is a multi-protein complex consisting of Argonaute-2 (AGO2), Dicer, and transactivation response element RNA-binding protein (TRBP). RISC unwinds the double-stranded RNA (dsRNA) into two single-stranded RNA (ssRNA) molecules. The passenger strand is degraded, while the guide strand serves as a template to recognize and bind to complementary mRNA. This interaction activates the endonuclease activity of AGO2, resulting in sequence-specific cleavage. Ultimately, this leads to mRNA degradation, thereby regulating gene expression [16].
siRNA refers to a group of exogenous short dsRNA molecules that are processed by the enzyme Dicer from longer fragments of dsRNA. Usually, siRNA molecules consist of approximately 19 to 25 nucleotides. siRNA molecules are highly specific in binding to target mRNA sequences and commonly silence a single gene [17]. The RISC complex containing siRNA can recycle and reuse itself, resulting in a cascade amplification effect. Through multiple iterations of this process, the overall level of the corresponding target mRNA is reduced, thereby inhibiting protein production.
microRNAs are another common class of endogenous noncoding RNAs, typically around 18 to 22 nucleotides in length [18]. A single microRNA can recognize multiple mRNA targets, and a single mRNA target can be recognized and regulated by multiple microRNAs. Therefore, microRNAs can simultaneously regulate multiple cellular signaling pathways, exerting a pivotal role in various cellular processes including proliferation, differentiation, metabolism, migration, and apoptosis [19, 20]. An increasing number of studies have demonstrated that the occurrence and progression of many diseases are associated with the abnormal expression of specific microRNAs. In many cancers, microRNAs play key roles by negatively regulating tumor suppressor genes or oncogenes [21–25]. Based on this, microRNA mimics and microRNA antagonists have become attractive tools and targets for novel therapeutic approaches.
microRNAs are initially transcribed by RNA polymerase II from noncoding DNA sequences in the nucleus, forming primary microRNAs (pri-microRNAs) with characteristic hairpin structures. Subsequently, they are recognized and processed into precursor microRNAs (pre-microRNAs) by the Drosha-DGCR8 complex. The generated pre-microRNAs are exported out of the nucleus through Exportin-5/Ran-GTP and further cleaved by the Dicer RNase, resulting in the formation of mature microRNAs, dsRNA fragments of about 20 base pairs in length, which can participate in the formation of the RISC and engage in the RNAi pathway [26]. Mature microRNAs are eventually integrated into the RISC, and they induce the cleavage or translation inhibition of the target mRNA by binding to complementary sequences in the 3’ untranslated region (3’ UTR) of the target mRNA, thereby blocking the expression of the target gene [27].
ASOs
ASOs are artificially synthesized short DNA or RNA molecules, typically consisting of 12 to 24 nucleotides. They engage in Watson–Crick base pairing with selected RNA sequences, forming hybrid complexes. By adopting different hybridization patterns with the target RNA, they trigger distinct mechanisms to regulate gene expression, ultimately leading to diverse functional outcomes.
Classic single-stranded ASOs primarily mediate endogenous RNA degradation through the dependence on RNase H. ASOs are predominantly synthetic DNA single strands that form DNA-RNA hybrids with the target RNA. Subsequently, the RNase H1 enzyme cleaves the RNA portion within the hybrid complex, resulting in RNA degradation [28].
In addition, ASOs can bind to various sites, including mRNA precursors, mature mRNA, microRNAs that regulate target mRNA, and regions on RNA that inhibit protein translation. This binding occurs through a steric hindrance mechanism known as spatial blocking, leading to diverse outcomes such as modification of pre-mRNA processing and splicing, alteration of mRNA sequences, and downregulation or upregulation of functional proteins [29, 30]. Chemical modifications of ASO nucleotides, bases, and backbone can improve pharmacokinetics and pharmacodynamics while reducing immunogenicity [31, 32].
mRNA
mRNA is a natural biological molecule that serves as a messenger in the translation of genetic information from genes into functional proteins. As a nucleic acid-based drug, mRNA exhibits diverse mechanisms of action. Firstly, mRNA can be translated into functional proteins, making it a promising alternative therapeutic approach in cancer treatment. mRNA delivery aims to upregulate the expression of target proteins, which can serve to supplement the absence or dysfunction of specific proteins or provide therapeutic proteins. This application is particularly common in genetic diseases or rare diseases.
Additionally, mRNA has emerged as a promising alternative to traditional vaccines due to its superior safety profile, high immunogenicity, relatively simple production process, and the potential for personalized medicine. When introduced into the body, mRNA can encode specific antigens, including tumor-associated antigens (TAAs) and tumor-specific antigens (TSAs) generated by cancer cell gene mutations. These antigens, once recognized by the immune system, elicit protective immune responses, efficiently inducing both humoral and cellular immune reactions and reducing the likelihood of tumor escape [33]. mRNA has the capacity to deliver multiple antigens and immunomodulatory agents simultaneously. This versatility allows for the development of multi-epitope vaccines that can target multiple antigens, enhancing the immune response and broadening the range of targeted diseases.
mRNA can also be utilized for cell therapy, where the conventional approach involves introducing exogenous mRNA into cells in vitro to modify their cellular characteristics or functions. The cells with the desired modifications are then reintroduced into the patient’s body to exert their therapeutic effects. In recent years, research has highlighted the development of mRNA-based transient chimeric antigen receptor T-cell immunotherapy (CAR-T). CAR-T is an immunotherapy that utilizes genetically modified T cells to express chimeric antigen receptors (CARs), enhancing their ability to recognize and attack cancer cells. Traditional CAR-T therapy involves extracting T cells from the patient’s body for genetic reprogramming, cultivating CAR-T cells, and subsequently reinfusing them into the patient’s body to exert their therapeutic effects. In contrast, mRNA-based transient CAR-T cell technology enables the direct delivery of modified mRNA encoding CARs into specific T cells, inducing cellular therapy within the body. Due to the transient nature of mRNA, this technology offers higher controllability and safety compared to traditional CAR-T therapy [34].
Furthermore, CRISPR-Cas9 mRNA provides a novel strategy for cancer gene therapy. In the traditional CRISPR-Cas system, guide RNA (gRNA) is employed to direct Cas proteins to specific genomic locations for editing. In CRISPR-Cas mRNA, gRNA and repair templates are integrated into the mRNA sequence, forming a gRNA-mRNA complex. This complex can be delivered into cancer cells to facilitate precise editing and repair of specific genes [35, 36].
Cellular uptake and intracellular transport of nucleic acid drugs
Small nucleic acid drugs face two main challenges when entering cells. Firstly, they have low stability within the body. Naked nucleic acids are susceptible to degradation by nucleases, can bind to serum proteins, can be engulfed by phagocytic cells, and can be cleared by the reticuloendothelial system [37, 38]. Secondly, the inherent negative charges, high molecular weight, and hydrophilicity of nucleic acids make it difficult for them to cross the plasma membrane by passive diffusion. Additionally, physiological barriers such as the vascular barrier, endothelial barrier, extracellular barrier, and cellular barrier impede the clinical application of nucleic acid drugs in cancer treatment [39]. Researchers have implemented various strategies and methods to overcome these challenges in nucleic acid delivery. One approach involves chemical modifications, such as phosphorothioation, 2’-O-methylation, and glycosylation, which enhance the stability of nucleic acids and reduce their degradation by nucleases. Another strategy utilizes cell-penetrating peptides (CPPs) that can either bind to nucleic acids or coat them onto nucleic acid carriers. This helps overcome the repulsion between negatively charged nucleic acids and cell membranes, ultimately improving the intracellular permeability of nucleic acid drugs and facilitating their uptake by cells. Additionally, targeted drug delivery systems have been developed to achieve precise delivery of nucleic acid drugs by specifically recognizing receptors or molecular markers on the surface of target cells [40].
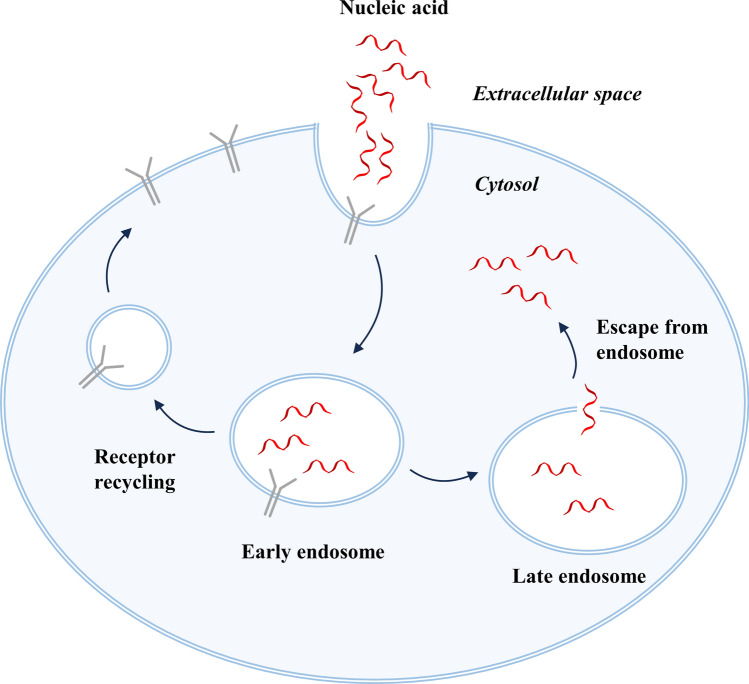
Upon reaching the cell surface, nucleic acid drugs are primarily internalized through endocytic processes, including non-specific internalization and receptor-mediated specific internalization. They are then transported through various membrane-bound compartments. Internalized nucleic acid drugs are initially transported to early/recycling endosomes, which serve as sorting centers for distributing the cargo to various destinations within the cell. They can be either diverted to late endosomes and lysosomes for degradation or recycled back to the plasma membrane. Many ligand–receptor complexes undergo dissociation in the early endosome, with the cargo remaining in the central lumen to be ultimately transported for lysosomal degradation, while the freed receptors migrate to tubular regions and are eventually recycled back to the cell surface through small shuttle vesicles (Fig. 2) [41–43].
Fig. 2.
Cellular uptake and intracellular transport of nucleic acid drugs
Therefore, nucleic acid drugs must cross the membrane barriers and escape from the endosomes to have the opportunity to reach the cytoplasm or further penetrate into the cell nucleus to exert their effects [44]. Enhancing the accumulation and expression of nucleic acid drugs in target tissues is one of the key factors for the success of nucleic acid therapeutics following systemic administration. Consequently, the concept of endosomal escape has gained prominence and is considered a restrictive step in achieving effective gene therapy. Nucleic acids within the endosomal compartments are considered pharmacologically inert, with only a small fraction of internalized nucleic acids being able to spontaneously escape into the cytoplasm and cell nucleus [45]. Intracellular transport of nucleic acid drugs involves a large amount of membrane fusion and fission, and locally generated membrane stress can lead to the formation of highly permeable non-bilayer lipid domains that allow the release of nucleic acids into the cytoplasm [46]. In addition to the endogenous escape of nucleic acids from endosomes, recent attempts in RNA delivery have actively promoted endosomal escape and efficient nucleic acid delivery by exploiting the acidic environment of endosomes and lysosomes or using external stimuli to alter or disrupt the endosomal barriers.
Nucleic acid delivery vectors
The ideal nucleic acid delivery system should be safe and well-tolerated, capable of overcoming extracellular and intracellular barriers, resistant to nucleases in the bloodstream, possess specific tumor-targeting ability with low off-target effects, have broad gene insertion capacity and high transfection efficiency, allow sustained gene expression, and promote endosomal escape of nucleic acid drugs upon entry into cells [47, 48].
