Abstract
Living kidney donors have been regarded as those people having earned the healthiest status level after having undergone scrutiny. Although one’s post-donation GFR is expected to fall to 50% of their pre-donation value, it is well documented that there is a compensatory increase in GFR which subsequently reaches approximately 60–70% of the donor’s pre-donation value. Data regarding gout/hyperuricemia in living kidney donors has remained scarce until now. This study involved kidney donors enrolled within the years 2000 to 2017, where those who were selected to be matched to those in group of case cohort by age, year of index date, gender and co-morbidity were considered as the control cohort. During the 17-year study period 2,716 participants were enrolled. Results revealed that kidney donors experienced a risk of new onset gout/ hyperuricemia (adjusted HR = 1.73; 95%CI = 1.27, 2.36), and new onset CKD (adjusted HR = 6.7; 95% CI = 4.4, 10.21) were found to be higher in kidney donors. Our findings suggest that people after kidney donation are significantly associated with a higher risk of new onset gout/hyperuricemia. Clinical professionals therefore need to be cautious of new onset gouy/hyperuricemia after donation surgery.
Keywords: Gout/hyperuricemia, Chronic kidney disease, Living kidney donors, Hypertension, Case cohort
Subject terms: Medical research, Nephrology, Risk factors
Introduction
Renal transplantation has been considered a favorable treatment for patients with end stage renal disease (ESRD) as compared with those undergoing treatment of dialysis1–3.
Generally speaking, living kidney donors have been regarded as the particular group within a population who have earned the healthiest status level after undergoing scrutiny. Upon completing complex medical examinations and undergoing surgery involving extensive detailed screening it has been determined that these donors have reached the healthiest status level when compared to those in the general population.
In general, one’s post-donation GFR is expected to fall to 50% of their pre-donation value, however, it is well documented that there is a compensatory increase in GFR which subsequently reaches approximately 60–70% of the donor’s pre-donation value4,21.
This compensatory increase in GFR results from a parallel increase in renal plasma flow (RPF), as well as pressure across the glomerular filtration barrier (Kf). This in turn leads to a modest increase in glomeruli hydraulic pressure, presumably not causing any damage to the glomerulus within the residual single kidney5,6 It has been found that the donor’s Glomerular filtration rate (GFR) after donation actually increases modestly over a period of time rather than declining, while that rate has been observed to decay in the general population, attributing to the aging process5 As a result, it has been optimistically believed that over the long-term, renal failure risk after living kidney donation was minimal to non-existent5
However, conflicting evidence exists that whether people after kidney donation are associated with a risk of mortality, ESRD, hypertension and pre-eclampsia7–15.
Likewise, uric acid is one of the major metabolites predominately excreted through the kidneys; therefore it is reasonably presumed that people after kidney donation should not be associated with disorders in uric acid metabolism.
However, metabolic disorders have been suggested to develop in people after kidney donation, including gout/hyperuricemia, kidney stones and hypertension, all of which are relevant to progression of renal failure2,16,17.
Data to elucidate on the association of new onset gout/hyperuricemia and CKD risk in living kidney donors remains scarce. Through use of a comprehensively integrated clinical database, we aim to determine the association between new onset gout/hyperuricemia and risk of end stage renal disease (ESRD) post-donation in living kidney donors.
Methods
Data source
In 1995, Taiwan’s government initiated a national health program named National Health Insurance (NHI). Its aim was to improve the national health and medical care within Taiwan. Medical records then began being stored in the National Health Insurance Research Database (NHIRD), which consists of data surrounding the demographic characteristics of the insured, records of inpatients and all outpatient orders, as well as the medicine and treatment provided from all medical care settings for more than 99% of the 23 million Taiwanese. The diagnostic codes of patients were based on the International Classification of Diseases, 9th and 10th edition, Clinical Modification (ICD-9-CM and ICD-10-CM).
Ethics statement
This study was approved by the Ethics Committee of China Medical University Hospital [Approval number: CMUH109-REC2-031(CR-2)] and conducted in accordance with the ethical principles of the Declaration of Helsinki. As the Taiwan National Health Insurance dataset is comprised of previously de-identified data for research usage, with all personal identifiable information being encrypted. Due to the retrospective nature of the study and anonymization of all data prior to analysis, the need for informed consent was waived by the Institutional Review Board of the NHRIs. All methods were performed in accordance with the applicable guidelines and regulations.
Study population
In present study, patients who had been diagnosed as being potential kidney donors through the disease codes ICD-9 and ICD-10. In Taiwan, people who have any stage of CKD would be totally excluded for living kidney donation. Patients with coding V59.4 in ICD-9 as well as Z52.4 in ICD-10 within the years 2000 to 2017 were enrolled as the case cohort18 On the other hand, those who do not have the target codes (V59.4 or Z52.4) were selected to match with the case cohort by gender, age (in every 5-year interval), year of index date, income in every month, urban level, hypertension, obesity, hyperlipidemia and chronic obstructive pulmonary disease (COPD) in order to find the control cohort. A ratio of 1:1 through propensity score matching analysis was applied18 The first prescription date of the donor has been defined as the index data of renal donors and that patient who were non-kidney donors was set as a random date after January 1, 2000. Patients younger than 20 years of age, those diagnosed with diabetes mellitus, arrhythmia, congestive heart failure or osteoporosis prior to the index date, along with those experiencing acute pyelonephritis (APN), gout, hyperuricemia, urinary tract stone calculi, hydro nephrons or chronic kidney disease (CKD) prior to the index date, as well as those with missing data regarding gender or age were all eliminated.
