Abstract
Background
Prostate cancer (PCa) is the most prevalent type of cancer in men in the UK. Exercise has been shown to improve the health and quality of life of PCa patients. Exercise should be easily accessible to men with PCa regardless of socioeconomic group or ethnicity. There is a need to better understand whether the current evidence base for exercise interventions is representative and inclusive of racial and ethnic minority men with PCa.
Methods
A systematic review of the literature was conducted according to PRISMA guidelines and prospectively registered via Prospero (ID: CRD42022384373). The MEDLINE Ovid, Cochrane Library and PubMed databases were searched from inception to December 2022. The search strategy keywords and MeSH terms used included the following: (1) exercise, (2) training, (3) prostate cancer, (4) ethnic and (5) diversity.
Results
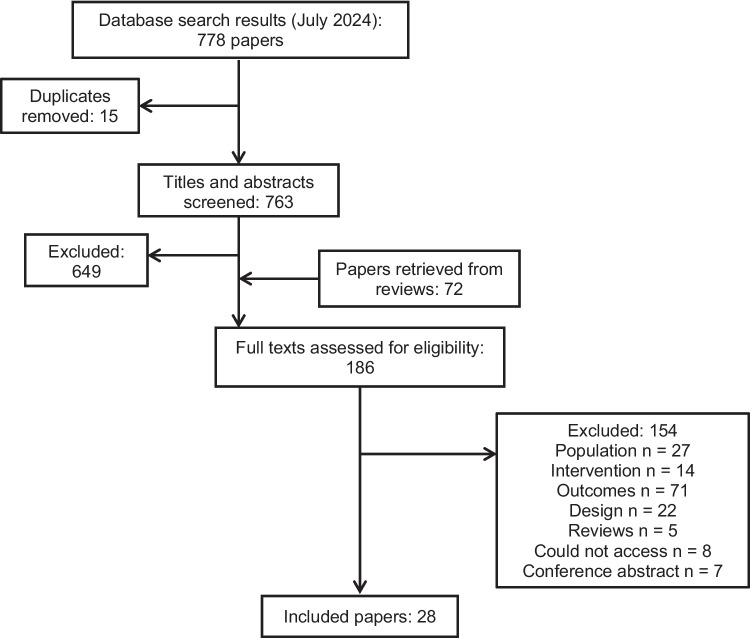
A total of 778 records were retrieved from database searches, of which 15 records were duplicates. A further 649 were eliminated following the screening of titles and abstracts. After full-text screening of 186 articles, 28 manuscripts were included for review.
Conclusion
This systematic review highlights that there is high heterogeneity in the reporting of participants’ ethnicity and there are low numbers of ethnic minority men included in PCa and exercise studies in the UK. Further work is required to understand why representation is lacking within PCa exercise trials in the UK and strategies are needed to achieve representation from all ethnic groups.
Implications for cancer survivors
Improved representation and reporting of ethnicity in exercise trials is vital to ensure the results are applicable to all patients.
Keywords: Prostate cancer, Exercise, Inclusivity, Ethnic diversity
Background
Prostate cancer (PCa) is the most prevalent type of cancer in men in the UK, with approximately 55,100 confirmed new cases each year and a further 1.4 million worldwide [1, 2]. Effective treatments for localised and metastatic PCa are recognised to have side effects which may be associated with negative impacts on patients’ quality of life, functional status and physical and mental health [3–6]. Despite advances in PCa management and improvements in outcomes driven by clinical trials, it is recognised that significant PCa health disparity remains due to a poorly understood, complex interplay of factors [7]. It is well documented that Black men are at a 2.1 times greater risk of being diagnosed with PCa (23.5–37.2%) compared to individuals of White (13.2–15.0%) or Asian (6.3–10.5%) ethnicity [8, 9]. Black men are more likely to be diagnosed at a younger age and with a more advanced stage PCa compared to men of other ethnicities. Advanced PCa can have a significant impact on patients’ survival, quality of life and ability to carry out activities of daily living effectively [10].
Exercise has been demonstrated to improve the health outcomes and quality of life of men with PCa [11–13]. The evidence supporting the benefits of exercise in men with PCa has become more compelling over the past decade, to the extent that exercise is recommended in current national and international PCa guidelines [14, 15]. The effect of aerobic, resistance and flexibility exercise on patients’ outcomes at different stages of the pathway and in multiple settings for example home, community and hospital has been researched over varying durations [16, 17]. Exercise during and after PCa treatment is safe and effective at improving important health outcomes, including improved aerobic capacity and body composition [6, 11, 18]. Varying exercise modalities have been shown to improve body composition, strength, blood pressure, blood biomarkers such as plasma triglycerides and fasting plasma glucose, aerobic capacity, quality of life and attenuate fatigue [11, 12, 19–21]. Interventions involving combined exercise modalities, such as aerobic and strength training concurrently, have been demonstrated to improve bone density [6].
Exercise opportunities should be easily accessible to men with PCa regardless of racial or ethnic minority status. Recent reports have highlighted that diversity and representation are poor in PCa clinical trials, with approximately 96% of men participating in PCa research being White [22]. There is a need to understand whether the present evidence base for exercise interventions is representative and inclusive of racial and ethnic minority men with PCa and, if not, explore the barriers and seek tailored acceptable interventions or strategies which are more inclusive. The aim of this systematic review is to examine current reporting of race and ethnicity data and strategies to increase inclusivity and demographic representation within PCa and exercise trials.
Methods
Protocol and registration
The preferred reporting items for systematic review and meta-analyses (PRISMA) guidelines were followed when conducting and reporting this prospectively registered systematic review (PROSPERO ID: CRD42022384373) [23].
Eligibility criteria
We included trials published in the English language that studied exercise interventions in PCa patients. Manuscripts were excluded if they were protocols, reports, conference abstracts, position statements or case series reports. Participants must have been aged > 16 years and have been diagnosed with PCa. Studies could include any form of exercise intervention in isolation or combined with other lifestyle factors such as nutritional supplementation or smoking cessation. Studies that included either details of participants’ ethnicity or strategies to ensure inclusion and representation were eligible.
Search strategy
The MEDLINE Ovid, Cochrane Library and PubMed databases were searched from inception to July 2024. The search strategy keywords and MeSH terms used included the following: (1) exercise, (2) training, (3) prostate cancer, (4) ethnic and (5) diversity. Reference lists of all relevant systematic reviews identified were searched for additional studies. All searches were conducted by the same author (RA), with search results collated using Rayyan software [24], and duplicates were removed. All titles, abstracts and full texts were screened by one reviewer (RA). Any uncertainty of a manuscript was resolved by a second reviewer (MF).
Data extraction
One author (RA) extracted data using Microsoft Excel which was checked by a second author (MF). Any disagreements were resolved via consensus with a third party (CR). Extracted data included study design, participant demographics, intervention details and data for all outcomes.
