Abstract
Background
Patients with Crohn’s disease (CD) or ulcerative colitis (UC) often cycle through conventional therapies (CT) with different mechanisms of action (MOA) before initiating advanced therapy (AT). We describe treatment patterns among patients with CD/UC.
Methods
Using Merative MarketScan Research databases, adult patients with CD/UC were identified from medical/pharmacy claims (2017–2021). Patients had ≥1 hospitalization or ≥2 outpatient visits (≥30 days apart within 1 year) for CD/UC. Two cohorts were established; cohort 1: Newly diagnosed patients (index date is the date of first diagnosis) and cohort 2: Patients initiating AT (index date is the date of first AT). First-line treatment patterns (cohort 1) and CT pathways before AT initiation (cohort 2) by the number of episodes (ie, adding a new therapy, switching to another therapy, or restarting the same therapy after ≥60 days) and MOA are reported.
Results
Among newly diagnosed patients in cohort 1 (CD: n = 1739; UC: n = 2740), 14.4% (CD) and 5.9% (UC) of patients had any AT use during the follow-up period (mean: 2.3 years; ≥ 77% initiated corticosteroids). Among patients in cohort 2 (CD: n = 2594; UC: n = 2431), the mean number of CT episodes before AT initiation was 4.0 ± 4.3 (CD) and 5.9 ± 5.0 (UC). Among those with ≥1 corticosteroid episode (CD: 82.2%; UC: 91.5%), the mean number of episodes was 4.6 ± 4.3 (CD) and 6.3 ± 5.0 (UC). Overall, 13.3% (CD) and 23.7% (UC) of patients cycled through 3 MOAs before AT initiation.
Conclusions
Despite treatment recommendations, few newly diagnosed CD/UC patients initiated AT as their first treatment. Moreover, patients cycled through multiple CTs before initiating AT.
Keywords: Crohn’s disease, ulcerative colitis, treatment patterns
Key Messages.
What is already known?
Previous reports demonstrated low uptake of advanced therapies in patients with moderate-to-severe Crohn’s disease or ulcerative colitis; however, recent updates to treatment recommendations suggest early use of advanced therapies in this population.
What is new here?
Despite updated treatment recommendations, patients newly diagnosed with Crohn’s disease or ulcerative colitis continue to receive multiple courses of conventional therapies, predominantly corticosteroids, before initiating an advanced therapy. In addition, few patients initiated advanced therapy as their first treatment.
How can this study help patient care?
These findings suggest that better uptake of treatment guidelines is needed to shift practice patterns and ensure that patients are not languishing on ineffective therapies.
Introduction
Inflammatory bowel disease (IBD), which encompasses both Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic inflammatory illness characterized by progressive inflammation of the gastrointestinal (GI) tract, resulting in numerous symptoms including abdominal pain, diarrhea, fever, weight loss, and fatigue.1,2 Aside from the troublesome disease-related symptoms, IBD has also been shown to greatly impact patients’ health-related quality of life (HRQoL). A recent meta-analysis of more than 2000 IBD patients showed lower scores on mental and physical HRQoL measures for IBD patients when compared to those without IBD.3,4 Likewise, patients with IBD had higher healthcare utilization, including a greater number of hospitalizations, emergency department visits, and surgeries than those without IBD.5,6 Data have shown that up to 30% of patients with UC, and 70% of patients with CD, will eventually require surgery to manage disease, further contributing to the high financial burden of disease.7
Currently, available treatment options are used to reduce disease symptoms, achieve remission, and prevent further damage.8 According to the selecting therapeutic targets in IBD (STRIDE) II initiative of the International Organization for the Study of IBD (IOIBD), current treat-to-target recommendations for the management of CD and UC focus on symptom remission and normalization of C-reactive protein levels in the short-term and improvement in patient HRQoL, absence of disability, and endoscopic healing in the long-term.9 Conventional treatments include 5-aminosalicylic acids (5-ASAs), systemic corticosteroids, and immunomodulators. For those patients with mild disease, conventional treatments may be sufficient to manage disease. However, for patients with moderate to severe disease, early use of advanced therapies (AT; ie, biological and small molecule therapies) is recommended.8,10 In fact, in prior and recent studies of patients with moderate to severe CD, early use of biologic therapies was associated with a dramatic improvement in surgery and steroid-free remission in addition to endoscopic remission,11,12 which also significantly reduces healthcare resources and associated costs.7,13 Thus, these findings highlight the benefits of early treatment with ATs in patients with more than mild disease.