Main outcomes and covariates
The development of gout/hyperuricemia, urinary tract stone calculi, CKD and end stage renal disease (ESRD) were all outcomes in our research. The covariates contained gender, age (20–34, 35–49, ≥ 50 years), monthly income (NT$ < 20,000, 20,000–38,999, ≥ 39,000), and urban level, which were all compared between the kidney donors and controls. The related comorbidities consisted of hypertension (ICD-9: 401–405 and ICD-10: I10-I15), hyperlipidemia (ICD-9: 272 and ICD-10: E78), and chronic obstructive pulmonary disease (COPD) (ICD-9: 491, 492, 496 and ICD-10: J41, J42, J43, J44), which had occurred prior to the index date. Furthermore, we also considered any related drug use, including Angiotensin-converting enzyme inhibitors (ACEIs), Angiotensin receptor blockers (ARBs), Fenofibrate and Statins, as confounding factors.
Statistical analysis
We demonstrated the categorical data of the study participants as both number and percentage, while the continuous variables were presented as mean and standard deviation (SD). The differences seen in all variables between the two cohorts were compared by the standardized mean difference (SMD), where the two cohorts were considered to have a negligible difference if the SMD value was less than 0.1. The incidence rates for both cohorts were calculated as the number of occurrences of outcomes divided by the total sum during the follow-up year (per 1000 person-years). The Cox proportional hazard regression model was used to estimate the unadjusted and multivariable-adjusted hazard ratios (cHR and aHR), with corresponding 95% confidence intervals (CIs) used for the risk between the two cohorts. We analyzed the cumulative incidence curves of the outcomes in kidney donors and comparison cohorts using the Kaplan–Meier method, while the log-rank test was utilized to compare the differences. All statistical analyses were presented using software SAS, version 9.4, while plotting was performed using software R, version 4.0. A significant level was determined to be a p-value less than 0.05.
Results
From 2000 to 2017, a total of 2716 patients were included in the present study, where both the kidney donor and non-donor groups contained 1358 subjects. The proportion of baseline characteristics, including gender, age, income in every month, the living level of urban, associated comorbidities and medications are shown in Table 1. As the table shows, female participants outnumbered male participants in both groups. Subjects aged above 50 years and those aged 35 to 49 were in the majority in the control and kidney donor groups, respectively. The distribution of urban levels for the two groups was significantly different, with both cohorts mainly being composed of subjects at a median level. The proportions of hypertension (28.72% vs 10.97%), hyperlipidemia (25.55% vs 9.20%) and COPD (21.94% vs 4.93%) were with a high level in the control group compared to that of the kidney donor group. Compared to the controls, fewer Renin angiotensin system inhibitor (RAS blockade) (non-donors: 30.71% vs donors: 14.21%) as well as statin (non-donors: 7.22% vs donors: 3.39%) users were presented in the kidney donor group.
Table 1.
Demographic and comorbidity comparison of kidney donors and nondonors at baseline.
| Variable | Kidney donors | SMD | |||
|---|---|---|---|---|---|
| No | Yes | ||||
| n = 1358 | n = 1358 | ||||
| n | % | n | % | ||
| Gender | |||||
| Female | 728 | 53.61 | 706 | 51.99 | 0.033 |
| Male | 630 | 46.39 | 652 | 48.01 | 0.033 |
| Age, years | |||||
| 20–34 | 398 | 29.31 | 435 | 32.03 | 0.059 |
| 35–49 | 373 | 27.47 | 521 | 38.37 | 0.234 |
| ≥ 50 | 587 | 43.23 | 402 | 29.60 | 0.286 |
| Mean, (SD) | 44.37 | (15.06) | 41.19 | (12.54) | 0.230 |
| Income, NTD/mo | |||||
| < 20,000 | 468 | 34.46 | 485 | 35.71 | 0.026 |
| 20,000–38,999 | 572 | 42.12 | 608 | 44.77 | 0.054 |
| ≥ 39,000 | 318 | 23.42 | 265 | 19.51 | 0.095 |
| Level of urbanization | |||||
| 1(highest) | 228 | 16.79 | 98 | 7.22 | 0.298 |
| 2 | 579 | 42.64 | 642 | 47.28 | 0.093 |
| 3(lowest) | 551 | 40.57 | 618 | 45.51 | 0.100 |
| Comorbidities | |||||
| Hypertension | 390 | 28.72 | 149 | 10.97 | 0.456 |
| Hyperlipidemia | 347 | 25.55 | 125 | 9.20 | 0.442 |
| COPD | 298 | 21.94 | 67 | 4.93 | 0.515 |
| Medication | |||||
| ACEI/ARB | 417 | 30.71 | 193 | 14.21 | 0.403 |
| Statins | 98 | 7.22 | 46 | 3.39 | 0.172 |
| Follow up, Mean (SD) | |||||
| APN | 8.50 | (4.83) | 5.74 | (5.36) | 0.540 |
| Gout, hyperuricemia | 8.28 | (4.85) | 5.54 | (5.26) | 0.541 |
| Urinary tract stone calculi | 8.42 | (4.87) | 5.89 | (5.40) | 0.493 |
| Hydro nephrons | 8.76 | (4.85) | 5.97 | (5.45) | 0.541 |
| CKD | 8.76 | (4.83) | 5.64 | (5.42) | 0.606 |
| ESRD | 8.85 | (4.87) | 6.00 | (5.46) | 0.551 |
| Osteoporosis | 8.63 | (4.88) | 5.90 | (5.42) | 0.529 |
SMD: standardized mean difference; SD: standard deviation.