Results
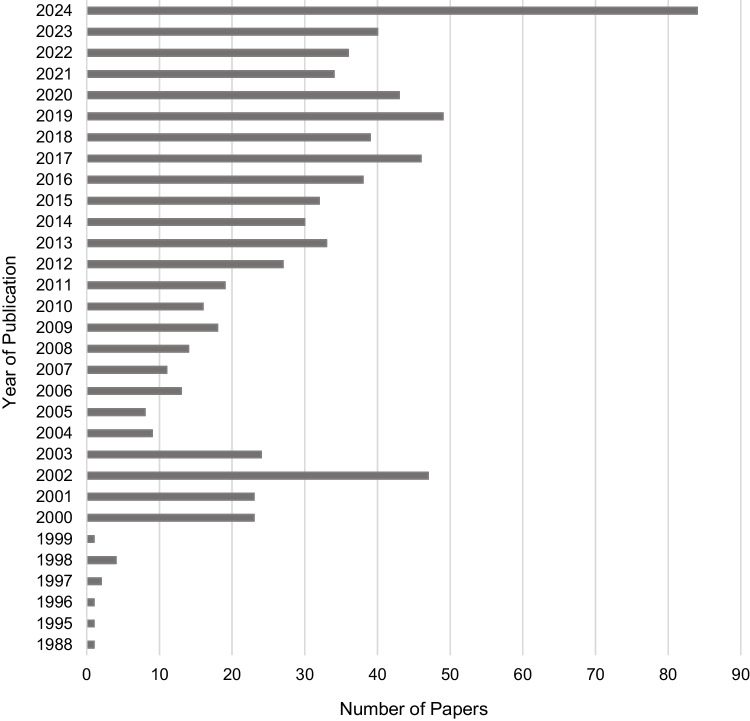
Seven hundred and seventy-eight records were retrieved from database searches, of which 15 records were duplicates. A further 649 were eliminated following a screening of titles and abstracts. After the full-text screening of 186 articles, 28 manuscripts were found to be eligible for inclusion in this review (Fig. 1) with an increase in the volume of papers published over the last three decades (Fig. 2).
Fig. 1.
PRISMA flow diagram
Fig. 2.
Volume of peer-reviewed academic articles published per year included in the screening
Characteristics of studies
The 28 studies included comprised 15,946 participants, with sample sizes ranging from 11 to 7328 per study. Summary details of the included studies and populations are presented in Table 1.
Table 1.
Characteristics of included studies
| Authors | Title | Country | Setting | Number of centres | Study Design | Treatment | Trial enrolment duration |
|---|---|---|---|---|---|---|---|
| Alibhai et al. [25] | A Phase II RCT of Three Exercise Delivery Methods in Men with PCa on ADT | CAN | Secondary, home | Multiple | RCT | ADT | 6 months |
| Ashton et al. [11] | Supported Progressive Resistance Exercise Training to Counter the Adverse Side Effects of Robot-Assisted RP: An RCT | UK | Home | Single | RCT | RP | 24 weeks |
| Au et al. [26] | Prehabilitation and Acute Postoperative Physical Activity in Patients Undergoing RP: A Secondary Analysis from An RCT | CAN | Home | Multiple | RCT | RP | Post-op day 1 until 7 days post-discharge |
| Ben-Josef et al. [27] | Yoga Intervention for Patients with PCa Undergoing External Beam RT: A Pilot Feasibility Study | USA | Secondary | Single | Feasibility | RT | 6–9 weeks dependent on the RT course |
| Brown et al. [28] | Feasibility of home‑based exercise training during adjuvant treatment for metastatic castrate‑resistant prostate cancer patients treated with an androgen receptor pathway inhibitor (EXACT) | UK | Home | Single | Feasibility | ADT | 12 weeks |
| Campo et al. [29] | Levels of Fatigue and Distress in Senior PCa Survivors Enrolled in a 12-Week RCT of Qigong | USA | Secondary, home | Single | RCT | Any | 12 weeks |
| Chan et al. [30] | Feasibility and Acceptability of a Remotely Delivered, Web-Based Behavioural Intervention for Men with PCa: Four-Arm Pilot RCT | USA | Home | Multiple | RCT | Any | 6 months |
| Dai et al. [31] | Vigorous Physical Activity is Associated with Lower Risk of Metastatic-Lethal Progression in PCa and Hypomethylation in the CRACR2A Gene | USA | Not stated | Not stated | Cohort | Any | Not stated |
| Dalla Via et al. [32] | Musculoskeletal Responses to Exercise Plus Nutrition in Men with PCa on ADT: A 12-Month RCT | AUS | Home, community | Multiple | RCT | ADT | 12 months |
| Dawson et al. [33] | Impact of Resistance Training on Body Composition and Metabolic Syndrome Variables During ADT for PCa: A Pilot RCT | USA | Home, community | Not stated | RCT | ADT | 12 weeks |
| Evans et al. [34] | Usability, Acceptability, and Safety Analysis of a Computer-Tailored Web-Based Exercise Intervention (Exercise Guide) for Individuals with Metastatic PCa: Multi-Methods Laboratory-Based Study | AUS | University | Not stated | Laboratory-based assessment | Any | Not stated |
| Fairman et al. [35] | Monitoring Resistance Exercise Intensity Using Ratings of Perceived Exertion in Previously Untrained Patients with PCa Undergoing ADT | USA | University | Single | Secondary analysis | ADT | Not stated |
| Faithfull et al. [36] | Obesity and Low Levels of Physical Activity Impact on Cardiopulmonary Fitness in Older Men After Treatment for PCa | UK | Secondary, university | Multiple | Cross-sectional | RP, RT or ADT | Single visit |
| Friedenreich et al. [37] | Physical Activity and Survival After PCa | CAN | Secondary, university | Multiple | Case–control | Any | 14 years |
| Gilbert et al. [38] | Effects of a Lifestyle Intervention on Endothelial Function in Men on Long-Term ADT for PCa | UK | Home, community | Not stated | RCT | ADT | 12 weeks |
| Hébert et al. [39] | A Diet, Physical Activity, and Stress Reduction Intervention in Men with Rising Prostate-Specific Antigen After Treatment for PCa | USA | Secondary | Multiple | RCT | RP, RT | 6 months |
| Jones et al. [40] | Effects of Nonlinear Aerobic Training on Erectile Dysfunction and Cardiovascular Function Following RP for Clinically Localized PCa | CAN | Secondary | Single | RCT | RP | 6 months |
| Langelier et al. [41] | Perceptions of Masculinity and Body Image in Men with PCa: The Role of Exercise | CAN | University | Single | Cross-sectional | Any | Not stated |
| Nilsen et al. [42] | Novel Methods for Reporting of Exercise Dose and Adherence: An Exploratory Analysis | USA | Secondary | Single | RCT | RP | 24 weeks |