The development of delivery vectors and related delivery technologies is a crucial foundation for the clinical application of nucleic acid therapeutics. Nucleic acid vectors can generally be classified into two main categories: viral vectors and non-viral vectors (Table 1).
Table 1.
Nucleic acid delivery vectors
| Delivery system | Classification | Advantages | Disadvantages |
|---|---|---|---|
| Viral vectors |
Adenovirus Adeno-associated virus, lentivirus Retrovirus Herpes simplex viruses |
High transduction efficiency High tolerance Standardized transduction procedures Long-term and stable gene expression |
Limited loading space High immunogenicity Risk of insertional mutagenesis, Potential carcinogenicity |
| Lipid-based nanoparticles |
Liposomes Lipid nanoparticles Solid lipid nanoparticles Nanostructured lipid carriers |
Good biocompatibility Easy preparation Large drug encapsulation space |
Toxicity and immunogenicity Limited stability |
| Polymer nanoparticles |
Chitosan nanoparticles PLGA nanoparticles, Dendrimers |
Good biocompatibility and biodegradability Ability to target specific cells Easy functionalization Controlled drug release |
Toxicity Non-degradability for some chitosan nanoparticles |
| Inorganic nanoparticles |
Metal nanoparticles Iron-based nanoparticles Bimetallic nanoparticles Carbon-based nanostructures Selenium nanoparticles Silica nanoparticles Quantum dots |
Easy surface modification High loading capacity and fast release rates Noninvasive imaging |
Potential toxicity Non-degradability |
| Extracellular vesicles and exosomes |
Exosomes Microvesicles Apoptotic bodies |
Good biocompatibility and biodegradability High permeability Low immunogenicity Long half-life High delivery efficiency |
Uncontrollable genomic or protein components Potential toxicity High cost |
| Nucleic acid conjugates |
GalNAc molecular conjugation Aptamer |
Low immunogenicity Ability to target specific cells Long-term and gene expression |
Low stability Potential toxicity Limited targets |
Viral vectors
Virus-based gene delivery systems are the earliest researched and utilized carriers, and they are currently the most commonly used carriers for nucleic acid therapy projects [49]. Viral vectors take advantage of the innate ability of wild-type viruses to efficiently load nucleic acids and transfer their genetic material to target host cells by inserting the viral genome in accordance with the natural mechanisms of viral infection and replication [50]. Numerous studies and experiments have shown that viral vectors exhibit higher transduction rates, delivery efficiency, as well as more stable and durable gene expression than non-viral delivery systems [51]. Common viral vectors include adenovirus, adeno-associated virus (AAV), lentivirus, retrovirus, and herpes simplex virus.
However, virus-based gene delivery systems have limited cargo capacity and require complex manufacturing procedures and expensive production costs. And more importantly, the high immunogenicity, insertional mutagenesis risk, potential toxicity, and carcinogenicity of viral vectors impose safety concerns that restrict their widespread use [52, 53].
Taking AAV as an example, the history of AAV being utilized for gene delivery can be traced back to the 1980s. AAV is an unenveloped, replication-defective single-stranded DNA virus that belongs to the family Parvoviridae [54]. Many studies have employed recombinant adeno-associated virus (rAAV), which are created by modifying the genome of non-pathogenic wild-type AAV to accommodate the insertion of therapeutic genes or other genetic material [55, 56].
rAAV is regarded as one of the most promising viral vectors for cancer therapy due to its high safety, good tolerability, long-term gene expression, diverse serotypes, and tissue specificity, and lower immunogenicity compared to other viral vectors [53]. Despite its low immunogenicity, rAAV can still induce immune responses due to its inherent characteristics. For instance, preexisting neutralizing antibodies (NAbs) in the body can neutralize and precipitate AAV virus, activation of Toll-like receptors (TLRs) can trigger innate immunity, and AAV capsid proteins or nucleic acids can stimulate the production of antibodies against themselves. Several studies have focused on engineering the AAV vector to mitigate immune responses and optimize drug safety and efficacy. This includes modifying the AAV capsid proteins, conjugating molecules on the virus surface, or encapsulating the viral particles [57–60].
Non-viral vectors
In comparison with viral vectors, non-viral vectors have higher safety profiles and specificity, but they often exhibit lower transfection efficiency and weaker transgene expression capabilities. However, with the continuous improvement of transfection efficiency and gene expression capabilities of various non-viral gene delivery systems in recent years, the number of non-viral vectors employed in clinical trials has gradually increased. Additionally, their structural characteristics provide high payload capacity, allowing for the delivery of large-sized genes, which is not achievable with viral vectors [61–63].
Lipid-based nanoparticles, polymer nanoparticles, and inorganic nanoparticles are the three most common types of non-viral nanoparticles. The enhanced permeability and retention (EPR) effect is generally considered the primary mechanism for the passive tumor targeting of nanoparticles. In tumor tissue, there is a significant increase in the density of capillaries, and the endothelial cell layer of blood vessels exhibits defects and fenestrations. These characteristics contribute to an enhanced permeability of blood vessels to larger particles and molecules. Additionally, the lymphatic drainage system in tumor tissues is often compromised, reducing drug clearance and allowing for enhanced nanoparticle retention in the tumor tissue. These inherent characteristics of tumor tissues are major factors contributing to the formation of EPR effect, enabling long-circulating nanoparticles with suitable diameters to extravasate from blood vessels and accumulate within tumor tissues [64, 65]. However, the results of both preclinical and clinical studies show that the delivery efficiency of nanomedicine to tumors is still unsatisfactory [66]. Recent studies by Wang et al. have demonstrated the presence of a dense basement membrane structure on the extravascular side of tumor blood vessels, severely hindering the ability of nanoparticles to penetrate across tumor vasculature. This results in the formation of “blood-pool” accumulation of nanoparticles outside tumor blood vessels and highlights the potential of inducing acute inflammation to enhance the extravasation of nanoparticles into tumor blood vessels [67]. On the other hand, active targeting involves the modification of nanoparticles with specific ligands that can actively recognize and bind to overexpressed receptors on the surface of tumor cells. Nanoparticles are often modified with sugars, peptides, antibodies, or aptamers to enable active targeting toward tumor cells [68].
Additionally, common non-viral carriers also include extracellular vesicles, exosomes, and nucleic acid conjugates, which will be further discussed later in the text.
Lipid-based nanoparticles
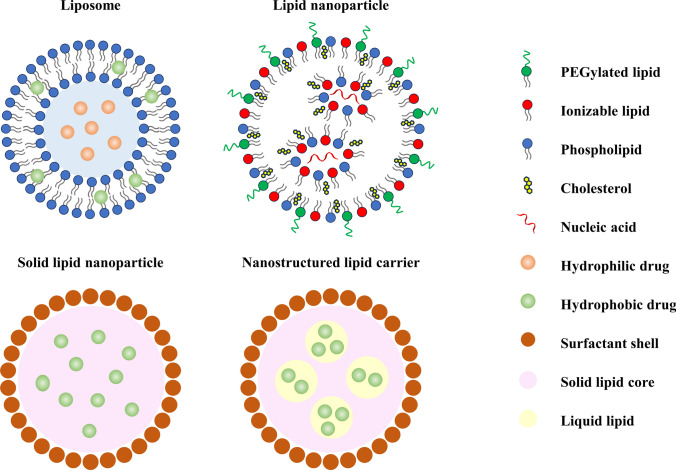
Lipid-based nanoparticles are among the most commonly utilized non-viral nucleic acid vectors in scientific research and clinical trials (Fig. 3) [69]. Lipids are amphiphilic molecules, meaning they possess both hydrophilic (water-loving) and hydrophobic (water-hating) properties. Structurally, lipids typically consist of a polar head group, a hydrophobic tail, and a linker connecting the two regions. The most common cationic headgroups are various amine groups, while the hydrophobic tails are typically composed of fatty chains or cholesterol. These two components are connected by chemical bonds such as ester bonds and ether bonds [70].
Fig. 3.
Schematic diagram of the structures and composition of various lipid-based nanoparticles
The concept of liposomes was introduced in 1965, describing structures with a typical spherical shape composed of a bilayer of amphiphilic phospholipids and an aqueous core. In contrast, the concept of “lipid nanoparticles (LNPs)” emerged in the early 1990s with the advent of nanotechnology. Therefore, liposomes are considered as the earliest form of LNPs.
Liposomes are composed of one or multiple lipid bilayers, and their core–shell nanostructure enables them to effectively encapsulate both hydrophobic and hydrophilic molecules. Hydrophilic drugs can be entrapped within the aqueous core, while hydrophobic drugs are enclosed within the lipophilic bilayer shell. Due to the fundamental role of lipids and phospholipids in cell membranes, liposomes share a similar structure to biological cells, resulting in excellent biocompatibility and facilitating cellular uptake of RNA [71, 72].
Subsequently, various types of lipids, such as cationic lipids, ionizable lipids, solid lipid nanoparticles (SLNs), and nanostructured lipid carriers (NLCs), have been extensively employed in the development of LNPs.
The head groups of cationic lipids possess permanent positive charges, which facilitate their interaction with the negatively charged phosphate groups on the sugar–phosphate backbone of nucleic acids. Through electrostatic adsorption and self-assembly, cationic lipids can further form lipoplexes (LPs) with nucleic acids, protecting nucleic acids from enzymatic degradation and enhancing their stability [73]. Additionally, the excess positive charges on the surface of LPs make it more readily approach and bind to negatively charged cell membranes, promoting cellular uptake. However, previous studies have demonstrated that the positive charges on the surface of nanoparticles have significant cytotoxicity [74–76].
Ionizable lipids can maintain neutrality at physiological pH, while in acidic environments, the amino groups in the head group of ionizable lipids become protonated and carry positive charges. The pH responsiveness of ionizable lipids reduces their interactions with anionic cell membranes and anionic serum proteins (such as albumin), thereby enhancing the biocompatibility of LNPs and reducing non-specific cytotoxicity and immunogenicity [77]. Upon administration into the body through intravenous injection or other routes, ionizable LNPs can seep out of blood vessels t and reach target tissues. LNPs adhere to the cell surface and are subsequently internalized through endocytosis. Within the endosomal environment, which is acidic, ionizable lipids that are trapped undergo protonation and carry positive charges, interacting with the negatively charged endosomal membrane, leading to membrane disruption and the release of nucleic acid drugs into the cytoplasm [78].
Apart from cationic and ionizable lipids, LNPs often incorporate other lipid components such as neutral helper lipids (e.g., distearoyl phosphorcholine, DSPC, and dioleoyl phosphatidyl ethanolamine, DOPE), cholesterol, and PEGylated lipids [79, 80]. These components can improve the stability, delivery efficiency, tolerability, and in vivo distribution of LNPs while assisting in the intracellular escape of nucleic acid molecules.
Helper lipids in LNPs play a structural role, which helps improve the stability of LNPs and enhance their delivery efficiency. Helper lipids with cylindrical geometry, such as DSPC, facilitate the formation of lamellar phases and stabilize the structure of lipid nanoparticles, thereby extending the half-life of the LNPs in circulation. Helper lipids with conical geometry, like DOPE, promote the transition from lamellar to hexagonal phases, disrupting the stability of endosomal membranes and allowing efficient escape of LNPs from endosomal vesicles [81]. Cholesterol exhibits strong membrane fusion properties, and its association with lipids improves stability, enhances transfection efficiency, and enhances the overall effect. Lipid formulations with higher cholesterol content have lower transition temperatures, favoring the lamellar-to-hexagonal phase transition, which promotes nucleic acid internalization and entry into the cytoplasm [82]. PEGylated lipids are used for immune evasion and increased stability. PEG lipids, as biocompatible and inert polymers, preferentially accumulate on the surface of LNPs, forming a steric hindrance barrier. This mechanism enhances the colloidal stability of LNPs, reduces non-specific binding with plasma proteins, thereby preventing uptake by macrophages and prolonging the circulation time of LNPs in the bloodstream. PEG-lipids can also be utilized to attach specific ligands onto the surface of nanoparticles for targeted delivery [83, 84].