The follow-up times for gout/hyperuricemia (8.28 ± 4.85 vs 5.54 ± 5.26), urinary tract stone calculi (8.42 ± 4.87 vs 5.89 ± 5.40), CKD (8.76 ± 4.83 vs 5.64 ± 5.42), ESRD (8.85 ± 4.87 vs 6.00 ± 5.46), hydro nephrons (8.76 ± 4.85 vs 5.97 ± 5.45), APN (8.50 ± 4.83 vs 5.74 ± 5.36) and osteoporosis (8.63 ± 4.88 vs 5.90 ± 5.42) within the kidney non-donor group were longer than that seen in the kidney donor group.
After being adjusted for associated factors, the risks for new onset gout/hyperuricemia (adjusted HR = 1.73; 95%CI = 1.27, 2.36), chronic kidney disease (adjusted HR = 6.70; 95% CI = 4.40, 10.21), and new onset gout/hyperuricemia with CKD (adjusted HR = 7.15; 95%CI = 2.94, 17.35) were remarkably high in the kidney donor group when compared to the controls, as seen in Table 2.
Table 2.
Incidence of outcomes in kidney donors and nondonors.
| Outcome | Kidney donors | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | |||||||||||
| N | PY | IR§ | N | PY | IR§ | cHR | (95% CI) | p-value | aHR$ | (95% CI) | p-value | |
| Gout/hyperuricemia | 112 | 11,244.69 | 9.96 | 92 | 7528.87 | 12.22 | 1.21 | (0.92, 1.60) | 0.172 | 1.73 | (1.27, 2.36)*** | < 0.001 |
| Gout with CKD | 11 | 11,971.71 | 0.92 | 22 | 8050.09 | 2.73 | 3.11 | (1.51, 6.42)** | 0.002 | 7.15 | (2.94, 17.35)*** | < 0.001 |
| CKD | 40 | 11,892.49 | 3.36 | 121 | 7665.10 | 15.79 | 4.68 | (3.27, 6.69)*** | < 0.001 | 6.7 | (4.4, 10.21)*** | < 0.001 |
| ESRD | n < 3 | 0.25 | n < 3 | 0.25 | 1.03 | (0.17, 6.19) | 0.9711 | 4.17 | (0.32, 55.12) | 0.278 | ||
| Urinary tract stone calculi | 77 | 11,438.16 | 6.73 | 27 | 7994.57 | 3.38 | 0.50 | (0.32, 0.78)** | 0.002 | 0.65 | (0.4, 1.05) | 0.076 |
| Hydro nephrons | 28 | 11,893.00 | 2.35 | 16 | 8101.80 | 1.97 | 0.85 | (0.46, 1.57) | 0.608 | 0.91 | (0.45, 1.82) | 0.783 |
| Osteoporosis | 46 | 11,721.27 | 3.92 | 21 | 8018.59 | 2.62 | 0.67 | (0.40, 1.12) | 0.124 | 1.17 | (0.63, 2.17) | 0.626 |
| APN | 158 | 11,538.10 | 13.69 | 113 | 7797.04 | 14.49 | 1.08 | (0.85, 1.37) | 0.539 | 1.26 | (0.95, 1.66) | 0.111 |
PY: person-years; IR: incidence rate; CI: confidence interval; HR: hazard ratio.
n < 3: In order to avoid the issue of security and security, the government stipulates that if the data variable unit is less than 3 units or the value of less than 3 units can be calculated, the data cannot be carried.
§: Per 1000 person-year.
$: Adjusted HR estimated by the model including the variables of gender, age, monthly income, level of urbanization, comorbidities, and medications.
*p < 0.05, **p < 0.01, ***p < 0.001.
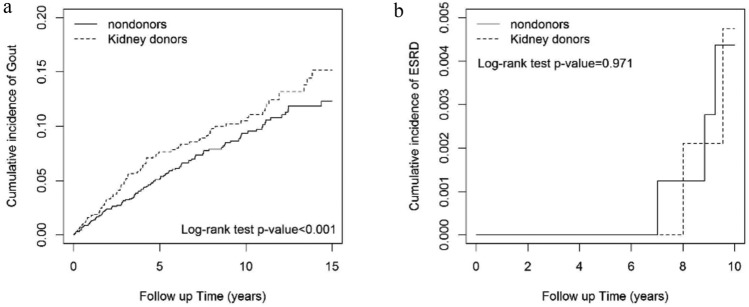
A higher incidence rates was noted in people after kidney donation while compared to those without donation both in gout/hyperuricemia and ESRD which was statically significantly (Fig. 1).