| Sajid et al. [43] | Novel Physical Activity Interventions for Older Patients with PCa on Hormone Therapy: A Pilot Randomized Study | USA | Home | Not stated | RCT | ADT | 12 weeks |
| Santa Mina et al. [44] | Exercise Effects on Adipokines and The IGF Axis in Men with PCa Treated with ADT: A Randomized Study | CAN | Home | Single | Prospective, randomised trial | ADT | 24 weeks |
| Santa Mina et al. [45] | Prehabilitation for RP: A Multicentre RCT | CAN | Home | Multiple | RCT | RP | 26 weeks |
| Stolley et al. [46] | Exploring Health Behaviours, Quality of Life, and Support Needs in African-American PCa Survivors: A Pilot Study to Support Future Interventions | USA | Community | Single | Cross-sectional | Any | Not stated |
| Taaffe et al. [47] | Effects of Different Exercise Modalities on Fatigue in PCa Patients Undergoing ADT: A Year-long RCT | AUS | University, community | Multiple | RCT | ADT | 12 months |
| Trinh et al. [48] | RiseTx: Testing the Feasibility of a Web Application for Reducing Sedentary Behaviour Among PCa Survivors Receiving ADT | CAN | Secondary, home | Multiple | Prospective, single-arm | ADT | 12 weeks |
| Wang et al. [49] | Recreational Physical Activity in Relation to PCa-specific Mortality Among Men with Nonmetastatic PCa | USA | Home | Multiple | Cohort | Any | Not stated |
| Winters-Stone et al. [50] | Benefits Of Partnered Strength Training for PCa Survivors and Spouses: Results from A RCT of The Exercising Together Project | USA | University | Single | RCT | Any | 6 months |
| Wolin et al. [51] | Risk of Urinary Incontinence Following RP: The Role of Physical Activity and Obesity | USA | Secondary | Single | Cross-sectional | RP | 50–74 weeks |
CAN Canada, UK United Kingdom, AUS Australia, USA United States of America, PCa prostate cancer, RCT randomised control trial, ADT androgen deprivation therapy, RT radiotherapy, RP radical prostatectomy
The majority of studies (21/28) were performed in North America. Eleven studies (12/28) were conducted at a single site and another 11/28 recruited patients from multiple sites with the remaining 5/28 not stating the number of recruiting sites. The trial enrolment duration varied from 1 day to 14 years with 3/28 studies stating a range of lengths depending on the patient and 6/28 studies not stating the trial duration.
Narrative synthesis
All studies included reported the ethnicity of participants. Four studies 4/28 (14.3%; [28, 34, 44, 47]) only reported including Caucasian individuals and one study 1/28 (3.6%; [46]) reported only including African American patients. The remaining studies 23/28 (82.1%; [11, 25–27, 29–33, 35–43, 45]) all recruited patients from multiple ethnicities. No studies included a statement regarding inclusivity as part of the published manuscripts.
Fifteen studies 15/28 (53.6%; [11, 25, 26, 29–31, 33, 38–40, 42, 43, 45, 47, 50]) adopted a randomised control trial design, four 4/28 (14.3%; [36, 41, 46, 51]) cross-sectional, two 2/28 (7.1%; [31, 49]) cohort, two prospective 2/28 (7.1%; [44, 48]), two feasibility 2/28 (7.1%; [27, 28]), laboratory analysis 1/28 (3.6%; [34]), secondary analysis 1/28 (3.6%; [35]) and case–control study 1/28 (3.6%; [37]). Ten studies 10/28 (35.7%; [25, 28, 32, 33, 35, 38, 43, 44, 47, 48]) only recruited patients on androgen deprivation therapy (ADT), nine studies 9/28 (32.1%; [29–31, 34, 37, 41, 46, 49, 50]) involved patients on any form of treatment and six studies 6/28 (21.4%; [11, 26, 40, 42, 45, 51]) recruited those who had undergone radical prostatectomy. One study 1/28 (3.6%; [27]) recruited those receiving radiotherapy only, one study 1/28 (3.6%; [39]) recruited either radical prostatectomy or radiotherapy and a single study 1/28 (3.6%; [36]) involved either ADT, radical prostatectomy or radiotherapy patients.
The exercise elements of the studies varied, and individual study exercise details are presented in Table 2. Seven studies 7/28 (25%; [26, 28, 30, 43–45, 49]) involved home-based or unsupervised exercise. Six studies 6/28 (21.4%; [27, 29, 34, 40, 42, 50]) reported exercise sessions that were supervised by an exercise trainer or exercise physiologist and a further six studies 6/28 (21.4%; [11, 25, 32, 33, 38, 47]) had a mix of supervision over the trial period. Nine studies 9/28 (32.1%; [31, 35–37, 39, 41, 46, 48, 51]) did not explicitly state whether exercise was supervised or unsupervised. Three studies 3/28 (10.7%; [11, 35, 50]) included resistance exercise interventions, two 2/28 (7.1%; [40, 42]) included aerobic exercise interventions and a further two studies 2/28 (7.1%; [27, 29]) included yoga or flexibility programmes. Eleven studies 11/28 (39.3%; [25, 26, 28, 32, 33, 36, 38, 43–45, 47]) investigated an intervention which used a mix of exercise modalities (i.e. aerobic, resistance and flexibility). Ten studies 10/28 (35.7%; [30, 31, 34, 37, 39, 41, 46, 48, 49, 51]) were physical activity-based and primarily involved activity tracking.
Table 2.