SLNs emerged in 1991 with the aim of providing biocompatibility, storage stability, and prevention of drug degradation. The core of SLNs consists of a solid lipid matrix, while the outer layer is enveloped by a bilayer membrane composed of surfactant molecules. SLNs have two notable drawbacks. Firstly, the compact lipid matrix network of SLNs results in limited drug encapsulation space, so that SLNs can only accommodate relatively small amounts of drugs. Secondly, the lipid matrix of SLNs undergoes polymorphic transformations during storage, causing the lipid crystals to rearrange into a more organized lattice and gradually expel the encapsulated drug. As a result, SLNs exhibit poor long-term drug retention. To overcome the limitations of low drug loading and drug expulsion in SLNs, researchers replaced a portion of the solid lipids in SLNs with liquid lipids, resulting in the development of NLCs. NLCs offer larger drug encapsulation space and longer drug retention time.
LNPs can be employed for the delivery of various nucleic acid therapeutics, primarily siRNA or mRNA. Woitok et al. utilized LNPs formulated with cationic amino lipids for the targeted delivery of siRNA against Jnk2 (siJnk2LNP). The loaded siRNA LNPs can adsorb apolipoprotein E (ApoE) on their surface, which is an endogenous ligand for the low-density lipoprotein receptor (LDLR) expressed on the outer membrane of hepatocytes. As a result, the formulation effectively accumulates in hepatic tissues and silences Jnk2 in liver cells, and ultimately reduces carcinogenesis in late-stage liver cancer models [85]. Zhang et al. employed a liposome-protamine lipoplex (CLPP) as a carrier to delivery mRNA encoding survivin-T34A. In these liposomes, protamine condenses the mRNA into a solid core while the liposomes provide a lipid bilayer shell, forming a core–shell structure. The nanoscale CLPP/mRNA formulation exhibited significant therapeutic effects in various colon cancer models [86].
Polymer nanoparticles
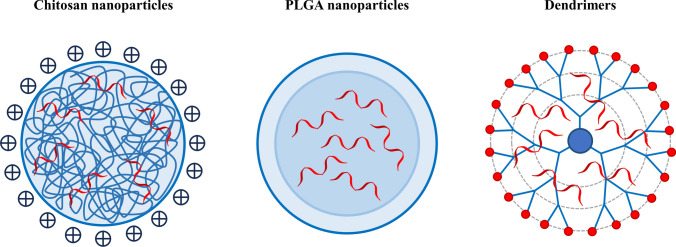
Polymer-based nanomaterials can be categorized into two main groups based on their source: natural polymers and synthetic polymers (Fig. 4). Natural polymers, such as dextran, chitosan, cyclodextrins, poly-l-lysine, and gelatin, are extracted or modified from natural sources. They exhibit excellent biocompatibility, biodegradability, and low production costs, making them widely utilized in the field of biomedical research. Among synthetic polymers, polylactic acid (PLA), poly(lactic-co-glycolic acid) (PLGA), polyethylenimine (PEI), and polyethylene glycol (PEG) are extensively employed as delivery vehicles for RNA due to their good biocompatibility, biodegradability and high stability. Furthermore, it is noteworthy that synthetic polymers can be easily functionalized with ligands for targeted delivery. Additionally, these polymers have responsive units capable of responding to chemical, biological, and physical stimuli, enabling the controlled release of cargo [87–89].
Fig. 4.
Illustration of commonly used polymer nanoparticles
Cationic polymers possess a high density of positive charges, allowing them to interact electrostatically with negatively charged nucleic acid molecules, forming compact and tightly packed polyelectrolyte complexes (PICs). These complexes effectively prevent the entry of nucleases [90]. Additionally, due to the nitrogen-containing structure of the polymers, cationic polymers can offer strong pH buffering capacity, which facilitates the escape of complexes from the endosome [91, 92]. However, certain synthetic polymers, such as PLGA, lack cationic units and exhibit low electrostatic interaction with RNA, making them unsuitable for direct nucleic acid delivery. To overcome this challenge, cationic modification can be performed on these polymers, or they can be co-assembled with cationic polymers to form nanostructures.
It should be noted that some commonly used cationic polymers such as PEI, PDMAEMA, and PLL are non-degradable and exhibit significant cytotoxicity. The cytotoxicity of cationic polymers is generally considered to be associated with their molecular size and positive charge density, that is, the transfection efficiency and cytotoxicity of cationic polymers increase with higher molecular weight. Therefore, achieving a balance between toxicity and transfection efficiency is a major challenge in the design of cationic polymers. To address this issue, conjugation of low molecular weight cationic polymers with chemical bonds such as ester bonds, amide bonds, or disulfide bonds can be utilized to develop highly efficient and low-toxicity biodegradable cationic polymers [93]. For instance, Dai et al. synthesized disulfide cross-linked polyethylenimine (PEI-SS) through oxidation and thiolation of low molecular weight PEI. PEI-SS serves as an efficient and low-toxicity non-viral vector for DNA/siRNA delivery. In cancer cells, the multimer is degraded by glutathione, releasing anti-miR-155 to effectively inhibit the progress of breast cancer [94].
Another strategy is to use biodegradable polymers, particularly proteins or polysaccharides derived from natural sources, such as albumin, chitosan, hyaluronic acid, and β-cyclodextrin. These biodegradable polymers have advantages in terms of biocompatibility and also possess natural affinity to specific tissues or cells. For example, tumor cells often overexpress hyaluronic acid receptors, while liver cells exhibit high expression of sialic acid glycoprotein receptors, allowing for targeted delivery [95, 96]. Xie et al. developed a novel therapeutic approach for the treatment of uveal melanoma (UM), a type of ocular melanoma, by developing a tricomplex consisting of hyaluronic acid-coated chitosan/siRNA. This tricomplex targeted the hypoxia-inducible factor 1α (HIF-1α) pathway. The HA-coated Chi/siRNA tricomplex demonstrated improved cellular uptake and endosomal escape capabilities due to the combined effects of hyaluronic acid and chitosan, while exhibiting low cytotoxicity. Furthermore, the tricomplex successfully inhibited the expression of HIF-1α mRNA and protein levels, leading to downregulation of ascular endothelial growth factor (VEGF) protein expression and effectively suppressing the progression and invasion of UM [97].
Dendrimers are a highly promising class of polymer nanoparticles. They are three-dimensional spherical molecules with a highly branched structure and nanoscale dimensions, formed by repetitive branching units connected to a core molecule. Their three-dimensional structure and chemical composition give them unique physical and chemical properties [98, 99]. Firstly, dendrimers typically consist of a core, inner branching units, and outer functional groups. Precise design of the dendrimer’s structure, shape, size, and functional groups can be achieved by controlling the polymerization of monomers. Secondly, dendrimers have a spacious internal cavity structure that can be utilized for drug encapsulation. This encapsulation method reduces potential drug toxicity, enhances stability, and facilitates targeted delivery and controlled release. Moreover, their shape and chemical composition allow for numerous functional groups on the surface, providing a variety of possibilities for modifications. By selecting highly reactive branching units or introducing specific functional groups at the core and terminal ends of the polymer, the dendrimer’s surface can be densely functionalized, resulting in excellent water solubility, high biocompatibility, and non-immunogenicity. Additionally, this offers opportunities for attachment of drugs, antibodies, genetic materials, and signaling molecules to the dendrimer’s surface [100].
Polyamidoamine (PAMAM) dendrimers are three-dimensional molecules composed of repeating branched chains of amide and amine groups. They are one of the earliest fully characterized, synthesized, and commercialized families of dendrimers. Similar to liposomal complexes, PAMAM dendrimers, carrying cationic groups, have a defined number of positively charged amine groups that allow for the connection to nucleic acids through ion interactions. Therefore, PAMAM dendrimers are capable of promoting internalization through adsorption-mediated endocytosis, effectively condensing nucleic acids using surface amino groups to protect them from enzymatic degradation, and utilizing a large number of tertiary amine groups in the core to provide pH buffering capacity. The ability of PAMAM dendrimers and similar carriers to deliver nucleic acids into tumor cells and produce specific therapeutic effects has been validated in multiple studies [101, 102]. Palombarini et al. constructed a hybrid nanocomplex called HumFt-PAMAM, using positively charged dendrimers that interacted electrostatically with the inner surface of hybrid ferritin. This complex delivered pre-microRNA into cells expressing the CD71 receptor, releasing it into the cytoplasm, where it was processed into mature microRNA, thereby inducing a phenotype resembling early differentiation in NB4 leukemia cells [103].
Inorganic nanoparticles
Inorganic nanoparticles exhibit a diverse range of species and possess distinct physical and chemical properties (Table 2) [104–106]. Inorganic nanoparticles can be easily surface-modified, allowing optimization of their degradation, biocompatibility, immunogenicity, nucleic acid loading capacity, pharmacokinetics, and biodistribution. In addition to their ability to load nucleic acid molecules, inorganic nanoparticles often possess unique functionalities, making them an attractive strategy for gene delivery [107–110]. Therefore, inorganic nanoparticles hold significant promise in the realm of medical applications, particularly in cancer therapy.
Table 2.
Advantages and disadvantages of different types of inorganic nanoparticles for drug delivery
| Delivery system | Examples | Advantages | Disadvantages |
|---|---|---|---|
| Metal NPs |
Gold NPs Silver NPs |
Unique optical properties and photothermal conversion effects Photothermal therapy Versatility of shape design |
High cost Poor stability Easy aggregation Potential toxicity |
| Iron-based NPs |
Iron-based nanocrystals (iron oxide NPs, iron-based alloys) Iron-based nanocomposites(amorphous iron NPs, MOFs) |
Superparamagnetism and magnetocaloric conversion effects Noninvasive imaging Magnetothermal therapy Synergistic therapy in conjunction with ferroptosis |
Poor stability Potential toxicity The need for surface modification |
| Bimetallic NPs |
Au–Ag bimetallic NPs Au–Pt bimetallic NPs Fe–Pt bimetallic NPs Pt–Pd bimetallic NPs |
Integration of multiple characteristics and functions Larger specific surface area and more active sites |
Complex preparation process Metal interactions and interface effects |
| CBNs |
Graphene and its oxide Graphdiyne Fullerene and its derivatives CNTs Carbon quantum dots |
Low immunotoxicity High load capacity and adsorption capacity Adjustable optical properties |
Potential biotoxicity Poor water solubility Low biodegradability |
| Selenium NPs |
Excellent antioxidant capacity Antitumor effect |
Complex preparation process Poor stability Potential toxicity |
|
| Silica NPs |
Adjustable pore size Easy modification and functionalization Good biocompatibility |
Complex preparation process Potential toxicity |
|
| QDs | Good Optical and electronic properties |
Complex preparation process Some quantum dots contain toxic heavy metals |
Metal nanoparticles, particularly gold nanoparticles and silver nanoparticles, exhibit unique optical properties and photothermal conversion effects. The photothermal effect generated by their surface plasmon resonance (SPR) can be tuned to the near-infrared (NIR) region, which matches the transparent window of biological tissues. This enables metal nanoparticles to efficiently absorb NIR light and convert it into heat, leading to localized hyperthermia and the destruction of cancer cells. This characteristic offers the potential for synergistic and combined approaches of gene therapy and photothermal therapy [111–113]. To alleviate the potential cytotoxicity of metal nanoparticles, a core–shell nanostructure can be formed by coating the metal core with a shell made of different materials. This approach aims to prevent the aggregation of metal nanoparticles, enhance their stability, and enable further customization and functionalization. Noble metals like gold or silver, as well as metal oxides, are commonly employed as core materials. The selection of shell composition depends on the specific application, spanning from organic polymers to inorganic compounds [114, 115].