Fig. 1.
The cumulative incidence of gout/hyperuricemia, and end stage renal disease (ESRD) for kidney donors was significantly higher than that seen in the non-donor cohort. (a) Cumulative incidence of Gout in kidney donors and controls (b) Cumulative incidence of ESRD in kidney donors and controls.
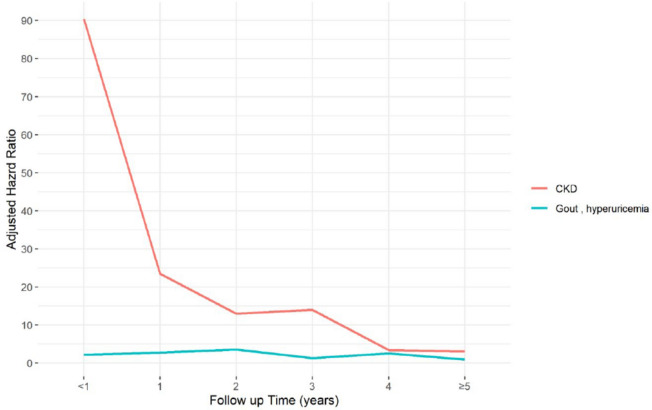
Due to its long duration, the follow-up period for the study was divided into six groups, < 1 year, 1–2, 2–3, 3–4, 4–5 years, and ≥ 5 years. Table 3 lists the risk of gout/hyperuricemia and CKD among the two cohorts for the different follow-up times. Compared with the kidney non-donors, the risk of new onset gout/hyperuricemia for kidney donors was higher (adjusted HR = 2.22; 95%CI = 1.03, 4.79) less than 1 year after the index date, as well as at 1–2 years after the index date (adjusted HR = 2.73; 95%CI = 1.23, 6.10). It further reached its peak 2–3 years after the index date (adjusted HR = 3.59; 95%CI = 1.41, 9.15). In addition, kidney donors experienced a higher risk of developing CKD than non-kidney donors within 1 year after the index date (adjusted HR = 83.20; 95%CI = 10.73, 646.31) and trended to decrease thereafter, reaching a risk level of 3.16 more than five years after the index date (95%CI = 1.77, 5.62). The trend surrounding the risk of gout/hyperuricemia and CKD is presented in Fig. 2. The flow chart of the study sample selection from Taiwan’s NHIRD is shown as Fig. 3.
Table 3.
The risk of outcomes by follow-up years in kidney donors and nondonors.
| Outcome | cHR | (95% CI) | p-value | aHR$ | (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Gout, hyperuricemia | ||||||
| < 1 year | 1.37 | (0.70, 2.69) | 0.358 | 2.22 | (1.03, 4.79)* | 0.043 |
| 1–2 years | 1.38 | (0.67, 2.82) | 0.379 | 2.73 | (1.23, 6.10)* | 0.014 |
| 2–3 years | 2.22 | (0.96, 5.13) | 0.062 | 3.59 | (1.41, 9.15)** | 0.008 |
| 3–4 years | 1.19 | (0.52, 2.76) | 0.678 | 1.39 | (0.55, 3.47) | 0.483 |
| 4–5 years | 1.60 | (0.68, 3.77) | 0.282 | 2.52 | (0.95, 6.68) | 0.063 |
| ≥ 5 years | 0.82 | (0.51, 1.33) | 0.418 | 0.97 | (0.57, 1.64) | 0.907 |
| CKD | ||||||
| < 1 year | 55.80 | (7.67, 405.78)*** | < 0.001 | 83.20 | (10.73, 646.31)*** | < 0.001 |
| 1–2 years | 20.10 | (2.65, 153.06)** | 0.004 | 23.40 | (2.80, 196.28)** | 0.004 |
| 2–3 years | 5.43 | (1.52, 19.47)** | 0.009 | 11.00 | (2.08, 58.72)** | 0.005 |
| 3–4 years | 5.46 | (1.52, 19.55)** | 0.009 | 11.70 | (1.93, 71.21)** | 0.007 |
| 4–5 years | 2.52 | (0.60, 10.54) | 0.206 | 3.11 | (0.64, 15.18) | 0.161 |
| ≥ 5 years | 2.34 | (1.45, 3.77)*** | < 0.001 | 3.16 | (1.77, 5.62)*** | < 0.001 |
CI: confidence interval; HR: hazard ratio.
$: Adjusted HR estimated by the model including the variables of gender, age, monthly income, level of urbanization, comorbidities, and medications.
* p < 0.05, ** p < 0.01, *** p < 0.001.
Fig. 2.
Adjusted hazard ratio and risk of gout/hyperuricemia and new onset CKD according to follow-up time in the kidney donor group.
