Abstract
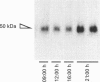
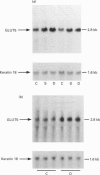
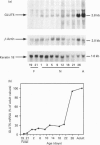
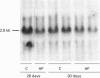
1. GLUT5 gene expression was studied in small intestine under a variety of conditions characterized by altered intestinal absorption of monosaccharides. 2. RNA-blotting studies showed that GLUT5 mRNA was abundantly expressed in rat and rabbit intestine and kidney, but it was not detected in heart or brown adipose tissue. GLUT5 mRNA levels were higher in the upper segments of the small intestine (duodenum and proximal jejunum) than in the lower segments (distal jejunum and ileum). 3. The intestinal expression of GLUT5 mRNA in rat proximal jejunum showed circadian rhythm. A 12-fold increase in GLUT5 mRNA levels was detected at the end of the light cycle and at the beginning of the dark cycle when compared with the early light period. In keeping with this, GLUT5 protein content in brush-border membranes was also increased at the beginning of the dark cycle compared with that in the light period. 4. In streptozotocin-induced diabetes an 80% increase in GLUT5 mRNA levels in mucosa from the proximal jejunum was detected under conditions in which enhanced intestinal absorption of monosaccharides has been reported. 5. The intestinal expression of GLUT5 mRNA showed regulation during perinatal development. Levels of GLUT5 mRNA were low during fetal life, increased progressively during the postnatal period and reached levels comparable with the adult state after weaning. Weaning on to a high-fat diet partially prevented the induction of GLUT5 gene expression. 6. Our results indicate that GLUT5 gene expression is tightly regulated in small intestine. Regulation involves maximal expression in the upper part of the small intestine, circadian rhythm, developmental regulation dependent on the fat and carbohydrate content in the diet at weaning and enhanced expression in streptozotocin-induced diabetes. Furthermore, changes observed in intestinal GLUT5 expression correlate with reported alterations in intestinal absorption of fructose. This suggests a regulatory role for GLUT5 in fructose uptake by absorptive enterocytes.
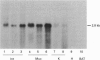