In addition to optical properties and photothermal conversion effects, iron-based nanomaterials also exhibit superparamagnetism and magnetocaloric conversion effects. When subjected to an external magnetic field, the interaction between electromagnetic radiation and the inorganic atoms in the core of the nanoparticles enables efficient targeting and entry of iron-based nanoparticles carrying genes into specific cells or tissues. They can convert the applied alternating magnetic field into heat, leading to localized heating of the surrounding tissue or cells. This localized heating can be utilized for the destruction of cancer cells, inhibition of tumor growth, and facilitation of drug release, known as magnetothermal therapy [116]. By employing these techniques, metal nanoparticles can be tracked and precisely located within the body, allowing for accurate targeting, simultaneous diagnosis, and monitoring of therapeutic functionalities in diseased tissues [117, 118]. Most of these delivery systems rely on surface-engineered cationic iron oxide nanoparticles, which are coupled with nucleic acid drugs through electrostatic interactions. Additionally, the small size and uniformity of iron oxide nanoparticles allow for better distribution throughout the entire tumor network, making them particularly suitable for the treatment of metastatic cancers [119]. Metal–organic frameworks (MOFs) are a type of organic–inorganic hybrid material. They are composed of metal ions or metal clusters at their cores, linked to organic ligands through coordination bonds. MOFs containing iron elements can induce ferroptosis by releasing ferrous/iron ions at the tumor site, triggering oxidative stress and lipid peroxidation, ultimately resulting in tumor cell death. Therefore, iron-containing MOFs possess unique advantages in synergistic therapy in conjunction with ferroptosis [120, 121].
Bimetallic nanoparticles are composed of two different metal elements, capable of forming various structures such as core–shell, alloy, or segregated structures. Bimetallic nanoparticles often demonstrate superior properties compared to monometallic nanoparticles. Firstly, they combine the characteristics of two different metals, facilitating the integration of multiple functionalities. Secondly, bimetallic nanoparticles possess a larger specific surface area. Moreover, their irregular arrangement and the formation of metal–polarity bonds create additional active sites, thereby further enhancing their catalytic and adsorption capabilities for efficient nucleic acid delivery [122]. Furthermore, the surface charge, optical properties, and biocompatibility of these nanoparticles can be finely tuned by adjusting the metal composition, structure, and surface modifications, providing greater versatility for their applications, providing greater versatility for their applications [123].
Carbon-based nanostructures (CBNs) originate from various isomers of carbon. The nanoscale size and porous structure of carbon-based nanoparticles endow them with a high specific surface area, providing abundant surface reaction sites. The presence of supramolecular π-π stacking characteristics effectively enhances the interaction between carbon nanoparticles and various biomolecules or substances [124, 125]. Furthermore, carbon-based nanoparticles exhibit excellent optical properties, including fluorescence, photothermal conversion, and light absorption capabilities, making them ideal materials for biological imaging and fluorescent probes. Carbon nanotubes (CNTs) are tubular structures formed by the rolling of graphene layers. Depending on the number of layers, carbon nanotubes can be classified into single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs). The unique structure of carbon nanotubes provides them with small volume, high specific surface area, and high aspect ratio (length/diameter), making them capable of accommodating various chemical substances and offering multiple sites for modification, such as for different drugs, targeting moieties, and imaging agents [126]. Fullerenes are another class of CBNs. They exist in hollow spherical or tubular structures, comprised of hexagonal and pentagonal carbon rings. The vesicle-like structure and their lipid-like nonpolar properties enable fullerenes to penetrate cell membranes. It is worth noting that research indicates the potential of fullerenes as drug carriers for delivering medications to the brain, despite the intricate and challenging process of crossing the blood–brain barrier due to their distorted structure [127]. Nevertheless, they hold promise as a therapeutic agent for facilitating brain tumor treatment. However, CBNs exhibit chemical stability and are difficult to degrade in the body due to the absence of specific metabolic enzymes. Administration of high doses of CBNs can potentially trigger adverse inflammatory responses and systemic toxicity [128]. Therefore, further research is needed to determine the toxicity and pharmacokinetics of various CBNs.
Selenium is an essential micronutrient for the human body. It exists in the form of selenoproteins. Selenium enzymes, such as peroxidases and reductases, rely on selenium as a catalytic site for their activity and function. Therefore, selenium nanoparticles possess excellent antioxidant properties, acting as potent scavengers of free radicals, protecting nucleic acid molecules from oxidative damage, and helping to reduce oxidative stress and cellular/tissue injury caused by reactive oxygen species [129, 130]. Additionally, several studies have indicated that selenium nanoparticles can inhibit tumor growth and proliferation through various mechanisms, including inducing cell apoptosis, inhibiting angiogenesis, and modulating signaling pathways [131].
Silica nanoparticles possess tunable nanopores and a hydrophilic surface that can be functionalized. For silica nanoparticles with smaller pore sizes, the pore dimensions are in close proximity to the size of nucleic acids, allowing for more precise and adjustable control over the release rate of nucleic acids. Conversely, larger-sized silica nanoparticles possess greater spatial capacity and faster release rates. The negatively charged surface of silica nanoparticles can be functionalized by incorporating various types of cationic macromolecules, such as PEI, dendrimers, and lipids. These functional modifications greatly influence drug loading, release rates, and biodistribution while reducing systemic toxicity.
Quantum dots are extremely small semiconductor particles that possess unique optical and electronic properties. In addition to their role in nucleic acid delivery, they can serve as highly efficient fluorescent probes, allowing real-time tracking of nucleic acid labeling and in vivo dynamic distribution [132].
However, the degradability of all inorganic materials has not been fully established, and potential toxicity remains a concern. Therefore, further extensive in vivo studies are necessary to address these issues.
Extracellular vesicles and exosomes
Extracellular vesicles (EVs) are double-layered lipid membrane particles naturally released from various types of cells, with diameters ranging from 20 to 30 nm to 10 µm [133, 134]. EVs can be classified into different types based on their size and biogenesis pathways, including exosomes (30–120 nm), microvesicles (100–1000 nm), and apoptotic bodies (> 1000 nm) [135]. These membrane-bound particles serve as natural mediators of intercellular communication, allowing the transfer of biologically active substances such as mRNA, microRNA, proteins, or antigens to target cells to transmit information and exert functional effects [136].
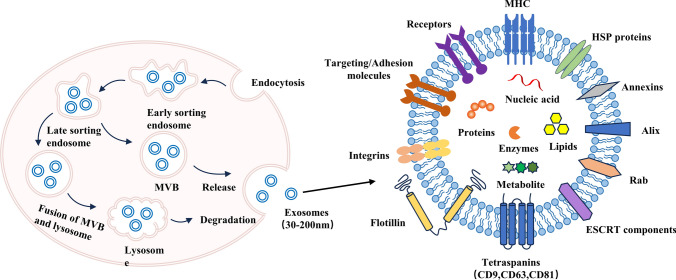
Currently, there is extensive research focusing on exosomes, which are promising carriers for delivering microRNAs and siRNAs [137]. This literature will primarily focus on exosomes (Fig. 5). Exosomes are postulated to be small vesicles formed through the inward budding of the multi-vesicular body (MVB) membrane. As mature MVBs fuse with the cell membrane, exosomes are released into the extracellular matrix [138]. Due to the size limitations of MVBs, exosomes typically have diameters ranging from 30 to 200 nm. Exosomes have a lipid bilayer membrane structure and contain various compounds, including proteins, lipids, nucleic acids, and sometimes organelles from their parental cells. The composition of these cellular components within exosomes is closely related to the physiological state of the producing cells, making them potential tools for disease diagnostics, such as early cancer screening. It is worth noting that the structure, function, and distribution of exosomes can be highly variable. Uncontrolled genomic or proteomic composition on their surface may induce undesired activities. Therefore, they can be a double-edged sword in the development and spread of cancer cell metastasis or in the diagnosis and treatment of tumor cells. Identifying the cellular source of exosomes used is key to reducing unnecessary risks.
Fig. 5.
Visual illustration of the biogenetic process and structure of exosomes
Compared to artificial carriers, exosomes, as natural vesicles, possess morphological and surface characteristics similar to native cells. They possess excellent biocompatibility, biodegradability, and the ability to penetrate biological barriers [139]. Exosomes can interact with macrophages through specific receptors, thereby avoiding phagocytosis and prolonging the half-life of drugs [140]. Consequently, exosomes significantly enhance drug delivery efficiency and therapeutic effectiveness [141]. Taken together, these outstanding features make exosomes an ideal drug delivery system with potential clinical applications.
Nucleic acid conjugates
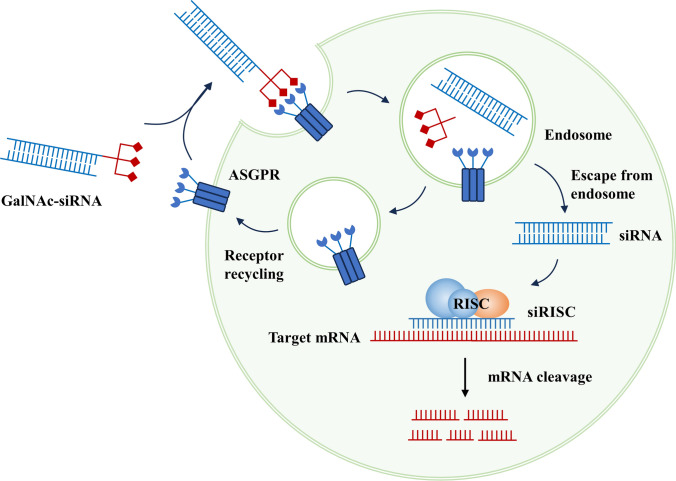
As early as the 1960s and 1970s, research discovered that lactose can bind to the receptor on the surface of the liver and undergo internalization. Scientists have utilized this property to deliver substances such as proteins, peptides, and small molecules into cells. The asialoglycoprotein receptor (ASGPR) is a glycoprotein receptor composed of multiple subunits with carbohydrate recognition domains, primarily expressed on the plasma membrane of liver cells. ASGPR is responsible for the clearance of targeted glycoproteins from the circulation. Both glycoproteins and glycoprotein mimetics can serve as ASGPR ligands, with N-acetylgalactosamine (GalNAc) being the strongest affinity ligand among them (Fig. 6) [142]. GalNAc, a lactose analog, can be covalently linked to the 3’ end of nucleic acids in a trivalent state, and it efficiently mediates endocytosis through binding with ASGPR. Nucleic acid molecules in nucleic acid conjugates often lack necessary protections. However, their stability can be significantly improved by chemical modifications at the 2’ position of nucleotides, as well as by replacing phosphodiester bonds with thiophosphoramidate linkages. Additionally, the design and modification positions of the molecular linkers are also crucial factors to consider [143].
Fig. 6.