Fig. 3.
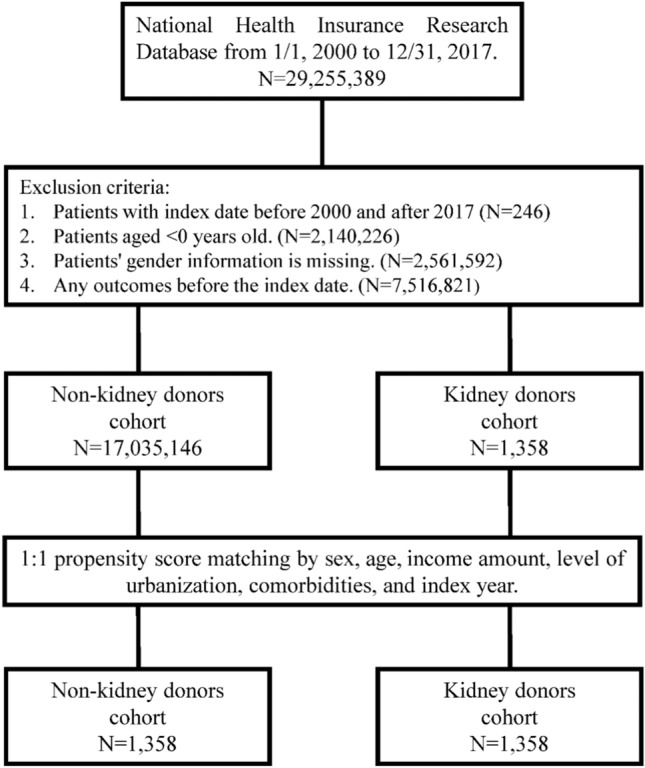
The flow chart of the study sample selection from NHIRD Taiwan.
Table 4 compares the incidence rates of new onset gout/hyperuricemia stratified by gender, age, monthly income, urban level, comorbidities and medications between the two cohorts, separately. As shown in Table 4, amongst male patients, the risk of new onset gout/hyperuricemia in kidney donors was higher when compared to the controls (adjusted HR = 1.75; 95%CI = 1.23, 2.51). For patients aged 20 to 34 and older or equal to 50, kidney donors had a higher hazard ratio when compared to kidney non-donors (age 20–34: adjusted HR = 2.07; 95%CI = 1.02, 4.18; age ≥ 50: adjusted HR = 1.99; 95%CI = 1.26, 3.13). Additionally, amongst subjects with a monthly income less than NT$ 20,000, or between NT$ 20,000 and 38,999, the risk of new onset gout/hyperuricemia in the kidney donor cohort was higher than that seen in the control group (< 20,000: adjusted HR = 1.90; 95%CI = 1.09, 3.29; 20,000–38,999: adjusted HR = 1.75; 95%CI = 1.10, 2.76). In regards to subjects having median and lowest urban levels, the risk of new onset gout/hyperuricemia for kidney donors was 1.62, 2.09 times to that of the controls, respectively (median: 95%CI = 1.01, 2.59 / lowest: 95%CI = 1.30, 3.35).
Table 4.
Incidence rates, hazard ratios and confidence intervals of Gout in kidney donors and non-donors, stratified by All variables.
| Gout | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-KD | KD | |||||||||||
| Variable | N | PY | IR§ | N | PY | IR§ | cHR | (95% CI) | p-value | aHR$ | (95% CI) | p-value |
| Gender | ||||||||||||
| Female | 34 | 5980.28 | 5.69 | 27 | 4596.32 | 5.87 | 1.01 | (0.61, 1.68) | 0.963 | 1.75 | (0.92, 3.31) | 0.086 |
| Male | 78 | 5264.41 | 14.82 | 65 | 2932.55 | 22.17 | 1.48 | (1.07, 2.06)* | 0.019 | 1.75 | (1.23, 2.51)** | 0.002 |
| Age, years | ||||||||||||
| 20–34 | 28 | 3591.94 | 7.80 | 22 | 2322.27 | 9.47 | 1.20 | (0.69, 2.10) | 0.523 | 2.07 | (1.02, 4.18)* | 0.043 |
| 35–49 | 33 | 3187.76 | 10.35 | 26 | 3004.53 | 8.65 | 0.84 | (0.50, 1.41) | 0.512 | 1.20 | (0.67, 2.15) | 0.540 |
| ≥ 50 | 51 | 4464.98 | 11.42 | 44 | 2202.06 | 19.98 | 1.71 | (1.14, 2.56)** | 0.009 | 1.99 | (1.26, 3.13)** | 0.003 |
| Income, NTD/mo | ||||||||||||
| < 20,000 | 36 | 4138.13 | 8.70 | 25 | 1914.18 | 13.06 | 1.51 | (0.90, 2.51) | 0.116 | 1.90 | (1.09, 3.29)* | 0.023 |
| 20,000–38,999 | 51 | 4606.83 | 11.07 | 48 | 3793.31 | 12.65 | 1.13 | (0.76, 1.67) | 0.549 | 1.75 | (1.10, 2.76)* | 0.017 |
| ≥ 39,000 | 25 | 2499.73 | 10.00 | 19 | 1821.39 | 10.43 | 1.02 | (0.56, 1.85) | 0.947 | 1.83 | (0.87, 3.87) | 0.114 |
| Level of urbanization | ||||||||||||
| 1 (highest) | 24 | 1945.46 | 12.34 | 7 | 487.11 | 14.37 | 1.14 | (0.49, 2.66) | 0.757 | 1.43 | (0.54, 3.80) | 0.474 |
| 2 | 44 | 4727.83 | 9.31 | 39 | 3685.18 | 10.58 | 1.13 | (0.73, 1.74) | 0.576 | 1.62 | (1.01, 2.59)* | 0.044 |
| 3 (lowest) | 44 | 4571.40 | 9.63 | 46 | 3356.58 | 13.70 | 1.39 | (0.92, 2.10) | 0.118 | 2.09 | (1.30, 3.35)** | 0.002 |
| Comorbidities | ||||||||||||
| Hypertension | 49 | 3148.29 | 15.56 | 13 | 652.28 | 19.93 | 1.24 | (0.67, 2.28) | 0.496 | 1.06 | (0.53, 2.10) | 0.878 |
| Hyperlipidemia | 38 | 2862.45 | 13.28 | 11 | 725.05 | 15.17 | 1.10 | (0.56, 2.17) | 0.772 | 1.25 | (0.61, 2.56) | 0.549 |
| COPD | 35 | 2515.72 | 13.91 | 6 | 335.34 | 17.89 | 1.11 | (0.47, 2.65) | 0.813 | 1.03 | (0.41, 2.58) | 0.950 |
| Medication | ||||||||||||
| ACEI/ARB | 61 | 3732.62 | 16.34 | 28 | 1320.38 | 21.21 | 1.29 | (0.83, 2.02) | 0.260 | 1.30 | (0.79, 2.14) | 0.296 |
| Statins | 14 | 637.33 | 21.97 | 4 | 233.39 | 17.14 | 0.75 | (0.25, 2.28) | 0.611 | 1.23 | (0.36, 4.23) | 0.742 |
PY: person-years; IR: incidence rate; CI: confidence interval; HR: hazard ratio; §: Per 1000 person-year.