Intervention and ethnicity data from included studies
| Authors | Age (mean ± SD) | Exercise details | Total sample size | Ethnicity |
|---|---|---|---|---|
| Alibhai et al. (2019) |
PT = 69.2 ± 7.3 Group = 71.5 ± 7.2 HOME = 69.6 ± 8.1 |
Three exercise delivery arms: (1) 1:1 supervised training (PT), (2) supervised group training (GROUP), (3) home-based smartphone-assisted training (HOME). 4–5 days per week of mixed modality exercise incorporating AET, RET and flexibility. A relative workload of 60–70% HR reserve was consistent across groups. The intensity was monitored using the 10-point RPE scale | 65 |
White (71.7%) Other (28.3%) |
| Ashton et al. (2021) |
EX = 64.6 ± 6.2 CON = 66.9 ± 6.8 |
Three weekly sessions of resistance band RET. 3 sets of 12–15 reps for 8–10 exercises targeting the major muscle groups. Exercises were performed with 30–60 s interpolated rest intervals until 3 sets of each exercise had been performed. Tapered supervision over the first 3 months | 42 |
White British (97.6%) Other (2.4%) |
| Au et al. (2019) |
EX = 61.4 ± 7.8 CON = 58.4 ± 6.1 |
Individualized, home-based, moderate-intensity AET and RET prescribed. Provided with exercise bands, an exercise mat and a stability ball to complete their program, in addition to a manual detailing their exercise prescription with supporting behaviour change strategies. Information and coaching on pelvic floor exercises to complete prior to surgery targeting earlier recovery of urinary control after surgery | 86 |
White/Caucasian (71.1%) Black/Afro-Caribbean/African (13.2%) Ashkenazi Jewish (2.6%) East and South Asian (7.9%) Arabic (2.6%) Hispanic (2.6%) |
| Ben-Josef et al. (2016) |
Mean (range) 66.4 (51–74) |
Classes were led by a trained instructor and lasted 75 min. A typical session included seated, standing and reclining poses. Yoga poses were modified and included the use of props to facilitate and adapt the poses for each participant. Sessions began with breathing and centring techniques | 15 (45 recruited) |
White (68.9%) Black (24.4%) Asian (6.7%) |
| Brown et al. (2023) | 71 ± 6 | Home-based intervention of progressive, moderate-intensity walking and RET, 2–5 times per week. RET was performed using body mass and dumbbells (or weighted household items depending on dumbbell accessibility). Participants were provided with a pedometer (Digi-Walker, Yamax) to determine step count during exercise, an exercise booklet to log sessions and RPE | 22 (30 recruited) | White (100%) |
| Campo et al. (2014) |
Median (range) EX = 72 (58–90) UC = 73 (61–93) |
The Qigong and non-aerobic stretching (UC) exercise classes were 60 min and held twice a week. Both groups received a DVD of progressive sessions of each intervention. Qigong classes were led by a certified instructor. Sessions began with a 5-min meditative focus on the breath, followed by sitting and standing exercises, and ended with a 5-min meditative focus on the breath. The study progressed with more time spent performing the standing exercises | 40 |
Non-Latino (96.6%) Latino (3.4%) White (93.1%) Non-white (6.9%) |
| Chan et al. (2020) |
Median (IQR) Level 1 = 70 (64–76) Level 2 = 70 (64–75) Level 3 = 70 (64–75) Level 4 = 70 (65–74) Total = 70 (65–75) |
Level 1 (reference group): received general information about exercise and diet and resources Level 2: same as level 1 and a personalised diet and exercise prescription, videos of recommended exercises and a weekly survey to track progress Level 3: same as level 2 and a Fitbit Alta with PA reports, supportive text messages and weekly web-based surveys to track progress Level 4: same as levels 3 and 2 optional 30-min calls with an exercise trainer/registered dietician |
202 |
White (92.6%) Black (2.5%) Asian (1.0%) Other (0.5%) More than one race (2.5%) Decline to answer (1.0%) |
| Dai et al. (2019) |
Median < 1 h per week = 60 1–3 h per week = 59 > 3 h per week = 60 |
Vigorous PA was defined to be any type of leisure time activity that lasts more than 20 min or works up for a sweat in the questionnaire. Data collected on the number of days in a week having vigorous PA in the year pre-diagnosis were analysed. Light and moderate PA data was not collected. Men were grouped into three categories of vigorous PA frequency in this analysis—greater than 3 times a week, 1–3 times a week or < 1 time per week vigorous PA | 1354 |
Caucasian (90.0%) African American (10.0%) |
| Dalla Via et al. (2021) |
EX = 71.4 ± 5.9 CON = 71.1 ± 6.6 |
Two gym-based sessions of AET, 5–6 RET exercises (2 sets, 8–12 reps at mod-hard intensity), three weight-bearing impact exercises (3 sets, 10–20 reps), two challenging balance/functional exercises (2 sets of 30–60 s) and two core stability exercises (2 sets, 10–15 reps). During the first 6 months, two weekly sessions were supervised by an exercise physiologist reduced to one. One weekly home-based session (20–60 min) using body weight and resistance bands. One sachet of a multi-nutrient supplement (powder mixed with 150 mL of water) and a vitamin D tablet was taken daily in addition to regular diet | 70 |
Caucasian (97.2%) Asian (1.4%) African (1.4%) |
| Dawson et al. (2018) |
TRAINPRO AND TRAIN = 68.6 ± 8.4 PRO AND STRETCH = 66.3 ± 9.0 |
TRAINPRO and TRAIN groups performed RET 3 days per week with a trainer. Sessions were ~ 50 min in duration and began with a 5-min warmup. Weekly training volume was divided so each muscle group was trained twice per week. PRO and STRETCH groups performed a home-based flexibility program 3 times per week. Each session matched the stretches performed by the TRAIN and TRAINPRO groups. PRO and STRETCH groups acted as CON and were given a stretching band and booklet detailing the exercises | 35 |
White (54.3%) African American (8.6%) Asian/Pacific Islander (25.7%) Hispanic (11.4%) |
| Evans et al. (2021) | 73.37 ± 6.7 | PA behaviour was measured using the modified Godin Leisure-Time Exercise Questionnaire. The weekly frequencies (longer than 15 min) of vigorous, moderate and light physical activities were weighted and summed to obtain a total score in units | 11 | Caucasian (100%) |
| Fairman et al. (2018) | 68.8 ± 9.07 | 1RM testing for chest press and leg extension after completing a warm-up set of 10–12 reps with roughly 10–20% of body weight, depending on patient characteristics and previous experience. Participants were asked to lift the weight once and to continue to perform single rep lifts with increasing weight, separated by a 3–5-min rest, until a max weight was reached | 77 |
White (92.2%) African American (6.5%) Asian (1.3%) |
| Faithfull et al. (2021) | 68.2 ± 7.4 | Grip strength measured upper body strength. A 30-s chair sit-to-stand time measured lower body strength. CPET pedalling frequency was self-selected within a given range. After a 2-min warm-up against no resistance, the intensity increased to 20–30 Watts/min. Men were encouraged to continue cycling to volitional exhaustion or a plateau in VO2 | 83 |
Caucasian (96.4%) Black British (3.6%) |
| Friedenreich et al. (2016) | Not stated | Interviews on lifetime PA were completed 4.3 ± 1.3 months post-diagnosis. The Lifetime Total Physical Activity Questionnaire was from childhood until diagnosis. Diet was reported for the year pre-diagnosis and height and weight for each decade of 20–60 years. Post-diagnosis PA was measured up to three times per participant using interviews and mail questionnaires | 830 |
White (95.0%) Other (5.0%) |
| Gilbert et al. (2016) |
EX = 70.1 ± 5.3 CON = 70.4 ± 9.2 |
Three sessions per week led by an exercise physiologist, tapering supervision over time. Sessions consisted of AET, RET and balance exercises. AET 30 min at 55–75% of age-predicted max HR or 11–13 RPE using ergometers and treadmills. RET 2–4 sets of 8–12 reps beginning at 60% 1RM. Advice on home exercise was provided. Healthy-eating seminars ~ 20 min delivered biweekly | 50 |