Biological mechanism of GalNAc-siRNAs for target RNA degradation
GalNAc-conjugated nucleic acid therapeutics primarily include GalNAc-ASO and GalNAc-siRNA. ASOs can be easily chemically modified to resist degradation by nucleases, and in the case of phosphorothioate modifications, they have a long-circulating half-life and are readily taken up by cells, making delivery carriers unnecessary. Moreover, to maintain their efficiency in entering the cell nucleus, they are generally not conjugated with GalNAc [144]. On the other hand, siRNAs are prone to degradation, so they often require carrier-mediated delivery techniques. Therefore, the common GalNAc-conjugated drugs are GalNAc-siRNAs. After subcutaneous injection, GalNAc-siRNA can efficiently enter the liver through the circulatory system. Subsequently, they are rapidly taken up by hepatocytes via the ASGPR on the cell surface. The ASGPR facilitates their entry into clathrin-coated vesicles, which are then transported to the endosomal compartment. As the pH in these compartments decreases, GalNAc-siRNA dissociates from ASGPR, allowing the receptor to recycle back to the cell membrane for reuse [145, 146]. The link between GalNAc and siRNA is also rapidly degraded and dissociated in the lumen. The siRNA accumulates in acidic intracellular compartments, slowly releases into the cytoplasm, and continuously loads onto the RISC, resulting in long-lasting inhibitory effects that can persist for months in preclinical species and humans [147]. Thus, GalNAc conjugation is an effective approach to increase siRNA accumulation in target organs and facilitate cellular uptake, significantly reducing the dosage required. Strong and sustained gene suppression can be achieved with small subcutaneous doses of the drug [148]. Compared to carrier systems such as lipid and polymeric complexes, nucleic acid conjugates have the advantages of smaller size, lower immunogenicity, and reduced clearance by the body. Brown et al. demonstrated that the same siRNA delivered in the form of lipid nanoparticles or GalNAc conjugates showed prolonged activity only with GalNAc-siRNA [149]. Despite the aforementioned advantages, GalNAc targeting is currently limited to liver cells, restricting the action of nucleic acid drugs to the liver, which represents a limitation in their application.
Concluding remarks and future perspectives
In summary, nucleic acid therapeutics are supported by a solid theoretical basis. It involves the exogenous introduction of nucleic acid fragments to directly intervene in the gene expression process, leading to the silencing or upregulation of target protein expression, thereby achieving the goal of alleviating or even treating diseases. Moreover, the design of drugs based on disease target genes has greatly expanded the applications of nucleic acid therapeutics. The mechanism of action and high specificity of nucleic acids demonstrate significant potential in cancer treatment.
However, nucleic acid drugs have inherent limitations such as low stability in the body, susceptibility to degradation by endogenous nucleases, and challenges in crossing various drug delivery barriers. In recent years, extensive research and rapid development of nucleic acid delivery systems have offered potential solutions to overcome these obstacles. These delivery systems can improve the stability of nucleic acid drugs, facilitate targeted cellular uptake of nucleic acid molecules, and assist their intracellular escape, thus expanding the applications of nucleic acid therapeutics.
It is unrealistic to expect a single “universal” carrier to address all nucleic acid delivery challenges due to the diverse types of nucleic acid drugs with different mechanisms of action and molecular sizes. Among the nucleic acid drugs mentioned in this article, ASOs can enhance their own stability through chemical modifications and do not strongly rely on carriers, while siRNA molecules are highly dependent on carriers. Small nucleic acid drugs such as microRNA and siRNA can be delivered using directly conjugated simple formulations, while larger nucleic acid drugs like mRNA require compression and encapsulation by carriers. Small nucleic acid molecules require carriers with high positive charge densities to form stable complexes, whereas large nucleic acid drugs have lower requirements for carrier charge densities. Although non-viral carriers have significantly improved their transfection abilities and performance, they still have a significant gap compared to viral carriers at the current stage. However, non-viral carriers have their irreplaceable advantages, such as safety, low immunogenicity, and the ability to carry large-sized nucleic acid drugs. With the development of novel nucleic acid drugs and advancements in delivery technologies, we can expect the future development of non-viral carriers.
Currently, several nucleic acid drugs have been approved and marketed worldwide, including ASOs, siRNAs, mRNA vaccines, and aptamers (Table 3). These drugs primarily address genetic disorders, metabolic diseases, and rare diseases. Mature delivery systems such as lipid-based nanoparticles and nucleic acid conjugates like GalNAc have been successfully applied in clinical nucleic acid drug delivery. As of the completion of this manuscript, no tumor-related indications have been approved. However, it is encouraging that some products have entered clinical trial phases and achieved preliminary results (Table 4). Lipid-based nanoparticles remain the main delivery system in ongoing clinical trials, possibly due to the yet-to-be-determined efficacy of novel nucleic acid drugs, necessitating established carriers to mitigate risks and variables.
Table 3.
Compilation of approved nucleic acid drugs
| Drugs | Indication | Drug class | Delivery system | Manufacturer | Approval year |
|---|---|---|---|---|---|
| Vitravene (Fomivirsen) | Cytomegalovirus retinitis | ASO | / | Novartis | 1998 |
| Pegaptanib (Macugen) | Neovascular age-related macular degeneration | Aptamer | / | Pfizer, EyePoint Pharmaceuticals | 2004 |
| Kynamro (Mipomersen) | Homozygous familial hypercholesterolemia | ASO | / | Ionis Pharmaceuticals | 2013 |
| Defibrotide (Defitelio) | Hepatic veno-occlusive disease | ASO | / | Jazz Pharmaceuticals | 2016 |
| Exondys 51 (Eteplirsen) | Duchenne muscular dystrophy | ASO | / | Sarepta Therapeutics | 2016 |
| Spinraza (Nusinersen) | Spinal muscular atrophy | ASO | / | Biogen, Ionis Pharmaceuticals | 2016 |
| Tegsedi (Inotersen) | Hereditary transthyretin amyloidosis | ASO | / | Akcea Therapeutics, Ionis Pharmaceuticals | 2018 |
| Onpattro (Patisiran) | Hereditary transthyretin amyloidosis | siRNA | LNP | Alnylam Pharmaceuticals | 2018 |
| Waylivra (Volanesorsen) | Familial chylomicronemia syndrome | ASO | / | Akcea Therapeutics, Ionis Pharmaceuticals | 2019 |
| Givlaari (Givosiran) | Acute intermittent porphyria | siRNA | GalNAc | Alnylam Pharmaceuticals | 2019 |
| Vyondys 53 (Golodirsen) | Duchenne muscular dystrophy | ASO | / | Sarepta Therapeutics | 2019 |
| Viltepso (Viltolarsen) | Duchenne muscular dystrophy | ASO | / | NS Pharma | 2020 |
| Oxlumo (Lumasiran) | Primary hyperoxaluria type 1 | siRNA | GalNAc | Alnylam Pharmaceuticals | 2020 |
| Leqvio (Inclisiran) | Primary hypercholesterolemia | siRNA | GalNAc | Alnylam Pharmaceuticals, Novartis | 2020 |
| Spikevax | Coronavirus disease 2019 | mRNA vaccines | LNP | Moderna | 2020 |
| Comiranty | Coronavirus disease 2019 | mRNA vaccines | LNP | Pfizer, BioNTech | 2020 |
| Amondys 45 (Casimersen) | Duchenne muscular dystrophy | ASO | / | Sarepta Therapeutics | 2021 |
| Amvuttra (Vutrisiran) | Hereditary transthyretin amyloidosis | siRNA | GalNAc | Alnylam Pharmaceuticals | 2022 |
| Tofersen (Qalsody) | Amyotrophic lateral sclerosis type 1 | ASO | / | Biogen, Ionis Pharmaceuticals | 2023 |
| Nedosiran (Rivfloza) | Primary hyperoxaluria type 1 | siRNA | GalNAc | Novo Nordisk | 2023 |
| Avacincaptad pegol (Izervay) | Age-related macular degeneration, geographic atrophy | Aptamer | / | Ocular Therapeutix | 2023 |
Table 4.
Some of ongoing clinical studies of nucleic acid therapeutics for cancer treatment
| NCT number | Drugs | Delivery System | Indications | Sponsor | Phase |
|---|---|---|---|---|---|
| NCT01591356 | EphA2 siRNA | Neutral liposome | Advanced malignant solid neoplasm | M.D. Anderson Cancer Center | Phase 1 |
| NCT03819387 | NBF-006 | LNP | Non-small cell lung, pancreatic, or colorectal cancer | Nitto BioPharma, Inc | Phase 1 |
| NCT04196257 | BP1001-A | Liposome | Advanced or recurrent solid tumors | Bio-Path Holdings, Inc | Phase 1 |
| NCT05267899 | WGI-0301 | LNP | Advanced hepatocellular carcinoma | Zhejiang Haichang Biotech Co., Ltd | Phase 2 |
| NCT05969041 | MT-302 (A) | LNP | Advanced/Metastatic epithelial tumors | Myeloid therapeutics | Phase 1 |
| NCT05497453 | OTX-2002 | LNP | Hepatocellular carcinoma and other solid tumor types known for association with the MYC oncogene | Omega Therapeutics | Phase 1 and 2 |
| NCT06389591 | RNA-LP vaccine | Lipid particles | Recurrent glioblastoma | University of Florida | Phase 1 |
| NCT04573140 | RNA-LP vaccine | Lipid particles | Newly diagnosed pediatric high-grade gliomas and adult glioblastoma | University of Florida | Phase 1 |
| NCT05660408 | RNA-LP vaccine | Lipid particles | Pulmonary osteosarcoma | University of Florida | Phase 1 and 2 |
| NCT03608631 | KRAS G12D siRNA | Exosome | Metastatic pancreatic adenocarcinoma | M.D. Anderson Cancer Center | Phase 1 |
Nonetheless, the continuous advancements in delivery technology are truly exciting. Leveraging the physical, chemical, and biological properties of the materials themselves, such as optical characteristics, superparamagnetism, and antioxidative abilities, delivery systems have developed unique functionalities beyond mere nucleic acid carriers. For example, noninvasive imaging techniques enable precise localization of pathological tissues and real-time monitoring of diagnostic and therapeutic functions. Photothermal therapy, magnetic hyperthermia, and ferroptosis offer possibilities for synergistic treatment with nucleic acid therapy. The differences in various stages of in vivo delivery of nucleic acid drugs and the complex tumor microenvironment suggest that a single carrier cannot fully meet delivery requirements. Composite materials provide greater advantages by offering more drug loading capacity and modification sites, integrating multiple characteristics and functions to address the complexity of the in vivo environment. Moving forward, interdisciplinary collaborations will continue to propel the progress of delivery technology as experts from diverse fields such as physics, biology, chemistry, and engineering work together, providing innovative ideas and solutions for the development of nucleic acid drug delivery systems.
In conclusion, although nucleic acid drugs for tumor-related indications have not been approved yet, we have high expectations for the application of nucleic acid therapy in cancer treatment through continuous improvement of delivery systems and the utilization of various delivery strategies. This will provide patients with more personalized and effective treatment options and contribute to significant breakthroughs and advancements in the field of oncology.
Acknowledgements
Not applicable.