$: Adjusted HR estimated by the model including the variables of gender, age, monthly income, level of urbanization, comorbidities, and medications; *p < 0.05, **p < 0.01, ***p < 0.001.
n < 3: In order to avoid the issue of security and security, the government stipulates that if the data variable unit is less than 3 units or the value of less than 3 units can be calculated, the data cannot be carried.
Discussion
In present study of a large-scale living kidney donor cohort over a long-term follow-up period, non-donor patients who had been selected from the same era and had a similar socio-economic status were matched with the controls by their date of donation as well as their demographic characteristics in order to compare the events between the groups through propensity-score matching analysis16.
First, our results have disclosed that there were risks of urological complications after donation and that these urinary tract complications included the risk of hydro-nephrosis and acute pyelonephritis (APN), as well as urinary tract stone, which was not much different from that seen in the general population. Literature review has revealed that there has been only one study performed regarding the renal calculi risk in people after donation, with that study showing a rate ratio of 0.75 in comparison with non-donors. Our findings show a lower risk, with an aHR of 0.65 in the donor group, which is much more consistent with the results seen in the previous study19 Moreover, our results suggest that other urinary complications were not different from those seen in non-donors, which reassures us on the safety of living kidney donation. This finding is novel and therefore has never been previously reported.
As we know, uric acid is one of the major metabolites predominately excreted through the kidneys. In present study, a 1.71-fold gout risk was observed in the living kidney donors after donation, which supports the findings seen in a study before20 It showed that a 1.6-fold increased risk of gouty after donation compared to a non-donor group20.
On the other hand, whether or not living kidney donors are likely to be associated with a higher risk of chronic kidney disease (CKD) or more prone to experiencing progression to end stage renal disease (ESRD) remains a great concern in regards to patients considering living kidney donation. After adjustment for associated confounders in multivariable analysis, an overall 6.67 times risk of new onset CKD was noted in patients after donation throughout the study period. Interestingly, after a time-variable risk analysis was performed showing that although a strikingly high risk of CKD in living donors occurred, most of that risk was observed within one year immediately after donation. Moreover, this risk analysis revealed that the trajectory of CKD risk rapidly declined in the shape of a sharp downward slope, while being extended during the follow-up time line. However, this new onset CKD risk would remain at an extremely relative steady status after two years which would not worsen further. Our finding is novel and has never been previously explored. A meta-analysis study of 5,048 donors reported a drop in renal function which glomerular filtration rates (GFR) varied from 59 to 30 ml/min (range 0–28%) during a 7-year follow-up time after donation21 Frequently, patients after kidney donation would be therefore regarded as a new chronic renal failure status22.
Our CKD risk analysis supports these findings. When compared with the matched control, our trajectory of CKD risk after donation in kidney donors represented a similar trend in GFR changes, thus supporting the findings seen in a previous study. More importantly, we saw very small case numbers (less than 3) in regards to absolute case numbers for ESRD in the donors from our study.