White (94.0%) Asian (4.0%) Black (2.0%) |
| Hébert et al. (2012) |
EX = 69.7 ± 8.8 CON = 71.1 ± 8.1 |
A single session where dietary and PA goals were discussed and set as well as 2.5-h group sessions conducted 3 times a week for the first 3 months. Monthly group booster sessions and progress calls continued for 3 months after. Participants were given daily ‘homework’ assignments that consisted of cooking, PA and stress reduction activities | 60 (47 analysed) |
White/European American (70.2%) Black/African American (29.8%) |
| Jones et al. (2014) |
EX = 58 ± 7 CON = 61 ± 5 |
AET of 72 supervised treadmill walking sessions 3 days a week. The intensity of each session alternated between five different doses of MET expenditure (i.e. VO2peak). The intensity was individualized to each patient based on workload (i.e. treadmill speed/grade) corresponding to a specific percent of VO2peak | 50 |
White (70.0%) Black (26.0%) Asian (4.0%) |
| Langelier et al. (2018) | 65.5 ± 8.5 | Questionnaires assessing demographic information, masculine values, body image, QoL and PA levels. The Godin’s Leisure Score Index of the Godin and Shephard Leisure Time Exercise Questionnaire was used to assess current PA levels | 50 |
Non-Hispanic White/Euro American (92.0%) Latino/Hispanic American (4.0%) East Asian/Indian American (2.0%) Middle Eastern/Arab American (2.0%) |
| Nilsen et al. (2018) |
EX = 58 ± 8 CON = 61 ± 5 |
AET of 72 supervised treadmill walking sessions 3 days a week. The intensity of each session alternated between five different doses of MET expenditure (i.e. VO2peak). The intensity was individualized to each patient based on workload (i.e. treadmill speed/grade) corresponding to a specific percent of VO2peak directly measured during the baseline or midpoint CPET | 50 |
White (76.0%) Black (24.0%) Asian (0.0%) |
| Sajid et al. (2016) |
Wii-Fit = 77.5 ± 6.7 EXCAP = 75.7 ± 9.5 CON = 71.8 ± 5 |
EXCAP: AET was walking at 60–70% of HR reserve and 3–5 RPE 5 days a week. Instructed to increase total daily steps by 5% and were encouraged to reach 10,000 steps a day using a pedometer. Progressive RET with bands provided low-moderate intensity 5 days a week and progressed. Wii-Fit: similar to EXCAP with a balance component and pedometer to calculate daily steps. Exercises of increasing intensity were unlocked as patients increased physical performance | 19 |
White (87.5%) African American (12.5%) |
| Santa Mina et al. (2013) |
AET = 70.6 ± 8.1 RET = 73.6 ± 8.8 |
RET group completed 10 exercises targeting major muscle groups using resistance bands, exercise mat and stability ball. Exercised 5 times per week for 60 min. AET exercised at moderate to vigorous intensity (60- 80% HR max) using a HR monitor provided. Exercised 5 times per week for 60 min | 26 | Caucasian (65.4%) |
| Santa Mina et al. (2018) |
EX = 61.2 ± 8.0 CON = 62.2 ± 6.9 |
Exercise prescriptions consisted of 60 min of unsupervised, home-based, moderate-intensity exercise 3–4 days per week. Also received an exercise manual, online videos, RET bands, stability ball, yoga mat and HR monitor. Completed daily pelvic floor muscle exercises. CON received pelvic floor exercises and lifestyle manual | 86 |
White/Caucasian (72.3%) Black/Afro-Caribbean/African (13.3%) Ashkenazi Jewish (1.2%) East and South Asian (4.8%) Southeast Asian (1.2%) Other (6.0%) Missing (1.2%) |
| Stolley et al. (2020) | 64.3 ± 4.0 | Completion of questionnaires on demographics, nutrition, physical activity patterns and QoL. Discussions focused on health behaviour change/needs, interests and preferences of lifestyle intervention. Godin Leisure Physical Activity Index asked about time spent engaged in light, moderate and strenuous PA over the past 7 days and engagement in RET per week | 22 | African American (100.0%) |
| Taaffe et al. (2017) |
ILRT = 68.9 ± 9.1 ART = 69.0 ± 9.3 DEL = 68.4 ± 9.1 |
ILRT: twice weekly supervised sessions of bounding/skipping/drop jumping/hopping/leaping. RET consisted of six exercises targeting major muscle groups. 2–4 sets of each exercise at 6–12RM. Home training twice weekly consisting of 2–4 circuits of skipping/hopping/leaping ART: twice weekly supervised sessions for the first 6 months. AET consisted of 20–30 min at 60–75% of estimated max HR using walking/jogging and stationary ergometers. RET during the initial 6 months was the same as the ILRT group. Encouraged to undertake home-based AET (e.g., walking/cycling) to accumulate 150 min/week. For the second 6 months, patients completed a home-based maintenance program UC/DEL received an information booklet about exercise for the first 6 months, followed by 6 months of twice weekly supervised exercise on a cycle ergometer at ~ 70% max HR and flexibility exercises |
159 | Caucasian (not stated) |
| Trinh et al. (2018) | 73.2 ± 7.3 | A wrist-worn activity tracker providing alerts to stand after prolonged sitting. The intervention consisted of five phases. Phases I-III (weeks 3–6) involved the progressive release of self-regulatory strategies (e.g., action planning) and changes in sitting time and step counts. Phase IV and V (weeks 9–12) received weekly reminders to encourage the use of RiseTx to practice Phases I–III strategies. Participants attempted to increase daily steps by + 1000 step increments above the previous phase | 46 |
White (80.4%) Black (8.7%) South Asian (4.3%) Southeast Asian (2.2%) Other (4.3%) |
| Wang et al. (2017) |
Median (IQR) at diagnosis 71 (67–75) |
Recreational PA per week during the past year was self-reported on the baseline questionnaire and on biennial follow-up questionnaires. METs were assigned to each of the seven activities as follows: 3.5 for walking, 3.5 for dancing, 4.0 for bicycling, 4.5 for aerobics, 6.0 for tennis or racquetball, 7.0 for jogging/running and 7.0 for lap swimming | 7328 pre-diagnosis, 5319 post-diagnosis |
White (97.4%) Black (1.5%) Other/unknown (1.1%) |
| Winters-Stone et al. (2016) |
Prostate cancer patient: EX = 70.6 ± 6.3 CON = 72.9 ± 8.0 Spouse: EX = 66.5 ± 7.2 CON = 69.7 ± 7.7 |
Couples assigned to Exercising Together (EX) attended 1-h group sessions twice a week delivered by an exercise physiologist. 5-min dynamic AET warm-up and 5–10 min stretching cool-down. RET 8–10 exercises with 8–15 reps of an exercise at intensities that progressed from 4 to 15% of body weight in a weighted vest for lower body and from a weight that could be lifted for 15 reps to a heavier weight that could be lifted for 8 reps for upper body exercises using free weights | 62 couples |
Caucasian (92.5%) Non-Hispanic (94.8%) |
| Wolin et al. (2010) |
Mean (range) 61 (39–79) |
Questionnaire on medical history and lifestyle factors including hours spent on vigorous activities (e.g., swimming, brisk walking). Urinary incontinence data were extracted at the first post-op visit at approximately 6 weeks (range 3 to 17) and at 58 weeks (range 50 to 74). Height and weight at surgery were used to calculate BMI | 589 |
White (95.0%) African American (4.0%) Hispanic (1.0%) |
SD standard deviation, EX exercise group, CON control group, AET aerobic exercise training, RET resistance exercise training, HR heart rate, IQR interquartile range, UC usual care group, RPE rating of perceived exertion, reps repetitions, s seconds, min minutes, UC usual care, PA physical activity, 1RM one repetition maximum, CPET cardiopulmonary exercise test, VO2 aerobic capacity, MET metabolic equivalent, QoL quality of life, ILRT impact loading and resistance training, ART aerobic and resistance training, DEL delayed exercise
Risk of bias
Risk of bias was assessed on all randomised studies and conducted by one author (RA). The Cochrane Risk of Bias tool [52] was used with risk of bias on the study level classified as ‘low’, ‘unclear’ or ‘high’ risk [53]. Table 3 shows a summary of the risk of bias for each of the included studies.