Despite the fact that ATs have been available to treat IBD for more than 2 decades, a previous report demonstrated that, over approximately 3 years of follow-up, significantly more patients with IBD followed treatment pathways consisting primarily of nonbiologic therapies and very few patients were ever initiated on biologic therapy.14 Furthermore, the second most commonly used treatment for CD was 5-ASA (only after corticosteroids), despite a lack of data to support the use of this drug class in most patients with CD.14 This suggests that the most effective therapies are underused and that there are barriers preventing optimized disease management. The current study had 2 objectives. First, treatment patterns among newly diagnosed patients with CD and UC were assessed. Second, the conventional treatment pathways leading to AT initiation, including corticosteroid use and number of different mechanisms of action (MOA) tried, among a larger population of patients with CD and UC were evaluated.
Materials and Methods
Study Design and Participants
Using the Merative MarketScan Research databases (Commercial Claims and Encounters database and Medicare Supplemental database; Ann Arbor, MI), adult patients with CD or UC were identified from medical/pharmacy claims based on International Classification of Diseases (ICD) 9th or 10th edition codes (ICD-9: CD, 555.xx; UC, 556.xx; ICD-10: CD, K50.x; UC, K51.x) between 2017 and 2021. The MarketScan databases contain individual-level, deidentified healthcare claims information from employer-sponsored private insurance plans, hospitals, and Medicare and Medicaid programs. These databases contain data on over 150 million patients across the United States. For this analysis, 2 cohorts were established to address 2 objectives (Supplementary Figure 1); objective 1 examined treatment patterns in patients newly diagnosed with CD or UC and objective 2 assessed treatment patterns leading to initiation of an AT among patients with CD or UC.
For cohort 1, eligible patients were aged ≥18 years and were newly diagnosed with CD or UC. The index date was defined as the first date of a CD or UC diagnosis. All patients had to have ≥1 hospitalization for CD or UC, or ≥2 outpatient visits for CD or UC that were ≥30 days apart within 1 year during January 2017—June 2020, and ≥12 months of continuous enrollment before and after the index date. Those with a prior IBD diagnosis or use of ATs prior to IBD diagnosis were excluded. Likewise, patients with ICD-9-CM/ICD-10-CM codes listing both conditions (ie, CD and UC) were excluded.
For cohort 2, eligible patients were aged ≥18 years and were initiating an AT. The index date was defined as the date of the first AT prescription. Eligible ATs were adalimumab, certolizumab, infliximab, golimumab, ustekinumab, vedolizumab, natalizumab, tofacitinib, and ozanimod. All patients included in the analysis had ≥1 hospitalization or ≥2 outpatient visits (≥30 days apart within 1 year) for CD or UC and were continuously enrolled for ≥2 years before and ≥1 year after the AT initiation date. Patients were excluded if they had a claim for an AT before the index date or if they had diagnosis codes for both CD and UC.
Outcomes
For both cohorts, baseline clinical and demographic characteristics were defined for patients with CD or UC. Conventional treatments were stratified by MOA, which were identified as 5-ASAs (ie, mesalamine, sulfasalazine, and olsalazine), immunomodulators (ie, azathioprine, 6-mercaptopurines, and methotrexate), and systemic corticosteroids (ie, betamethasone, cortisone, desoxycorticosterone, dexamethasone, hydrocortisone, methylprednisolone, paramethasone, prednisolone, prednisone, and triamcinolone) and budesonide.
For objective 1, the proportion of newly diagnosed patients initiating any treatment within the follow-up period is reported by MOA. Likewise, the first treatments used after following CD or UC diagnosis are reported by MOA. For this objective, the time to first AT, defined as the time in days between the index date (ie, diagnosis of CD or UC) and the first date of an AT treatment, is also reported.
For objective 2, therapy use after diagnosis, stratified by MOA is reported; baseline demographic and clinical characteristics are also presented based on the number of MOAs attempted prior to AT initiation. The mean time on conventional therapies (CT) and time to first AT, as well as the total number of episodes of conventional therapy use, defined as the addition of a new therapy, a switch to a different therapy, or re-start of the same therapy after ≥60 days, and the number of MOAs of conventional therapy used are reported.
Statistical Analysis of Data
Mean and standard deviation (SD) are reported for continuous variables, and frequency and percentage for categorical outcomes. Chi-square test was used to compare categorical variables and analysis of variance was used to compare continuous variables. Statistical significance was set as a P-value < .05. Conventional treatment pathways before AT initiation are described by Sankey diagrams showing the number of patients per pathway. Findings are reported by classifying conventional therapy based on episodes and MOA.