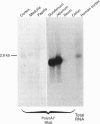
Full text
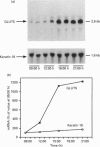
PDF

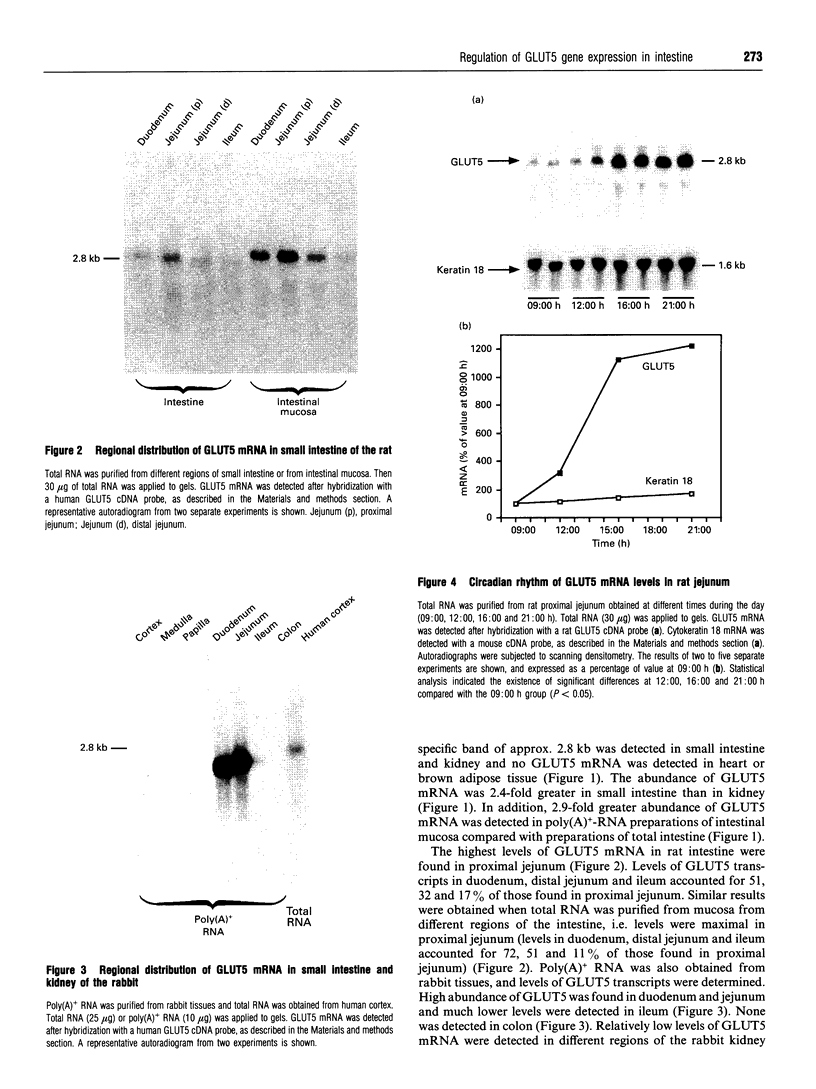
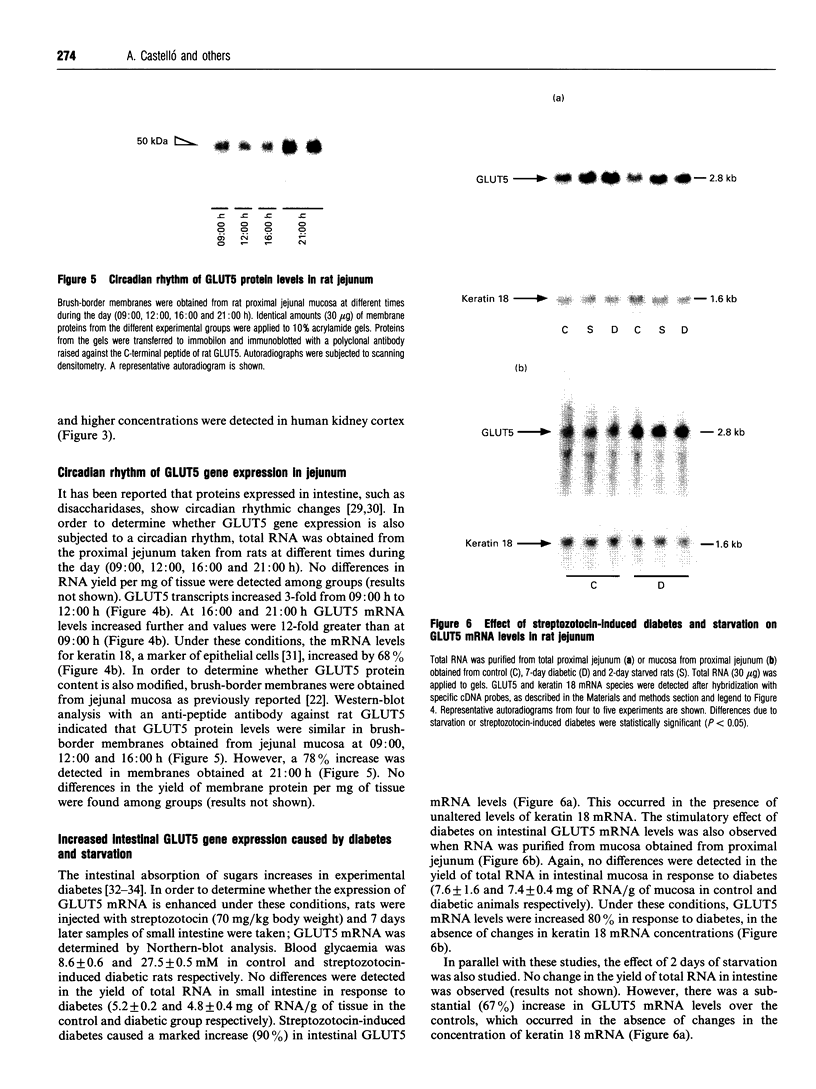
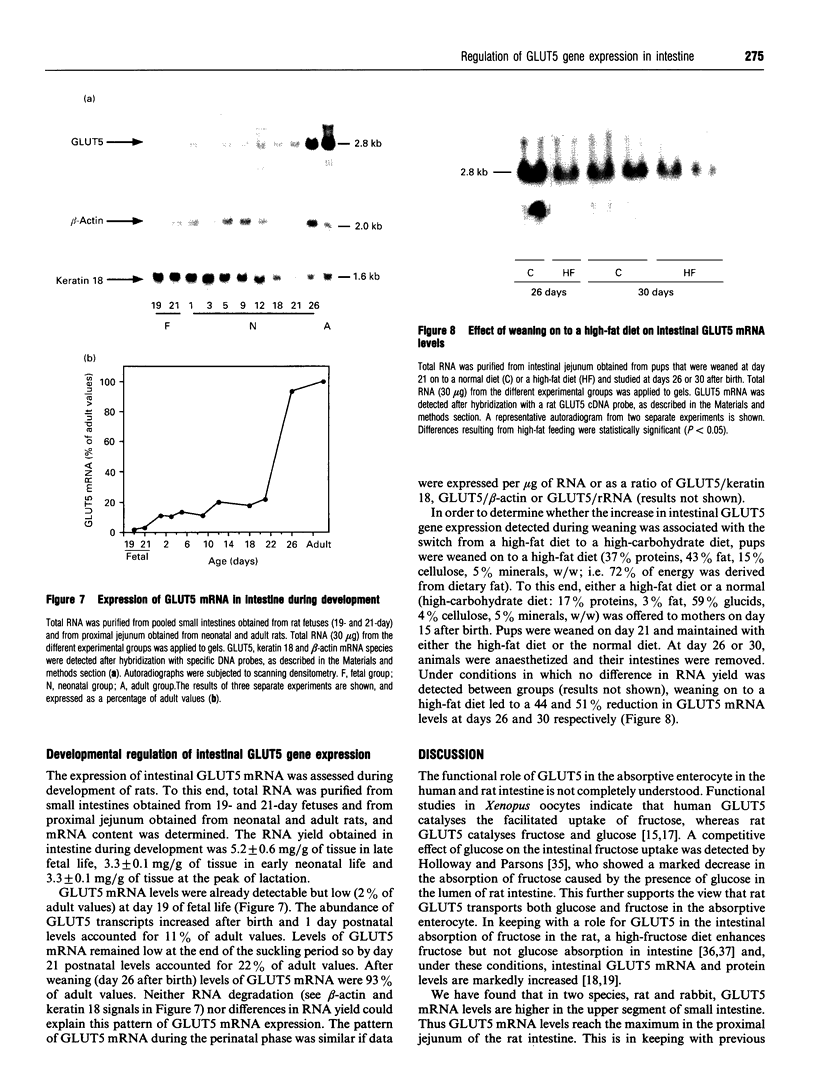


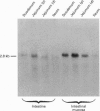
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrad A. D., Lawrence A. L., Hazelwood R. L. Fasting and alloxan diabetes effects of intestinal transport of monosaccharides. Am J Physiol. 1970 Oct;219(4):860–864. doi: 10.1152/ajplegacy.1970.219.4.860. [DOI] [PubMed] [Google Scholar]
- Beam H. E., Henning S. J. Development of the circadian rhythm of jejunal sucrase activity in the weanling rat. Am J Physiol. 1978 Oct;235(4):E437–E442. doi: 10.1152/ajpendo.1978.235.4.E437. [DOI] [PubMed] [Google Scholar]
- Bode C., Eisenhardt J. M., Haberich F. J., Bode J. C. Influence of feeding fructose on fructose and glucose absorption in rat jejunum and ileum. Res Exp Med (Berl) 1981;179(2):163–168. doi: 10.1007/BF01851984. [DOI] [PubMed] [Google Scholar]
- Buddington R. K., Diamond J. M. Ontogenetic development of monosaccharide and amino acid transporters in rabbit intestine. Am J Physiol. 1990 Oct;259(4 Pt 1):G544–G555. doi: 10.1152/ajpgi.1990.259.4.G544. [DOI] [PubMed] [Google Scholar]
- Burant C. F., Bell G. I. Mammalian facilitative glucose transporters: evidence for similar substrate recognition sites in functionally monomeric proteins. Biochemistry. 1992 Oct 27;31(42):10414–10420. doi: 10.1021/bi00157a032. [DOI] [PubMed] [Google Scholar]
- Burant C. F., Takeda J., Brot-Laroche E., Bell G. I., Davidson N. O. Fructose transporter in human spermatozoa and small intestine is GLUT5. J Biol Chem. 1992 Jul 25;267(21):14523–14526. [PubMed] [Google Scholar]
- CRANE R. K. An effect of alloxan-diabetes on the active transport of sugars by rat small intestine, in vitro. Biochem Biophys Res Commun. 1961 Apr 28;4:436–440. doi: 10.1016/0006-291x(61)90304-7. [DOI] [PubMed] [Google Scholar]