With regards to time-variable risk analysis for new onset gout/hyperuricemia in living kidney donors, the trajectory of risk for gout/hyperuricemia presented itself as being a modest increase in a plateau shape over the long observational period. The findings suggest that new onset gout/hyperuricemia after donation is substantially associated with lifelong CKD risk in living kidney donors. Comparisons showed that a remarkably high 7.15-fold increase was noted in new onset hyperuricemia with CKD in living kidney donors which reached a statistical significance. These findings are novel and have therefore never been reported previously.
Limitations
Our findings were robust; nevertheless, certain limitations needed to be elucidated further. First, biochemistry data such as serum creatinine, serum uric acid, and image reports including abdominal data cannot be obtained through the NHIRD. However, an early chronic kidney disease (CKD) care national program has been implemented throughout Taiwan. Therefore, the absolute risk of CKD or ESRD is less likely to be underestimated. Second, medical records regarding lifestyle, including smoking habits, blood pressure and obesity could not be discussed further. To eliminate any bias, proximity including COPD lung disease, hypertension and hyperlipidemia were included as confounders. Third, although medication prescriptions for the subjects were totally covered through the insurance plan of NHI, adherence to one’s medication schedule could not be evaluated, possibly resulting in an underestimation on medication influence retrospectively. Even if every possible associated factor was included and offered as our best efforts, it would still be likely that some potential variables were biased due to the inherent nature of a retrospective cohort study. Therefore, randomized control studies are still warranted in the future.
Conclusions
Our findings suggest that people after kidney donation are significantly associated with a new higher gout/hyperuricemia risk. Over a long period of time post donation, new onset gout/hyperuricemia risk remains persistent in a plateau shape in living kidney donors, suggesting that new onset hyperuricemia would likely contribute substantially to lifelong CKD risk in people after kidney donation. Clinical professionals must remain cautious for new onset hyperuricemia after surgery in people who donate kidney.
Acknowledgements
This study is supported in part by the Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW112-TDU-B-212-144004), and China Medical University Hospital (DMR-111-105; DMR-112-087; DMR-113-009). We are grateful to the Health Data Science Center of China Medical University Hospital for providing administrative, technical and funding support. The funders had no role in the study design, data collection or analysis, the decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
Abbreviations
- GFR
Glomerular filtration rate
- ESRD
End stage renal disease
- RPF
Renal plasma flow
- APN
Acute pyelonephritis
- CKD
Chronic kidney disease
- NHIRD
National Health Insurance Research Database
- ICD-9-CM
International Classification of Diseases, 9th edition, Clinical Modification
- ICD-10-CM
International Classification of Diseases, 10th edition, Clinical Modification
- COPD
Chronic obstructive pulmonary disease
- ACEI
Angiotensin-converting enzyme inhibitors
- SD
Standard deviation
- SMD
Standardized mean difference
- CIs
Confidence intervals
Author contributions
All authors have contributed significantly, and all are in agreement with regards to the content of the manuscript: Conception/Design: Y.-N. K., T.-M. Y.; Provision of study materials: Y.-N. K., T.-M. Y.; Collection and/or assembly of data: C.-L. L.; Data analysis and interpretation: Y.-N. K., S.-T. H., I-K. W., Y.-W. C., C.-L. L., B. K L., C.-Y. L., T.-M. Y.; Manuscript writing: Y.-N. K., S.-T. H., I-K. W., Y.-W. C., C.-L. L., B. K L., C.-Y. L., T.-M. Y.; Final approval of the manuscript: Y.-N. K., S.-T. H., I-K. W., Y.-W. C., C.-L. L., B. K L., C.-Y. L., T.-M. Y.