Table 3.
Risk of bias
| Authors | Bias arising from the randomization process | Bias arising from deviations from the intervention | Bias due to missing data | Bias in measurement of outcome | Bias in selection of reported result | Overall risk of bias |
|---|---|---|---|---|---|---|
| Alibhai et al. (2019) | - | - | - | - | ? | ? |
| Ashton et al. (2021) | - | - | - | ? | ? | ? |
| Au et al. (2019) | - | ? | ? | ? | ? | ? |
| Campo et al. (2014) | ? | ? | - | - | ? | ? |
| Chan et al. (2020) | - | ? | - | - | ? | ? |
| Dalla Via et al. (2021) | - | ? | - | ? | ? | ? |
| Dawson et al. (2018) | - | ? | - | ? | ? | ? |
| Fairman et al. (2018) | ? | ? | ? | ? | ? | ? |
| Gilbert et al. (2016) | - | ? | - | - | ? | ? |
| Hébert et al. (2021) | ? | ? | - | ? | ? | ? |
| Jones et al. (2014) | ? | ? | - | - | ? | ? |
| Nilsen et al. (2018) | - | - | - | ? | ? | ? |
| Sajid et al. (2016) | - | - | - | - | ? | ? |
| Santa Mina et al. (2013) | - | ? | - | ? | ? | ? |
| Santa Mina et al. (2018) | - | ? | - | ? | ? | ? |
| Taaffe et al. (2017) | - | ? | - | ? | ? | ? |
| Winters-Stone et al. (2016) | - | - | - | - | ? | ? |
+, high risk; ?, unclear risk; -, low risk
Discussion
Summary of findings
This review highlights the lack of reporting of patient ethnicity in PCa clinical trials involving exercise. Only 28 manuscripts reported ethnicity and none of the studies included a statement regarding strategies for ensuring inclusion or representative sampling. For example, studies may want to consider who the under-served groups are within the delivery area and barriers they face, plan for digital exclusion, recruit a sample who represents those who live with the specific condition, use language carefully or involve those under-served in the planning phase [54]. In the papers included in this review, it is evident that there is inconsistency in how ethnicity is reported making the results of the studies difficult to apply to the general PCa population. Accordingly, there is a greater need for those developing and running clinical trials in PCa and subsequently academic research outputs to adopt standardised terminology when it comes to describing and reporting the race and ethnicity of participants in exercise research, for example using those listed on countries official websites [55, 56]. To the authors’ knowledge, this is the first systematic review investigating ethnicity reporting in PCa and exercise trials and it therefore acts as a baseline for future practice.
Only one pilot trial has been included in this review that specifically recruited black men and this was in an African American community population. This mixed-methods study explored the quality of life, dietary and physical activity habits of African American PCa survivors [46]. The findings suggest that exercise interventions involving supervised strength training that are group-based; increase knowledge, skills-building and social support; address financial challenges and are easily accessed; these exercise interventions will help address some of the barriers in this underserved population [46]. Recognising that black men are at an increased risk of PCa, there is a clear need to design trials to fulfil the needs of black men, or at the very least further investigate qualitatively their views on exercise interventions and barriers. A study in 2017 explored the acceptability, barriers and facilitators to lifestyle interventions in African Caribbean PCa survivors and found that a PCa diagnosis, alongside ageing, heightened men’s awareness of their health [57]. They concluded that lifestyle interventions which enhance men’s independence and are framed as helping to regain fitness and aid post-treatment recovery are appealing and acceptable to African Caribbean PCa survivors [57].
Some of the barriers to research participation have been explored in other clinical populations and include a lack of childcare, mistrust, financial constraints, relatives’ influence and beliefs, lack of communication and cultural awareness between research staff and patients [58, 59]. The under-representation of ethnic minority groups in clinical trials affects the generalisability of study findings and ultimately contributes to exacerbating bias and inequities in access to healthcare if public health policies based on such evidence are implemented. It is possible that different ethnic groups respond in distinct ways to an intervention due to variations in physiology and/or disease state. Therefore, by studying the effects of an intervention in multiple ethnic groups, we can be sure that the outcomes are applicable to all.
Strengths and limitations
This systematic review has been conducted rigorously with regard to methodology and in line with the PRISMA guidelines. However, the main findings of this systematic review need to be considered in the context of some key limitations. For example, it is difficult to ascertain reasons for low recruitment numbers and, even when ethnicity was reported, recruitment strategy was not acknowledged in the manuscripts. Additionally, we must acknowledge that the lack of diversity may be due to the single centre studies being conducted in predominantly Caucasian areas, however, it is important that this is considered in their limitations section. More work is needed therefore to understand the barriers and facilitators of different ethnic groups to exercise research. Furthermore, recruitment strategies to ensure an inclusive sample need to be employed alongside reporting of participant ethnicities needs to be improved,
Implications for research and practice
The findings from this systematic review demonstrate that whilst there are many studies into the benefits of exercise within PCa patients, there are clear disparities between studies on the reporting of participants’ ethnicities and overall low numbers of ethnic minority men included. This systematic review suggests that representation is achieved in a few trials. Work needs to be performed to understand why representation is lacking in PCa exercise trials in the UK and action is needed to address this. Future studies may want to explore the barriers patients from different ethnic groups face when taking part in clinical trials and any potential bias within the recruitment process. Studies should include defined strategies to recruit a representative study sample and report this within the methods section of the manuscript in accordance with NIHR INCLUDE [54] to ensure the results are applicable and representative of the patient group. Additionally, authors should clearly report the ethnicity of participants within the demographic information of manuscripts to allow the reader to properly interpret the results in the context of the patients included.