- Camps M., Castelló A., Muñoz P., Monfar M., Testar X., Palacín M., Zorzano A. Effect of diabetes and fasting on GLUT-4 (muscle/fat) glucose-transporter expression in insulin-sensitive tissues. Heterogeneous response in heart, red and white muscle. Biochem J. 1992 Mar 15;282(Pt 3):765–772. doi: 10.1042/bj2820765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasena G., Sunitha I., Lau C., Nanthakumar N. N., Henning S. J. Expression of sucrase-isomaltase mRNA along the villus-crypt axis in the rat small intestine. Cell Mol Biol. 1992 May;38(3):243–254. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Colville C. A., Seatter M. J., Jess T. J., Gould G. W., Thomas H. M. Kinetic analysis of the liver-type (GLUT2) and brain-type (GLUT3) glucose transporters in Xenopus oocytes: substrate specificities and effects of transport inhibitors. Biochem J. 1993 Mar 15;290(Pt 3):701–706. doi: 10.1042/bj2900701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csáky T. Z., Fischer E. Intestinal sugar transport in experimental diabetes. Diabetes. 1981 Jul;30(7):568–574. doi: 10.2337/diab.30.7.568. [DOI] [PubMed] [Google Scholar]
- Davidson N. O., Hausman A. M., Ifkovits C. A., Buse J. B., Gould G. W., Burant C. F., Bell G. I. Human intestinal glucose transporter expression and localization of GLUT5. Am J Physiol. 1992 Mar;262(3 Pt 1):C795–C800. doi: 10.1152/ajpcell.1992.262.3.C795. [DOI] [PubMed] [Google Scholar]
- Fedorak R. N. Adaptation of small intestinal membrane transport processes during diabetes mellitus in rats. Can J Physiol Pharmacol. 1990 May;68(5):630–635. doi: 10.1139/y90-092. [DOI] [PubMed] [Google Scholar]
- Fedorak R. N., Cheeseman C. I., Thomson A. B., Porter V. M. Altered glucose carrier expression: mechanism of intestinal adaptation during streptozocin-induced diabetes in rats. Am J Physiol. 1991 Oct;261(4 Pt 1):G585–G591. doi: 10.1152/ajpgi.1991.261.4.G585. [DOI] [PubMed] [Google Scholar]
- Fedorak R. N., Thomson A. B., Porter V. M. Adaptation of intestinal glucose transport in rats with diabetes mellitus occurs independent of hyperphagia. Can J Physiol Pharmacol. 1991 Aug;69(8):1143–1148. doi: 10.1139/y91-167. [DOI] [PubMed] [Google Scholar]
- Ferraris R. P., Casirola D. M., Vinnakota R. R. Dietary carbohydrate enhances intestinal sugar transport in diabetic mice. Diabetes. 1993 Nov;42(11):1579–1587. doi: 10.2337/diab.42.11.1579. [DOI] [PubMed] [Google Scholar]
- Ferraris R. P., Hsiao J., Hernandez R., Hirayama B. Site density of mouse intestinal glucose transporters declines with age. Am J Physiol. 1993 Feb;264(2 Pt 1):G285–G293. doi: 10.1152/ajpgi.1993.264.2.G285. [DOI] [PubMed] [Google Scholar]