Abbreviations
- ASO
Antisense oligonucleotide
- RNAi
RNA interference
- DC
Dendritic cell
- RISC
RNA-induced silencing complex
- AGO2
Argonaute-2
- TRBP
Transactivation response element RNA-binding protein
- dsRNA
Double-stranded RNA
- ssRNA
Single-stranded RNA
- pri-microRNA
Primary microRNA
- pre-microRNA
Precursor microRNA
- 3’ UTR
3’ Untranslated region
- TAA
Tumor-associated antigen
- TSA
Tumor-specific antigen
- CAR-T
Chimeric antigen receptor T-cell immunotherapy
- CAR
Chimeric antigen receptor
- gRNA
Guide RNA
- CPP
Cell-penetrating peptide
- AAV
Adeno-associated virus
- rAAV
Recombinant adeno-associated virus
- Nabs
Neutralizing antibodies
- TLR
Toll-like receptor
- EPR
Enhanced permeability and retention effect
- LNP
Lipid nanoparticle
- SLN
Solid lipid nanoparticle
- NLC
Nanostructured lipid carrier
- LP
Lipoplex
- DSPC
Distearoyl phosphorcholine
- DOPE
Dioleoyl phosphatidyl ethanolamine
- siJnk2LNP
The LNP formulated with cationic amino lipids for the targeted delivery of siRNA against Jnk2
- ApoE
Apolipoprotein E
- LDLR
Low-density lipoprotein receptor
- CLPP
Liposome-protamine lipoplex
- PLA
Polylactic acid
- PLGA
Poly(lactic-co-glycolic acid)
- PEI
Polyethylenimine
- PEG
Polyethylene glycol
- PIC
Polyelectrolyte complex
- PEI-SS
Disulfide cross-linked polyethylenimine
- UM
Uveal melanoma
- HIF-1α
Hypoxia-inducible factor 1α
- VEGF
Vascular endothelial growth factor
- PAMAM
Polyamidoamine
- SPR
Surface plasmon resonance
- NIR
Near-infrared
- MOF
Metal–organic framework
- CBN
Carbon-based nanostructure
- CNT
Carbon nanotube
- SWCNT
Single-walled carbon nanotube
- MWCNT
Multi-walled carbon nanotube
- EV
Extracellular vesicle
- MVB
Multi-vesicular body
- ASGPR
Asialoglycoprotein receptor
- GalNAc
N-acetylgalactosamine
Author contributions
L.Z. and W.L. conceived the study; W.L. carried out investigation and validation; J.W. was involved in methodology, project administration, supervision, and writing-reviewing and editing; and L.Z. was responsible for software, visualization, and writing-original draft preparation. All authors contributed to the study conception and design. All authors read and approved the final manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data and material availability
No datasets were generated or analyzed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Leqi Zhang and Wenting Lou have contributed equally to this work.
References
- 1.Zhou Z, Liu X, Zhu D, Wang Y, Zhang Z, Zhou X, et al. Nonviral cancer gene therapy: delivery cascade and vector nanoproperty integration. Adv Drug Deliv Rev. 2017;115:115–54. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Li X, Huang L. Non-viral nanocarriers for siRNA delivery in breast cancer. J Control Release: Off J Control Release Soc. 2014;190:440–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yahya EB, Alqadhi AM. Recent trends in cancer therapy: a review on the current state of gene delivery. Life Sci. 2021;269:119087. [DOI] [PubMed] [Google Scholar]
- 4.Siddique S, Chow JCL. Application of nanomaterials in biomedical imaging and cancer therapy. Nanomaterials (Basel, Switzerland). 2020;10(9):1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunbar CE, High KA, Joung JK, Kohn DB, Ozawa K, Sadelain M. Gene therapy comes of age. Science (New York, NY). 2018;359(6372):eaan4672. [DOI] [PubMed] [Google Scholar]
- 6.Munagala R, Aqil F, Jeyabalan J, Kandimalla R, Wallen M, Tyagi N, et al. Exosome-mediated delivery of RNA and DNA for gene therapy. Cancer Lett. 2021;505:58–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labatut AE, Mattheolabakis G. Non-viral based miR delivery and recent developments. Euro J Pharm Biopharm: Off J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2018;128:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakraborty C, Sharma AR, Sharma G, Doss CGP, Lee SS. Therapeutic miRNA and siRNA: moving from bench to clinic as next generation medicine. Mol Ther Nucleic Acids. 2017;8:132–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lächelt U, Wagner E. Nucleic acid therapeutics using polyplexes: a journey of 50 years (and beyond). Chem Rev. 2015;115(19):11043–78. [DOI] [PubMed] [Google Scholar]
- 10.Mohammadinejad R, Dehshahri A, Sagar Madamsetty V, Zahmatkeshan M, Tavakol S, Makvandi P, et al. In vivo gene delivery mediated by non-viral vectors for cancer therapy. J Control Release: Off J Control Release Soc. 2020;325:249–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kara G, Calin GA, Ozpolat B. RNAi-based therapeutics and tumor targeted delivery in cancer. Adv Drug Deliv Rev. 2022;182:114113. [DOI] [PubMed] [Google Scholar]
- 12.Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Pérez-Gracia JL, et al. Cytokines in clinical cancer immunotherapy. Br J Cancer. 2019;120(1):6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalathil SG, Thanavala Y. High immunosuppressive burden in cancer patients: a major hurdle for cancer immunotherapy. Cancer Immunol Immunother: CII. 2016;65(7):813–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munn DH, Bronte V. Immune suppressive mechanisms in the tumor microenvironment. Curr Opin Immunol. 2016;39:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anfossi S, Babayan A, Pantel K, Calin GA. Clinical utility of circulating non-coding RNAs–an update. Nat Rev Clin Oncol. 2018;15(9):541–63. [DOI] [PubMed] [Google Scholar]
- 16.Iwasaki S, Sasaki HM, Sakaguchi Y, Suzuki T, Tadakuma H, Tomari Y. Defining fundamental steps in the assembly of the Drosophila RNAi enzyme complex. Nature. 2015;521(7553):533–6. [DOI] [PubMed] [Google Scholar]
- 17.Xin Y, Huang M, Guo WW, Huang Q, Zhang LZ, Jiang G. Nano-based delivery of RNAi in cancer therapy. Mol Cancer. 2017;16(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holoch D, Moazed D. RNA-mediated epigenetic regulation of gene expression. Nat Rev Genet. 2015;16(2):71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Y, Tang Y, Xie S, Zheng X, Zhang S, Mao J, et al. Chimeric peptide supramolecular nanoparticles for plectin-1 targeted miRNA-9 delivery in pancreatic cancer. Theranostics. 2020;10(3):1151–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desantis V, Saltarella I, Lamanuzzi A, Melaccio A, Solimando AG, Mariggiò MA, et al. MicroRNAs-based nano-strategies as new therapeutic approach in multiple myeloma to overcome disease progression and drug resistance. Int J Mol Sci. 2020;21(9):3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rupaimoole R, Calin GA, Lopez-Berestein G, Sood AK. miRNA deregulation in cancer cells and the tumor microenvironment. Cancer Discov. 2016;6(3):235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu H, Lei C, He Q, Pan Z, Xiao D, Tao Y. Nuclear functions of mammalian MicroRNAs in gene regulation, immunity and cancer. Mol Cancer. 2018;17(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhattacharya S, Chalk AM, Ng AJ, Martin TJ, Zannettino AC, Purton LE, et al. Increased miR-155-5p and reduced miR-148a-3p contribute to the suppression of osteosarcoma cell death. Oncogene. 2016;35(40):5282–94. [DOI] [PubMed] [Google Scholar]
- 24.Craig VJ, Cogliatti SB, Imig J, Renner C, Neuenschwander S, Rehrauer H, et al. Myc-mediated repression of microRNA-34a promotes high-grade transformation of B-cell lymphoma by dysregulation of FoxP1. Blood. 2011;117(23):6227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L, Liao Y, Tang L. MicroRNA-34 family: a potential tumor suppressor and therapeutic candidate in cancer. J Exp Clin Cancer Res: CR. 2019;38(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136(4):642–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin YX, Wang Y, Blake S, Yu M, Mei L, Wang H, et al. RNA nanotechnology-mediated cancer immunotherapy. Theranostics. 2020;10(1):281–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bennett CF, Swayze EE. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu Rev Pharmacol Toxicol. 2010;50:259–93. [DOI] [PubMed] [Google Scholar]
- 29.Liang XH, Sun H, Shen W, Wang S, Yao J, Migawa MT, et al. Antisense oligonucleotides targeting translation inhibitory elements in 5’ UTRs can selectively increase protein levels. Nucleic Acids Res. 2017;45(16):9528–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang XH, Shen W, Sun H, Migawa MT, Vickers TA, Crooke ST. Translation efficiency of mRNAs is increased by antisense oligonucleotides targeting upstream open reading frames. Nat Biotechnol. 2016;34(8):875–80. [DOI] [PubMed] [Google Scholar]
- 31.Craig K, Abrams M, Amiji M. Recent preclinical and clinical advances in oligonucleotide conjugates. Expert Opin Drug Deliv. 2018;15(6):629–40. [DOI] [PubMed] [Google Scholar]
- 32.Khvorova A, Watts JK. The chemical evolution of oligonucleotide therapies of clinical utility. Nat Biotechnol. 2017;35(3):238–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guevara ML, Persano S, Persano F. Lipid-based vectors for therapeutic mRNA-based anti-cancer vaccines. Curr Pharm Des. 2019;25(13):1443–54. [DOI] [PubMed] [Google Scholar]
- 34.Rurik JG, Tombácz I, Yadegari A, Méndez Fernández PO, Shewale SV, Li L, et al. CAR T cells produced in vivo to treat cardiac injury. Science (New York, NY). 2022;375(6576):91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karim ME, Haque ST, Al-Busaidi H, Bakhtiar A, Tha KK, Holl MMB, et al. Scope and challenges of nanoparticle-based mRNA delivery in cancer treatment. Arch Pharmacal Res. 2022;45(12):865–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang D, Wang G, Yu X, Wei T, Farbiak L, Johnson LT, et al. Enhancing CRISPR/Cas gene editing through modulating cellular mechanical properties for cancer therapy. Nat Nanotechnol. 2022;17(7):777–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karim ME, Shetty J, Islam RA, Kaiser A, Bakhtiar A, Chowdhury EH. Strontium sulfite: a new pH-responsive inorganic nanocarrier to deliver therapeutic siRNAs to cancer cells. Pharmaceutics. 2019;11(2):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karim ME, Tha KK, Othman I, Borhan Uddin M, Chowdhury EH. Therapeutic potency of nanoformulations of siRNAs and shRNAs in animal models of cancers. Pharmaceutics. 2018;10(2):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Gao DY, Huang L. In vivo delivery of miRNAs for cancer therapy: challenges and strategies. Adv Drug Deliv Rev. 2015;81:128–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen H, Mittal V, Ferrari M, Chang J. Delivery of gene silencing agents for breast cancer therapy. Breast cancer Res: BCR. 2013;15(3):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30(17):3481–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pandey E, Harris EN. Chloroquine and cytosolic galectins affect endosomal escape of antisense oligonucleotides after Stabilin-mediated endocytosis. Mol Therapy Nucleic Acids. 2023;33:430–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldenring JR. Recycling endosomes. Curr Opin Cell Biol. 2015;35:117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Juliano RL, Carver K. Cellular uptake and intracellular trafficking of oligonucleotides. Adv Drug Deliv Rev. 2015;87:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Varkouhi AK, Scholte M, Storm G, Haisma HJ. Endosomal escape pathways for delivery of biologicals. J Control Release: Off J Control Release Soc. 2011;151(3):220–8. [DOI] [PubMed] [Google Scholar]