Conclusion
This systematic review highlights that there is high heterogeneity in the reporting of participants’ ethnicity within PCa trials involving exercise. Additionally, it has also demonstrated that there are low numbers of ethnic minority men included in PCa and exercise studies in the UK and a lack of reporting of ethnicities in published papers. As such, further work is required to understand why representation is lacking within PCa exercise trials in the UK and strategies are needed to achieve representation. Future studies should seek to explore the barriers PCa patients from different ethnic groups face when taking part in clinical trials in the UK.
Author contribution
All authors contributed to the study conception and design. RA conducted the database searches with data extraction supported by MF. All authors contributed to the writing of the manuscripts. All authors read and approved the final manuscript.
Funding
This project was partially supported by the University of Derby Knowledge Exchange Impact Accelerator Fund.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cancer Research UK. Prostate cancer statistics. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer#heading-Zero. Accessed 17 July 2024
- 2.Wang L et al (2022) Prostate cancer incidence and mortality: global status and temporal trends in 89 countries from 2000 to 2019. Front Public Health 10:811044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paterson C et al (2015) Identifying the unmet supportive care needs of men living with and beyond prostate cancer: a systematic review. Eur J Oncol Nurs 19(4):405–418 [DOI] [PubMed] [Google Scholar]
- 4.Paterson C et al (2022) The effects of multimodal prehabilitation interventions in men affected by prostate cancer on physical, clinical and patient reported outcome measures: a systematic review. Semin Oncol Nurs 38(5):151333 [DOI] [PubMed] [Google Scholar]
- 5.Aning JJ et al (2018) Detailed analysis of patient-reported lower urinary tract symptoms and effect on quality of life after robotic radical prostatectomy. Urol Oncol 36(8):364.e15-364.e22 [DOI] [PubMed] [Google Scholar]
- 6.Campbell KL et al (2019) Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc 51(11):2375–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowder D et al (2022) Racial disparities in prostate cancer: a complex interplay between socioeconomic inequities and genomics. Cancer Lett 531:71–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lloyd T et al (2015) Lifetime risk of being diagnosed with, or dying from, prostate cancer by major ethnic group in England 2008–2010. BMC Med 13(1):171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delon C et al (2022) Differences in cancer incidence by broad ethnic group in England, 2013–2017. Br J Cancer 126(12):1765–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chornokur G et al (2011) Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer. Prostate 71(9):985–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashton RE et al (2021) Supported progressive resistance exercise training to counter the adverse side effects of robot-assisted radical prostatectomy: a randomised controlled trial. Support Care Cancer 29(8):4595–4605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bourke L et al (2018) Exercise training as a novel primary treatment for localised prostate cancer: a multi-site randomised controlled phase II study. Sci Rep 8(1):8374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banerjee S et al (2018) Vigorous intensity aerobic interval exercise in bladder cancer patients prior to radical cystectomy: a feasibility randomised controlled trial. Support Care Cancer 26(5):1515–1523 [DOI] [PubMed] [Google Scholar]
- 14.Mottet N, Cornford P, van den Bergh RCN, Briers E, Expert Patient Advocate, De Santis M, Gillessen S, Grummet J, Henry AM, van der Kwast TH, Lam TB, Mason MD, O'Hanlon S, Oprea-Lager De, Ploussard G, van der Poel HG, Rouviere O, Schoots IG, Tilki D, Wiegel T (2022) EAU guidelines on prostate cancer. Available from: https://uroweb.org/guidelines/prostate-cancer/chapter/introduction. Accessed 12 Feb 2024
- 15.NICE (2014) Physical activity: exercise referral schemes - public health guideline [PH54]. Available from: https://www.nice.org.uk/guidance/ph54. Accessed 12 Feb 2024
- 16.Bourke L et al (2016) Exercise for men with prostate cancer: a systematic review and meta-analysis. Eur Urol 69(4):693–703 [DOI] [PubMed] [Google Scholar]
- 17.Andersen MF, J Midtgaard, ED Bjerre (2022) Do patients with prostate cancer benefit from exercise interventions? A systematic review and meta-analysis. Int J Environ Res Public Health 19(2): 972 [DOI] [PMC free article] [PubMed]
- 18.Hart NH, Galvão DA, Newton RU (2017) Exercise medicine for advanced prostate cancer. Curr Opin Support Palliat Care 11(3):247–257 [DOI] [PubMed] [Google Scholar]
- 19.Hasenoehrl T et al (2015) The effects of resistance exercise on physical performance and health-related quality of life in prostate cancer patients: a systematic review. Support Care Cancer 23(8):2479–2497 [DOI] [PubMed] [Google Scholar]
- 20.Keilani M et al (2017) Effects of resistance exercise in prostate cancer patients: a meta-analysis. Support Care Cancer 25(9):2953–2968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hvid T et al (2016) Effect of a 2-year home-based endurance training intervention on physiological function and PSA doubling time in prostate cancer patients. Cancer Causes Control 27(2):165–174 [DOI] [PubMed] [Google Scholar]
- 22.Rencsok EM et al (2020) Diversity of enrollment in prostate cancer clinical trials: current status and future directions. Cancer Epidemiol Biomarkers Prev 29(7):1374–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Page MJ et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan — a web and mobile app for systematic reviews. Syst Rev 5:210 [DOI] [PMC free article] [PubMed]
- 25.Alibhai SMH et al (2019) A phase II randomized controlled trial of three exercise delivery methods in men with prostate cancer on androgen deprivation therapy. BMC Cancer 19(1):2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Au D et al (2019) Prehabilitation and acute postoperative physical activity in patients undergoing radical prostatectomy: a secondary analysis from an RCT. Sports Med Open 5(1):18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ben-Josef AM et al (2016) Yoga intervention for patients with prostate cancer undergoing external beam radiation therapy: a pilot feasibility study. Integr Cancer Ther 15(3):272–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown M, Murphy MH, McAneney H, McBride K, Crawford F, Cole A, O'Sullivan JM, Jain S, Prue G (2023) Feasibility of home‑based exercise training during adjuvant treatment for metastatic castrate‑resistant prostate cancer patients treated with an androgen receptor pathway inhibitor (EXACT). Supportive Care Cancer 31(7):442 [DOI] [PMC free article] [PubMed]