- Furuya S., Yugari Y. Daily rhythmic change of L-histidine and glucose absorptions in rat small intestine in vivo. Biochim Biophys Acta. 1974 May 24;343(3):558–564. doi: 10.1016/0304-4165(74)90274-8. [DOI] [PubMed] [Google Scholar]
- Hediger M. A., Coady M. J., Ikeda T. S., Wright E. M. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. 1987 Nov 26-Dec 2Nature. 330(6146):379–381. doi: 10.1038/330379a0. [DOI] [PubMed] [Google Scholar]
- Holloway P. A., Parsons D. S. Absorption and metabolism of fructose by rat jejunum. Biochem J. 1984 Aug 15;222(1):57–64. doi: 10.1042/bj2220057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundal H. S., Ahmed A., Gumà A., Mitsumoto Y., Marette A., Rennie M. J., Klip A. Biochemical and immunocytochemical localization of the 'GLUT5 glucose transporter' in human skeletal muscle. Biochem J. 1992 Sep 1;286(Pt 2):339–343. doi: 10.1042/bj2860339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T. S., Hwang E. S., Coady M. J., Hirayama B. A., Hediger M. A., Wright E. M. Characterization of a Na+/glucose cotransporter cloned from rabbit small intestine. J Membr Biol. 1989 Aug;110(1):87–95. doi: 10.1007/BF01870995. [DOI] [PubMed] [Google Scholar]
- Inagaki O., Mori H., Shimomura R., Inoue S., Fujita Y. Circadian rhythms of intestinal disaccharidases in experimental uremia in rats. Nephron. 1982;30(4):345–347. doi: 10.1159/000182514. [DOI] [PubMed] [Google Scholar]
- Inukai K., Asano T., Katagiri H., Ishihara H., Anai M., Fukushima Y., Tsukuda K., Kikuchi M., Yazaki Y., Oka Y. Cloning and increased expression with fructose feeding of rat jejunal GLUT5. Endocrinology. 1993 Nov;133(5):2009–2014. doi: 10.1210/endo.133.5.8404647. [DOI] [PubMed] [Google Scholar]
- Karasov W. H., Diamond J. M. Adaptive regulation of sugar and amino acid transport by vertebrate intestine. Am J Physiol. 1983 Oct;245(4):G443–G462. doi: 10.1152/ajpgi.1983.245.4.G443. [DOI] [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mantych G. J., James D. E., Devaskar S. U. Jejunal/kidney glucose transporter isoform (Glut-5) is expressed in the human blood-brain barrier. Endocrinology. 1993 Jan;132(1):35–40. doi: 10.1210/endo.132.1.8419132. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Takao Y., Akazawa S., Yano M., Uotani S., Kawasaki E., Takino H., Yamasaki H., Okuno S., Yamaguchi Y. Developmental change of facilitative glucose transporter expression in rat embryonal and fetal intestine. Biochem Biophys Res Commun. 1993 Jun 30;193(3):1275–1282. doi: 10.1006/bbrc.1993.1763. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Hase K., Takagi T., Fujii T., Taketani Y., Minami H., Oka T., Nakabou Y. Differential responses of intestinal glucose transporter mRNA transcripts to levels of dietary sugars. Biochem J. 1993 Oct 1;295(Pt 1):211–215. doi: 10.1042/bj2950211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K., Hase K., Taketani Y., Minami H., Oka T., Nakabou Y., Hagihira H. Developmental changes in intestinal glucose transporter mRNA levels. Biochem Biophys Res Commun. 1992 Mar 16;183(2):626–631. doi: 10.1016/0006-291x(92)90528-s. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Hase K., Taketani Y., Minami H., Oka T., Nakabou Y., Hagihira H. Diabetes and glucose transporter gene expression in rat small intestine. Biochem Biophys Res Commun. 1991 Dec 31;181(3):1110–1117. doi: 10.1016/0006-291x(91)92053-m. [DOI] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Nudel U., Zakut R., Shani M., Neuman S., Levy Z., Yaffe D. The nucleotide sequence of the rat cytoplasmic beta-actin gene. Nucleic Acids Res. 1983 Mar 25;11(6):1759–1771. doi: 10.1093/nar/11.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen W. A., Rosenberg I. H. Intestinal transport of sugars and amino acids in diabetic rats. J Clin Invest. 1970 Jan;49(1):96–105. doi: 10.1172/JCI106227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand E. B., Depaoli A. M., Davidson N. O., Bell G. I., Burant C. F. Sequence, tissue distribution, and functional characterization of the rat fructose transporter GLUT5. Am J Physiol. 1993 Jun;264(6 Pt 1):G1169–G1176. doi: 10.1152/ajpgi.1993.264.6.G1169. [DOI] [PubMed] [Google Scholar]
- Redman R. S., Sweney L. R. Changes in diet and patterns of feeding activity of developing rats. J Nutr. 1976 May;106(5):615–626. doi: 10.1093/jn/106.5.615. [DOI] [PubMed] [Google Scholar]