Results
Objective 1: Treatment Patterns Among Patients Newly Diagnosed With CD or UC (Cohort 1)
Baseline patient demographic and clinical characteristics
A total of 1739 and 2740 patients were newly diagnosed with CD or UC, respectively (Table 1). Among patients newly diagnosed with CD, the mean (±SD) age was 47.9 ± 16.5 years and 55.2% of patients were female. One-third of patients (33.6%; n = 585) had the disease in the small intestine, while 53.0% (n = 922) had an unspecified CD location at baseline. Nearly one-third of patients with CD (30.5%; n = 530) had experienced an intestinal stricture, and 3.5% (n = 61) and 1.6% (n = 27) had experienced a fistula or perianal abscess, respectively. The mean Charlson Comorbidity Index (CCI) score was 1.2 ± 1.8 and 2.1% (n = 36) had extraintestinal manifestations (EIMs). Among patients newly diagnosed with UC, the mean age was 51.9 ± 16.1 years and 61.6% (n = 1689) were female. More than half (61.8%) of patients did not have a specified disease location; 24.7% (n = 678) had pancolitis; 2.0% (n = 54) and 1.2% (n = 33) had experienced a fistula or perianal abscess, respectively. The mean CCI was 1.5 ± 2.0 and few patients (<8%) reported disease-related complications or EIMs (1.3%; n = 35).
Table 1.
Baseline characteristics among patients newly diagnosed with CD or UC (cohort 1).
| Characteristics among patients with CD | CD N = 1739 | UC N = 2740 |
|---|---|---|
| Age at the index date, mean ± SD | 47.9 ± 16.5 | 51.9 ± 16.1 |
| Female, n (%) | 960 (55.2) | 1689 (61.6) |
| Last CD disease location category in the baseline, n (%) | ||
| Large intestine | 158 (9.1) | |
| Small and large intestine | 74 (4.3) | |
| Small intestine | 585 (33.6) | |
| Unspecified | 922 (53.0) | |
| Last UC disease location category in the baseline, n (%) | ||
| Universal colitis | 678 (24.7) | |
| Left-sided | 238 (8.7) | |
| Proctitis | 54 (2.0) | |
| Proctosigmoiditis/rectosigmoiditis | 76 (2.8) | |
| Unspecified | 1694 (61.8) | |
| Region, n (%) | ||
| Midwest | 361 (23.2) | 566 (22.9) |
| Northeast | 302 (19.4) | 475 (19.2) |
| South | 726 (46.7) | 1140 (46.0) |
| West | 167 (10.7) | 296 (12.0) |
| Insurance, n (%) | ||
| Commercial | 1517 (87.2) | 2273 (83.0) |
| Medicare (including medicare advantage) | 222 (12.8) | 467 (17.0) |
| Baseline CCI, mean ± SD | 1.2 ± 1.8 | 1.5 ± 2.0 |
| Number of treated days to AT initiation, mean ± SD | 162.6 ± 197.6 | 251.1 ± 325.5 |
| CD disease complications, n (%) | ||
| Fistula | 61 (3.5) | |
| Intestinal abscess | 115 (6.6) | |
| Intestinal stricture | 530 (30.5) | |
| Perianal abscess | 27 (1.6) | |
| Stoma | 29 (1.7) | |
| UC disease complications, n (%) | ||
| Fistula | 54 (2.0) | |
| Intestinal abscess | 76 (2.8) | |
| Intestinal stricture | 195 (7.1) | |
| Perianal abscess | 33 (1.2) | |
| Stoma | 34 (1.2) | |
| EIMs, n (%) | 36 (2.1) | 35 (1.3) |
| Baseline presence of IBD-related ED visit,an (%) | 643 (37.0) | 1,068 (39.0) |
| Baseline presence of IBD-related hospitalization,an (%) | 335 (19.3) | 659 (24.1) |
aED visits and hospitalizations were determined by ICD-9/10 codes (ie, ICD-9: CD, 555.xx; UC, 556.xx; ICD-10: CD, K50.x; UC, K51.x).
Abbreviations: AT, advanced therapy; CCI, Charlson Comorbidity Index; CD, Crohn’s disease; ED, emergency department; EIM, extraintestinal manifestation; IBD, inflammatory bowel disease; ICD-9/10, International Classification of Diseases (ICD)-9/10; SD, standard deviation; UC, ulcerative colitis.