- 46.Juliano RL. The delivery of therapeutic oligonucleotides. Nucleic Acids Res. 2016;44(14):6518–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Neill CP, Dwyer RM. Nanoparticle-based delivery of tumor suppressor microRNA for cancer therapy. Cells. 2020;9(2):521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manikandan C, Kaushik A, Sen D. Viral vector: potential therapeutic for glioblastoma multiforme. Cancer Gene Ther. 2020;27(5):270–9. [DOI] [PubMed] [Google Scholar]
- 49.Babaei M, Eshghi H, Abnous K, Rahimizadeh M, Ramezani M. Promising gene delivery system based on polyethylenimine-modified silica nanoparticles. Cancer Gene Ther. 2017;24(4):156–64. [DOI] [PubMed] [Google Scholar]
- 50.Young LS, Searle PF, Onion D, Mautner V. Viral gene therapy strategies: from basic science to clinical application. J Pathol. 2006;208(2):299–318. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Bruggeman KF, Franks S, Gautam V, Hodgetts SI, Harvey AR, et al. Is viral vector gene delivery more effective using biomaterials? Adv Healthcare Mater. 2021;10(1):e2001238. [DOI] [PubMed] [Google Scholar]
- 52.Sung YK, Kim SW. Recent advances in the development of gene delivery systems. Biomater Res. 2019;23:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naso MF, Tomkowicz B, Perry WL 3rd, Strohl WR. Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs: Clin Immunother, Biopharm Gene Ther. 2017;31(4):317–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burdett T, Nuseibeh S. Changing trends in the development of AAV-based gene therapies: a meta-analysis of past and present therapies. Gene Ther. 2023;30(3–4):323–35. [DOI] [PubMed] [Google Scholar]
- 55.Li L, Vasan L, Kartono B, Clifford K, Attarpour A, Sharma R, et al. Advances in recombinant adeno-associated virus vectors for neurodegenerative diseases. Biomedicines. 2023;11(10):2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fajardo-Serrano A, Rico AJ, Roda E, Honrubia A, Arrieta S, Ariznabarreta G, et al. Adeno-associated viral vectors as versatile tools for neurological disorders: focus on delivery routes and therapeutic perspectives. Biomedicines. 2022;10(4):746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gross DA, Tedesco N, Leborgne C, Ronzitti G. Overcoming the challenges imposed by humoral immunity to AAV vectors to achieve safe and efficient gene transfer in seropositive patients. Front Immunol. 2022;13:857276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hamilton BA, Wright JF. Challenges posed by immune responses to AAV vectors: addressing root causes. Front Immunol. 2021;12:675897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li C, Samulski RJ. Engineering adeno-associated virus vectors for gene therapy. Nat Rev Genet. 2020;21(4):255–72. [DOI] [PubMed] [Google Scholar]
- 60.Taghian T, Marosfoi MG, Puri AS, Cataltepe OI, King RM, Diffie EB, et al. A safe and reliable technique for CNS delivery of AAV vectors in the cisterna magna. Mol Ther: J Am Soc Gene Ther. 2020;28(2):411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Che J, Xue Y, Feng J, Bai G, Yuan W. Comparison of biological responses of polymers based on imine and disulfide backbones for siRNA delivery. ACS Appl Mater Interfaces. 2018;10(6):5196–202. [DOI] [PubMed] [Google Scholar]
- 62.Wang S, Huang R. Non-viral nucleic acid delivery to the central nervous system and brain tumors. J Gene Med. 2019;21(7):e3091. [DOI] [PubMed] [Google Scholar]
- 63.Shim G, Kim D, Le QV, Park GT, Kwon T, Oh YK. Nonviral delivery systems for cancer gene therapy: strategies and challenges. Curr Gene Ther. 2018;18(1):3–20. [DOI] [PubMed] [Google Scholar]
- 64.Xiong Q, Lee GY, Ding J, Li W, Shi J. Biomedical applications of mRNA nanomedicine. Nano Res. 2018;11(10):5281–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guan S, Rosenecker J. Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems. Gene Ther. 2017;24(3):133–43. [DOI] [PubMed] [Google Scholar]
- 66.Danhier F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J Control Release: Off J Control Release Soc. 2016;244(Pt A):108–21. [DOI] [PubMed] [Google Scholar]
- 67.Wang Q, Liang Q, Dou J, Zhou H, Zeng C, Pan H, et al. Breaking through the basement membrane barrier to improve nanotherapeutic delivery to tumours. Nat Nanotechnol. 2023;19:95. [DOI] [PubMed] [Google Scholar]
- 68.Sato Y, Sakurai Y, Kajimoto K, Nakamura T, Yamada Y, Akita H, et al. Innovative technologies in nanomedicines: from passive targeting to active targeting/from controlled pharmacokinetics to controlled intracellular pharmacokinetics. Macromol Biosci. 2017;17(1):1600179. [DOI] [PubMed] [Google Scholar]
- 69.Lujan H, Griffin WC, Taube JH, Sayes CM. Synthesis and characterization of nanometer-sized liposomes for encapsulation and microRNA transfer to breast cancer cells. Int J Nanomed. 2019;14:5159–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65(1):36–48. [DOI] [PubMed] [Google Scholar]
- 71.Fitzgerald K, White S, Borodovsky A, Bettencourt BR, Strahs A, Clausen V, et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N Engl J Med. 2017;376(1):41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang J, Bahreman A, Daudey G, Bussmann J, Olsthoorn RC, Kros A. Drug delivery via cell membrane fusion using lipopeptide modified liposomes. ACS Cent Sci. 2016;2(9):621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ando H, Fukushima M, Eshima K, Hasui T, Shimizu T, Ishima Y, et al. A novel intraperitoneal therapy for gastric cancer with DFP-10825, a unique RNAi therapeutic targeting thymidylate synthase, in a peritoneally disseminated xenograft model. Cancer Med. 2019;8(17):7313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wei X, Shao B, He Z, Ye T, Luo M, Sang Y, et al. Cationic nanocarriers induce cell necrosis through impairment of Na(+)/K(+)-ATPase and cause subsequent inflammatory response. Cell Res. 2015;25(2):237–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ranjbar S, Zhong XB, Manautou J, Lu X. A holistic analysis of the intrinsic and delivery-mediated toxicity of siRNA therapeutics. Adv Drug Deliv Rev. 2023;201:115052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gokita K, Inoue J, Ishihara H, Kojima K, Inazawa J. Therapeutic potential of LNP-mediated delivery of miR-634 for cancer therapy. Mol Ther Nucleic Acids. 2020;19:330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sato Y, Hashiba K, Sasaki K, Maeki M, Tokeshi M, Harashima H. Understanding structure-activity relationships of pH-sensitive cationic lipids facilitates the rational identification of promising lipid nanoparticles for delivering siRNAs in vivo. J Control Release: Off J Control Release Soc. 2019;295:140–52. [DOI] [PubMed] [Google Scholar]
- 78.Moro M, Di Paolo D, Milione M, Centonze G, Bornaghi V, Borzi C, et al. Coated cationic lipid-nanoparticles entrapping miR-660 inhibit tumor growth in patient-derived xenografts lung cancer models. J Control Release: Off J Control Release Soc. 2019;308:44–56. [DOI] [PubMed] [Google Scholar]
- 79.Wu L, Wang W, Tian J, Qi C, Cai Z, Yan W, et al. Engineered mRNA-expressed bispecific antibody prevent intestinal cancer via lipid nanoparticle delivery. Bioengineered. 2021;12(2):12383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang H, Liu S, Jia L, Chu F, Zhou Y, He Z, et al. Nanostructured lipid carriers for MicroRNA delivery in tumor gene therapy. Cancer Cell Int. 2018;18:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12(11):967–77. [DOI] [PubMed] [Google Scholar]
- 83.Pasut G, Paolino D, Celia C, Mero A, Joseph AS, Wolfram J, et al. Polyethylene glycol (PEG)-dendron phospholipids as innovative constructs for the preparation of super stealth liposomes for anticancer therapy. J Control Release: Off J Control Release Soc. 2015;199:106–13. [DOI] [PubMed] [Google Scholar]
- 84.Cullis PR, Hope MJ. Lipid nanoparticle systems for enabling gene therapies. Mol Ther: J Am Soc Gene Ther. 2017;25(7):1467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Woitok MM, Zoubek ME, Doleschel D, Bartneck M, Mohamed MR, Kießling F, et al. Lipid-encapsulated siRNA for hepatocyte-directed treatment of advanced liver disease. Cell Death Dis. 2020;11(5):343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang X, Men K, Zhang Y, Zhang R, Yang L, Duan X. Local and systemic delivery of mRNA encoding survivin-T34A by lipoplex for efficient colon cancer gene therapy. Int J Nanomed. 2019;14:2733–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zamboni CG, Kozielski KL, Vaughan HJ, Nakata MM, Kim J, Higgins LJ, et al. Polymeric nanoparticles as cancer-specific DNA delivery vectors to human hepatocellular carcinoma. J Control Release: Off J Control Release Soc. 2017;263:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kumar V, Mundra V, Peng Y, Wang Y, Tan C, Mahato RI. Pharmacokinetics and biodistribution of polymeric micelles containing miRNA and small-molecule drug in orthotopic pancreatic tumor-bearing mice. Theranostics. 2018;8(15):4033–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zheng B, Chen L, Pan CC, Wang JZ, Lu GR, Yang SX, et al. Targeted delivery of miRNA-204-5p by PEGylated polymer nanoparticles for colon cancer therapy. Nanomedicine (Lond). 2018;13(7):769–85. [DOI] [PubMed] [Google Scholar]
- 90.Valencia-Serna J, Kucharski C, Chen M, Kc R, Jiang X, Brandwein J, et al. siRNA-mediated BCR-ABL silencing in primary chronic myeloid leukemia cells using lipopolymers. J Control Release: Off J Control Release Soc. 2019;310:141–54. [DOI] [PubMed] [Google Scholar]
- 91.Cosco D, Cilurzo F, Maiuolo J, Federico C, Di Martino MT, Cristiano MC, et al. Delivery of miR-34a by chitosan/PLGA nanoplexes for the anticancer treatment of multiple myeloma. Sci Rep. 2015;5:17579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Conte R, Valentino A, Di Cristo F, Peluso G, Cerruti P, Di Salle A, et al. Cationic polymer nanoparticles-mediated delivery of miR-124 impairs tumorigenicity of prostate cancer cells. Int J Mol Sci. 2020;21(3):869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen J, Guo Z, Jiao Z, Lin L, Xu C, Tian H, et al. Poly(l-glutamic acid)-based zwitterionic polymer in a charge conversional shielding system for gene therapy of malignant tumors. ACS Appl Mater Interfaces. 2020;12(17):19295–306. [DOI] [PubMed] [Google Scholar]
- 94.Dai Y, Zhang X. MicroRNA delivery with bioreducible polyethylenimine as a non-viral vector for breast cancer gene therapy. Macromol Biosci. 2019;19(4):e1800445. [DOI] [PubMed] [Google Scholar]
- 95.Kim T, Hyun HN, Heo R, Nam K, Yang K, Kim YM, et al. Dual-targeting RNA nanoparticles for efficient delivery of polymeric siRNA to cancer cells. Chem Commun (Camb). 2020;56(49):6624–7. [DOI] [PubMed] [Google Scholar]