- Semenza G., Kessler M., Hosang M., Weber J., Schmidt U. Biochemistry of the Na+, D-glucose cotransporter of the small-intestinal brush-border membrane. The state of the art in 1984. Biochim Biophys Acta. 1984 Sep 3;779(3):343–379. doi: 10.1016/0304-4157(84)90016-9. [DOI] [PubMed] [Google Scholar]
- Shepherd P. R., Gibbs E. M., Wesslau C., Gould G. W., Kahn B. B. Human small intestine facilitative fructose/glucose transporter (GLUT5) is also present in insulin-responsive tissues and brain. Investigation of biochemical characteristics and translocation. Diabetes. 1992 Oct;41(10):1360–1365. doi: 10.2337/diab.41.10.1360. [DOI] [PubMed] [Google Scholar]
- Solberg D. H., Diamond J. M. Comparison of different dietary sugars as inducers of intestinal sugar transporters. Am J Physiol. 1987 Apr;252(4 Pt 1):G574–G584. doi: 10.1152/ajpgi.1987.252.4.G574. [DOI] [PubMed] [Google Scholar]
- Stevenson N. R., Sitren H. S., Furuya S. Circadian rhythmicity in several small intestinal functions is independent of use of the intestine. Am J Physiol. 1980 Mar;238(3):G203–G207. doi: 10.1152/ajpgi.1980.238.3.G203. [DOI] [PubMed] [Google Scholar]
- Thorens B., Cheng Z. Q., Brown D., Lodish H. F. Liver glucose transporter: a basolateral protein in hepatocytes and intestine and kidney cells. Am J Physiol. 1990 Dec;259(6 Pt 1):C279–C285. doi: 10.1152/ajpcell.1990.259.2.C279. [DOI] [PubMed] [Google Scholar]
- Toloza E. M., Diamond J. Ontogenetic development of nutrient transporters in rat intestine. Am J Physiol. 1992 Nov;263(5 Pt 1):G593–G604. doi: 10.1152/ajpgi.1992.263.5.G593. [DOI] [PubMed] [Google Scholar]
- Traber P. G. Regulation of sucrase-isomaltase gene expression along the crypt-villus axis of rat small intestine. Biochem Biophys Res Commun. 1990 Dec 31;173(3):765–773. doi: 10.1016/s0006-291x(05)80853-8. [DOI] [PubMed] [Google Scholar]
- Umbach J. A., Coady M. J., Wright E. M. Intestinal Na+/glucose cotransporter expressed in Xenopus oocytes is electrogenic. Biophys J. 1990 Jun;57(6):1217–1224. doi: 10.1016/S0006-3495(90)82640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E. M., Turk E., Zabel B., Mundlos S., Dyer J. Molecular genetics of intestinal glucose transport. J Clin Invest. 1991 Nov;88(5):1435–1440. doi: 10.1172/JCI115451. [DOI] [PMC free article] [PubMed] [Google Scholar]