- 29.Campo RA et al (2014) Levels of fatigue and distress in senior prostate cancer survivors enrolled in a 12-week randomized controlled trial of Qigong. J Cancer Surviv 8(1):60–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan JM et al (2020) Feasibility and acceptability of a remotely delivered, web-based behavioral intervention for men with prostate cancer: four-arm randomized controlled pilot trial. J Med Internet Res 22(12):e19238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai JY et al (2019) Vigorous physical activity is associated with lower risk of metastatic-lethal progression in prostate cancer and hypomethylation in the CRACR2A gene. Cancer Epidemiol Biomarkers Prev 28(2):258–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalla Via J et al (2021) Musculoskeletal responses to exercise plus nutrition in men with prostate cancer on androgen deprivation: a 12-month RCT. Med Sci Sports Exerc 53(10):2054–2065 [DOI] [PubMed] [Google Scholar]
- 33.Dawson JK et al (2018) Impact of resistance training on body composition and metabolic syndrome variables during androgen deprivation therapy for prostate cancer: a pilot randomized controlled trial. BMC Cancer 18(1):368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evans HE et al (2021) Usability, acceptability, and safety analysis of a computer-tailored web-based exercise intervention (ExerciseGuide) for individuals with metastatic prostate cancer: multi-methods laboratory-based study. JMIR Cancer 7(3):e28370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fairman CM et al (2018) Monitoring resistance exercise intensity using ratings of perceived exertion in previously untrained patients with prostate cancer undergoing androgen deprivation therapy. J Strength Cond Res 32(5):1360–1365 [DOI] [PubMed] [Google Scholar]
- 36.Faithfull S et al (2021) Obesity and low levels of physical activity impact on cardiopulmonary fitness in older men after treatment for prostate cancer. Eur J Cancer Care (Engl) 30(6):e13476 [DOI] [PubMed] [Google Scholar]
- 37.Friedenreich CM et al (2016) Physical activity and survival after prostate cancer. Eur Urol 70(4):576–585 [DOI] [PubMed] [Google Scholar]
- 38.Gilbert SE et al (2016) Effects of a lifestyle intervention on endothelial function in men on long-term androgen deprivation therapy for prostate cancer. Br J Cancer 114(4):401–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hébert JR et al (2012) A diet, physical activity, and stress reduction intervention in men with rising prostate-specific antigen after treatment for prostate cancer. Cancer Epidemiol 36(2):e128–e136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones LW et al (2014) Effects of nonlinear aerobic training on erectile dysfunction and cardiovascular function following radical prostatectomy for clinically localized prostate cancer. Eur Urol 65(5):852–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Langelier DM et al (2018) Perceptions of masculinity and body image in men with prostate cancer: the role of exercise. Support Care Cancer 26(10):3379–3388 [DOI] [PubMed] [Google Scholar]
- 42.Nilsen TS et al (2018) Novel methods for reporting of exercise dose and adherence: an exploratory analysis. Med Sci Sports Exerc 50(6):1134–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sajid S et al (2016) Novel physical activity interventions for older patients with prostate cancer on hormone therapy: a pilot randomized study. J Geriatr Oncol 7(2):71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santa Mina D et al (2013) Exercise effects on adipokines and the IGF axis in men with prostate cancer treated with androgen deprivation: a randomized study. Can Urol Assoc J 7(11–12):E692–E698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santa Mina D et al (2018) Prehabilitation for radical prostatectomy: a multicentre randomized controlled trial. Surg Oncol 27(2):289–298 [DOI] [PubMed] [Google Scholar]
- 46.Stolley MR et al (2020) Exploring health behaviors, quality of life, and support needs in African-American prostate cancer survivors: a pilot study to support future interventions. Support Care Cancer 28(7):3135–3143 [DOI] [PubMed] [Google Scholar]
- 47.Taaffe DR et al (2017) Effects of different exercise modalities on fatigue in prostate cancer patients undergoing androgen deprivation therapy: a year-long randomised controlled trial. Eur Urol 72(2):293–299 [DOI] [PubMed] [Google Scholar]
- 48.Trinh L et al (2018) RiseTx: testing the feasibility of a web application for reducing sedentary behavior among prostate cancer survivors receiving androgen deprivation therapy. Int J Behav Nutr Phys Act 15(1):49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y et al (2017) Recreational physical activity in relation to prostate cancer-specific mortality among men with nonmetastatic prostate cancer. Eur Urol 72(6):931–939 [DOI] [PubMed] [Google Scholar]
- 50.Winters-Stone KM et al (2016) Benefits of partnered strength training for prostate cancer survivors and spouses: results from a randomized controlled trial of the Exercising Together project. J Cancer Surviv 10(4):633–644 [DOI] [PubMed] [Google Scholar]
- 51.Wolin KY et al (2010) Risk of urinary incontinence following prostatectomy: the role of physical activity and obesity. J Urol 183(2):629–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Higgins JPT et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Julian PT Higgins, JT Jacqueline Chandler, Miranda Cumpston, Tianjing Li, Matthew J Page, Vivian A Welch (2019) Cochrane Handbook for systematic reviews of interventions, 2nd ed, Wiley-Blackwell [DOI] [PMC free article] [PubMed]
- 54.NIHR (2020) Improving inclusion of under-served groups in clinical research: guidance from the NIHR-INCLUDE project. UK: NIHR. 14th March 2023]. Available from: www.nihr.ac.uk/documents/improving-inclusion-of-under-served-groups-in-clinical-research-guidance-from-include-project/25435. Accessed 12 Feb 2024
- 55.Jenson E, Jones N, Orozco K, Medina L, Peery M, Bolender B, Battle K (2022) Measuring racial and ethnic diversity for the 2020 census. Available from: https://www.census.gov/newsroom/blogs/random-samplings/2021/08/measuring-racial-ethnic-diversity-2020-census.html. Accessed 23 July 2024
- 56.UK Government (N.D) List of ethnic groups. Available from: https://www.ethnicity-facts-figures.service.gov.uk/style-guide/ethnic-groups/. Accessed 23 July 2024
- 57.Er V et al (2017) Barriers and facilitators to healthy lifestyle and acceptability of a dietary and physical activity intervention among African Caribbean prostate cancer survivors in the UK: a qualitative study. BMJ Open 7(10):e017217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Waheed W et al (2015) Overcoming barriers to recruiting ethnic minorities to mental health research: a typology of recruitment strategies. BMC Psychiatry 15(1):101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duma N et al (2018) Representation of minorities and women in oncology clinical trials: review of the past 14 years. J Oncol Pract 14(1):e1–e10 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.