- 96.Kozielski KL, Ruiz-Valls A, Tzeng SY, Guerrero-Cázares H, Rui Y, Li Y, et al. Cancer-selective nanoparticles for combinatorial siRNA delivery to primary human GBM in vitro and in vivo. Biomaterials. 2019;209:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xie L, Yang Y, Shen J. Efficient inhibition of uveal melanoma via ternary siRNA complexes. Int J Pharm. 2020;573:118894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kalomiraki M, Thermos K, Chaniotakis NA. Dendrimers as tunable vectors of drug delivery systems and biomedical and ocular applications. Int J Nanomed. 2016;11:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oddone N, Lecot N, Fernández M, Rodriguez-Haralambides A, Cabral P, Cerecetto H, et al. In vitro and in vivo uptake studies of PAMAM G45 dendrimers in breast cancer. J Nanobiotechnol. 2016;14(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim Y, Park EJ, Na DH. Recent progress in dendrimer-based nanomedicine development. Arch Pharmacal Res. 2018;41(6):571–82. [DOI] [PubMed] [Google Scholar]
- 101.Tarach P, Janaszewska A. Recent advances in preclinical research using PAMAM dendrimers for cancer gene therapy. Int J Mol Sci. 2021;22(6):2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Surekha B, Kommana NS, Dubey SK, Kumar AVP, Shukla R, Kesharwani P. PAMAM dendrimer as a talented multifunctional biomimetic nanocarrier for cancer diagnosis and therapy. Colloids Surf, B. 2021;204:111837. [DOI] [PubMed] [Google Scholar]
- 103.Palombarini F, Masciarelli S, Incocciati A, Liccardo F, Di Fabio E, Iazzetti A, et al. Self-assembling ferritin-dendrimer nanoparticles for targeted delivery of nucleic acids to myeloid leukemia cells. J Nanobiotechnol. 2021;19(1):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang R, Degirmenci V, Xin H, Li Y, Wang L, Chen J, et al. PEI-coated Fe3O4 nanoparticles enable efficient delivery of therapeutic siRNA targeting REST into glioblastoma cells. Int J Mol Sci. 2018;19(8):2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Villar-Alvarez E, Leal BH, Cambón A, Pardo A, Martínez-Gonzalez R, Fernández-Vega J, et al. Triggered RNAi therapy using metal inorganic nanovectors. Mol Pharm. 2019;16(8):3374–85. [DOI] [PubMed] [Google Scholar]
- 106.Zhao R, Li T, Zheng G, Jiang K, Fan L, Shao J. Simultaneous inhibition of growth and metastasis of hepatocellular carcinoma by co-delivery of ursolic acid and sorafenib using lactobionic acid modified and pH-sensitive chitosan-conjugated mesoporous silica nanocomplex. Biomaterials. 2017;143:1–16. [DOI] [PubMed] [Google Scholar]
- 107.Arami H, Khandhar A, Liggitt D, Krishnan KM. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem Soc Rev. 2015;44(23):8576–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stephen ZR, Dayringer CJ, Lim JJ, Revia RA, Halbert MV, Jeon M, et al. Approach to rapid synthesis and functionalization of iron oxide nanoparticles for high gene transfection. ACS Appl Mater Interfaces. 2016;8(10):6320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mu Q, Kievit FM, Kant RJ, Lin G, Jeon M, Zhang M. Anti-HER2/neu peptide-conjugated iron oxide nanoparticles for targeted delivery of paclitaxel to breast cancer cells. Nanoscale. 2015;7(43):18010–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.He X, Yin F, Wang D, Xiong LH, Kwok RTK, Gao PF, et al. AIE featured inorganic-organic core@shell nanoparticles for high-efficiency siRNA delivery and real-time monitoring. Nano Lett. 2019;19(4):2272–9. [DOI] [PubMed] [Google Scholar]
- 111.Esteban-Fernández de Ávila B, Angell C, Soto F, Lopez-Ramirez MA, Báez DF, Xie S, et al. Acoustically propelled nanomotors for intracellular siRNA delivery. ACS Nano. 2016;10(5):4997–5005. [DOI] [PubMed] [Google Scholar]
- 112.Wayteck L, Xiong R, Braeckmans K, De Smedt SC, Raemdonck K. Comparing photoporation and nucleofection for delivery of small interfering RNA to cytotoxic T cells. J Control Release: Off J Control Release Soc. 2017;267:154–62. [DOI] [PubMed] [Google Scholar]
- 113.Hosseini SA, Kardani A, Yaghoobi H. A comprehensive review of cancer therapies mediated by conjugated gold nanoparticles with nucleic acid. Int J Biol Macromol. 2023;253(Pt 5):127184. [DOI] [PubMed] [Google Scholar]
- 114.Guo X, Mao F, Wang W, Yang Y, Bai Z. Sulfhydryl-modified Fe3O4@SiO2 core/shell nanocomposite: synthesis and toxicity assessment in vitro. ACS Appl Mater Interfaces. 2015;7(27):14983–91. [DOI] [PubMed] [Google Scholar]
- 115.Kowalska A, Adamska E, Grobelna B. Medical applications of silver and gold nanoparticles and core-shell nanostructures based on silver or gold core: recent progress and innovations. ChemMedChem. 2024;19(12):e202300672. [DOI] [PubMed] [Google Scholar]
- 116.Wu X, Zhang H. Therapeutic strategies of iron-based nanomaterials for cancer therapy. Biomed Mater (Bristol, England). 2021;16(3):032003. [DOI] [PubMed] [Google Scholar]
- 117.Daldrup-Link HE. Ten things you might not know about iron oxide nanoparticles. Radiology. 2017;284(3):616–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin B, Lu L, Wang Y, Zhang Q, Wang Z, Cheng G, et al. Nanomedicine directs neuronal differentiation of neural stem cells via silencing long noncoding RNA for stroke therapy. Nano Lett. 2021;21(1):806–15. [DOI] [PubMed] [Google Scholar]
- 119.Revia RA, Stephen ZR, Zhang M. Theranostic nanoparticles for RNA-based cancer treatment. Acc Chem Res. 2019;52(6):1496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang J, Dai D, Zhang X, Teng L, Ma L, Yang YW. Multifunctional metal-organic framework (MOF)-based nanoplatforms for cancer therapy: from single to combination therapy. Theranostics. 2023;13(1):295–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gong Y, Hu X, Chen M, Wang J. Recent progress of iron-based nanomaterials in gene delivery and tumor gene therapy. J Nanobiotechnol. 2024;22(1):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Niu G, Gao F, Wang Y, Zhang J, Zhao L, Jiang Y. Bimetallic nanomaterials: a promising nanoplatform for multimodal cancer therapy. Molecules (Basel, Switzerland). 2022;27(24):8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gilroy KD, Ruditskiy A, Peng HC, Qin D, Xia Y. Bimetallic nanocrystals: syntheses, properties, and applications. Chem Rev. 2016;116(18):10414–72. [DOI] [PubMed] [Google Scholar]
- 124.Bagheri B, Surwase SS, Lee SS, Park H, Faraji Rad Z, Trevaskis NL, et al. Carbon-based nanostructures for cancer therapy and drug delivery applications. J Mater Chem B. 2022;10(48):9944–67. [DOI] [PubMed] [Google Scholar]
- 125.Zhuang WR, Wang Y, Cui PF, Xing L, Lee J, Kim D, et al. Applications of π-π stacking interactions in the design of drug-delivery systems. J Control Release : Off J Control Release Soc. 2019;294:311–26. [DOI] [PubMed] [Google Scholar]
- 126.Sharma M, Alessandro P, Cheriyamundath S, Lopus M. Therapeutic and diagnostic applications of carbon nanotubes in cancer: recent advances and challenges. J Drug Target. 2024;32(3):287–99. [DOI] [PubMed] [Google Scholar]
- 127.Kazemzadeh H, Mozafari M. Fullerene-based delivery systems. Drug Discovery Today. 2019;24(3):898–905. [DOI] [PubMed] [Google Scholar]
- 128.Kobayashi N, Izumi H, Morimoto Y. Review of toxicity studies of carbon nanotubes. J Occup Health. 2017;59(5):394–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xu C, Qiao L, Guo Y, Ma L, Cheng Y. Preparation, characteristics and antioxidant activity of polysaccharides and proteins-capped selenium nanoparticles synthesized by Lactobacillus casei ATCC 393. Carbohyd Polym. 2018;195:576–85. [DOI] [PubMed] [Google Scholar]
- 130.Maiyo F, Singh M. Selenium nanoparticles: potential in cancer gene and drug delivery. Nanomedicine (Lond). 2017;12(9):1075–89. [DOI] [PubMed] [Google Scholar]
- 131.Xiao X, Deng H, Lin X, Ali ASM, Viscardi A, Guo Z, et al. Selenium nanoparticles: properties, preparation methods, and therapeutic applications. Chem Biol Interact. 2023;378:110483. [DOI] [PubMed] [Google Scholar]
- 132.Hassan S, Prakash G, Ozturk A, Saghazadeh S, Sohail MF, Seo J, et al. Evolution and clinical translation of drug delivery nanomaterials. Nano Today. 2017;15:91–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.O’Brien KP, Khan S, Gilligan KE, Zafar H, Lalor P, Glynn C, et al. Employing mesenchymal stem cells to support tumor-targeted delivery of extracellular vesicle (EV)-encapsulated microRNA-379. Oncogene. 2018;37(16):2137–49. [DOI] [PubMed] [Google Scholar]
- 134.Gong C, Tian J, Wang Z, Gao Y, Wu X, Ding X, et al. Functional exosome-mediated co-delivery of doxorubicin and hydrophobically modified microRNA 159 for triple-negative breast cancer therapy. J Nanobiotechnol. 2019;17(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zheng Y, Hasan A, Nejadi Babadaei MM, Behzadi E, Nouri M, Sharifi M, et al. Exosomes: multiple-targeted multifunctional biological nanoparticles in the diagnosis, drug delivery, and imaging of cancer cells. Biomed Pharmacother = Biomed Pharmacother. 2020;129:110442. [DOI] [PubMed] [Google Scholar]
- 137.Shahabipour F, Barati N, Johnston TP, Derosa G, Maffioli P, Sahebkar A. Exosomes: nanoparticulate tools for RNA interference and drug delivery. J Cell Physiol. 2017;232(7):1660–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhao X, Wu D, Ma X, Wang J, Hou W, Zhang W. Exosomes as drug carriers for cancer therapy and challenges regarding exosome uptake. Biomed Pharmacother = Biomed Pharmacother. 2020;128:110237. [DOI] [PubMed] [Google Scholar]
- 139.Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharmaceutica Sinica B. 2016;6(4):287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wan Y, Wang L, Zhu C, Zheng Q, Wang G, Tong J, et al. Aptamer-conjugated extracellular nanovesicles for targeted drug delivery. Can Res. 2018;78(3):798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jiang XC, Gao JQ. Exosomes as novel bio-carriers for gene and drug delivery. Int J Pharm. 2017;521(1–2):167–75. [DOI] [PubMed] [Google Scholar]
- 142.Willoughby JLS, Chan A, Sehgal A, Butler JS, Nair JK, Racie T, et al. Evaluation of GalNAc-siRNA conjugate activity in pre-clinical animal models with reduced asialoglycoprotein receptor expression. Mol Ther: J Am Soc Gene Ther. 2018;26(1):105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Debacker AJ, Voutila J, Catley M, Blakey D, Habib N. Delivery of oligonucleotides to the liver with GalNAc: from research to registered therapeutic drug. Mol Ther: J Am Soc Gene Ther. 2020;28(8):1759–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shen X, Corey DR. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res. 2018;46(4):1584–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nair JK, Willoughby JL, Chan A, Charisse K, Alam MR, Wang Q, et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J Am Chem Soc. 2014;136(49):16958–61. [DOI] [PubMed] [Google Scholar]
- 146.Huang Y. Preclinical and clinical advances of GalNAc-decorated nucleic acid therapeutics. Mol Ther Nucleic Acids. 2017;6:116–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zimmermann TS, Karsten V, Chan A, Chiesa J, Boyce M, Bettencourt BR, et al. Clinical proof of concept for a novel hepatocyte-targeting GalNAc-siRNA conjugate. Mol Ther: J Am Soc Gene Ther. 2017;25(1):71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kulkarni JA, Witzigmann D, Thomson SB, Chen S, Leavitt BR, Cullis PR, et al. The current landscape of nucleic acid therapeutics. Nat Nanotechnol. 2021;16(6):630–43. [DOI] [PubMed] [Google Scholar]
- 149.Brown CR, Gupta S, Qin J, Racie T, He G, Lentini S, et al. Investigating the pharmacodynamic durability of GalNAc-siRNA conjugates. Nucleic Acids Res. 2020;48(21):11827–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.