Data availability
The datasets generated and/or analysed during the current study are available in the Taiwan Ministry of Health and Welfare (MOHW) repository. The Taiwan Ministry of Health and Welfare (MOHW) holds the dataset in this study and needed to approve our application for accessing the current data. Any researcher who is interested in accessing this dataset can submit an application form to the MOHW requesting access (MOHW, Email: stcarolwu@mohw.gov.tw). The Taiwan Ministry of Health and Welfare address is: No. 488, Sec. 6, Zhongxiao E. Rd., Nangang Dist., Taipei City 115, Taiwan (R.O.C.). Phone: + 886–2-8590–6848. All relevant data within the paper are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schold, J. D. et al. Comorbidity burden and perioperative complications for living kidney donors in the United States. Clin. J. Am. Soc. Nephrol.8, 1773–1782 (2013). 10.2215/CJN.12311212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lentine, K. L. & Segev, D. L. Understanding and communicating medical risks for living kidney donors: A matter of perspective. J. Am. Soc. Nephrol.28, 12–24 (2017). 10.1681/ASN.2016050571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matas, A. J. et al. OPTN/SRTR 2013 annual data report: Kidney. Am. J. Transpl.15(Suppl 2), 1–34 (2015). 10.1111/ajt.13195 [DOI] [PubMed] [Google Scholar]
- 4.Ogata, M. et al. Consequences of kidney donation by age in Japanese living kidney donors: A single-center study. Clin. Exp. Nephrol.28(7), 664–673 (2024). 10.1007/s10157-024-02476-9 [DOI] [PubMed] [Google Scholar]
- 5.Blantz, R. C. & Steiner, R. W. Benign hyperfiltration after living kidney donation. J. Clin. Invest.125, 972–974 (2015). 10.1172/JCI80818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lenihan, C. R. et al. Longitudinal study of living kidney donor glomerular dynamics after nephrectomy. J. Clin. Invest.125, 1311–1318 (2015). 10.1172/JCI78885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mjøen, G., Øyen, O., Holdaas, H., Midtvedt, K. & Line, P. D. Morbidity and mortality in 1022 consecutive living donor nephrectomies: benefits of a living donor registry. Transplantation88, 1273–1279 (2009). 10.1097/TP.0b013e3181bb44fd [DOI] [PubMed] [Google Scholar]
- 8.Segev, D. L. et al. Perioperative mortality and long-term survival following live kidney donation. JAMA303, 959–966 (2010). 10.1001/jama.2010.237 [DOI] [PubMed] [Google Scholar]
- 9.Lentine, K. L. et al. Perioperative complications after living kidney donation: A national study. Am. J. Transpl.16, 1848–1857 (2016). 10.1111/ajt.13687 [DOI] [PubMed] [Google Scholar]
- 10.Boudville, N. et al. Meta analysis: Risk for hypertension in living kidney donors. Ann. Intern. Med.145, 185–196 (2006). 10.7326/0003-4819-145-3-200608010-00006 [DOI] [PubMed] [Google Scholar]
- 11.Garg, A. X. et al. Cardiovascular disease and hypertension risk in living kidney donors: An analysis of health administrative data in Ontario Canada. Transplantation86, 399–406 (2008). 10.1097/TP.0b013e31817ba9e3 [DOI] [PubMed] [Google Scholar]
- 12.Lentine, K. L. et al. Understanding antihypertensive medication use after living kidney donation through linked national registry and pharmacy claims data. Am. J. Nephrol.40, 174–183 (2014). 10.1159/000365157 [DOI] [PubMed] [Google Scholar]
- 13.Garg, A. X. et al. Gestational hypertension and preeclampsia in living kidney donors. N Engl. J. Med.372, 124–133 (2015). 10.1056/NEJMoa1408932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reisaeter, A. V., Røislien, J., Henriksen, T., Irgens, L. M. & Hartmann, A. Pregnancy and birth after kidney donation: The Norwegian experience. Am. J. Transpl.9, 820–824 (2009). 10.1111/j.1600-6143.2008.02427.x [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim, H. N. et al. Pregnancy outcomes after kidney donation. Am. J. Transpl.9, 825–834 (2009). 10.1111/j.1600-6143.2009.02548.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matas, A. J. & Rule, A. D. Long-term medical outcomes of living kidney donors. Mayo Clin. Proc.97, 2107–2122 (2022). 10.1016/j.mayocp.2022.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen, J. et al. Assessment of post donation outcomes in US living kidney donors using publicly available data sets. JAMA Netw. Open2, e191851 (2019). 10.1001/jamanetworkopen.2019.1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu T. Risk of hyperuricemia and chronic kidney disease in living kidney donors: a nationwide population-based cohort study with propensity score matching analysis AJT, Volume 23, Issue 6, Supplement 1.
- 19.Thomas, S. M. et al. Risk of kidney stones with surgical intervention in living kidney donors. Am. J. Transpl.13, 2935–2944 (2013). 10.1111/ajt.12446 [DOI] [PubMed] [Google Scholar]
- 20.Lam, N. N. et al. Gout after living kidney donation: A matched cohort study. Am. J. Kidney Dis.65, 925–932 (2015). 10.1053/j.ajkd.2015.01.017 [DOI] [PubMed] [Google Scholar]
- 21.Garg, A. X. et al. Proteinuria and reduced kidney function in living kidney donors: A systematic review, meta-analysis, and meta-regression. Kidney Int.70, 1801–1810 (2006). 10.1038/sj.ki.5001819 [DOI] [PubMed] [Google Scholar]
- 22.Wan, R. K., Spalding, E., Winch, D., Brown, K. & Geddes, C. C. Reduced kidney function in living kidney donors. Kidney Int.71, 1077 (2007). 10.1038/sj.ki.5002252 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are available in the Taiwan Ministry of Health and Welfare (MOHW) repository. The Taiwan Ministry of Health and Welfare (MOHW) holds the dataset in this study and needed to approve our application for accessing the current data. Any researcher who is interested in accessing this dataset can submit an application form to the MOHW requesting access (MOHW, Email: stcarolwu@mohw.gov.tw). The Taiwan Ministry of Health and Welfare address is: No. 488, Sec. 6, Zhongxiao E. Rd., Nangang Dist., Taipei City 115, Taiwan (R.O.C.). Phone: + 886–2-8590–6848. All relevant data within the paper are available from the corresponding author upon reasonable request.