Treatment patterns among newly diagnosed patients
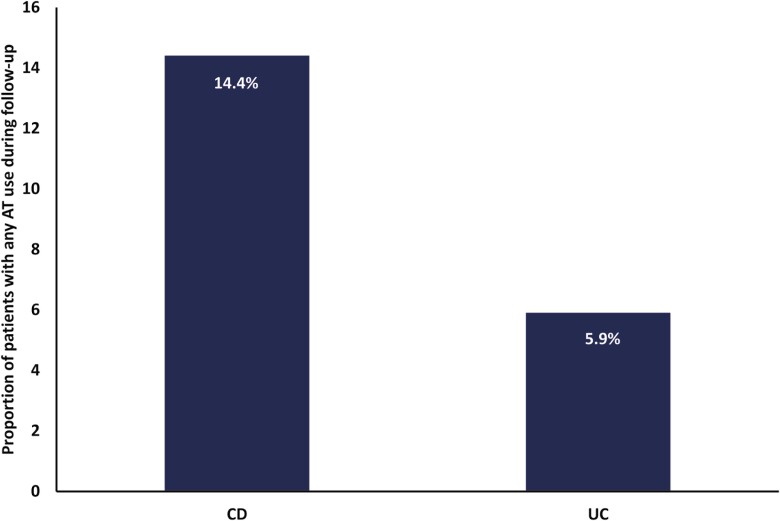
Overall, 65% of patients with CD or UC initiated an eligible therapy (ie, 5-ASA, immunomodulator, systemic steroid, or AT) during a mean follow-up of 2.3 years (Table 2). Of patients newly diagnosed with CD or UC who started treatment (CD: N = 1123; UC: N = 1788), 80.4% (n = 903) and 76.6% (n = 1370) of patients with CD and UC, respectively, initiated corticosteroid monotherapy following diagnosis. Few patients initiated monotherapy with 5-ASAs (CD: 4.3%; UC: 8.1%), immunomodulators (CD: 1.9%; UC: 1.2%), or an AT (CD: 4.5%; UC: 0.4%) after their diagnosis. Among those patients initiating multiple therapies, 3.7% and 10.6% of patients with CD and UC, respectively, initiated 5-ASAs with corticosteroids. Less than 2% of patients with CD and UC-initiated corticosteroids with immunomodulators; 2.1% of patients with CD and 1.0% of patients with UC-initiated corticosteroids with an AT. Overall, only 14.4% (n = 251) of patients with CD, and 5.9% (n = 161) of patients with UC, had any use of an AT (ie, alone or with other therapies) during the follow-up period (Figure 1).
Table 2.
First-line treatments among patients newly diagnosed with CD and UC (cohort 1).
| CD (N = 1739) | UC (N = 2740) | |
|---|---|---|
| Follow-up from first diagnosis [years], mean ± SD | 2.3 ± 1.0 | 2.3 ± 1.0 |
| Patients initiating a treatment, n (%) | 1123 (64.6) | 1788 (65.3) |
| First-line therapy after diagnosis, n (%) | ||
| 1 MOA | ||
| 5-ASA only | 48 (4.3) | 144 (8.1) |
| Immunomodulators | 21 (1.9) | 21 (1.2) |
| Corticosteroidsa | 903 (80.4) | 1370 (76.6) |
| ATb | 50 (4.5) | 7 (0.4) |
| 2 MOAs | ||
| 5-ASA and immunomodulators | 1 (0.1) | 1 (0.1) |
| Corticosteroidsa and immunomodulators | 20 (1.8) | 17 (1.0) |
| 5-ASA and corticosteroidsa | 42 (3.7) | 189 (10.6) |
| 5-ASA and ATb | –– | –– |
| ATb and immunomodulators | 4 (0.4) | –– |
| ATb and corticosteroids | 23 (2.1) | 18 (1.0) |
| 3 MOAs | ||
| 5-ASA and immunomodulators and corticosteroids | 4 (0.4) | 6 (0.3) |
| ATb and 5-ASA and corticosteroids | 2 (0.2) | 13 (0.7) |
| ATsb and immunomodulators and corticosteroids | 5 (0.5) | –– |
| 4 MOAs | ||
| ATb and 5-ASA and corticosteroids and immunomodulators | –– | 2 (0.1) |
| Time to AT [days], mean ± SD | 162.6 ± 197.6 | 251.1 ± 325.5 |
| Any use of AT in the follow-up, n (%) | 251 (14.4) | 161 (5.9) |
aIncludes systemic oral or injectable corticosteroids only (ie, betamethasone, budesonide, cortisone, desoxycorticosterone, dexamethasone, hydrocortisone, methylprednisolone, paramethasone, prednisolone, prednisone, triamcinolone).
bIncludes adalimumab, certolizumab, infliximab, golimumab, ustekinumab, vedolizumab, natalizumab, tofacitinib, and ozanimod.
Abbreviations: ASA, aminosalicylic acid; AT, advanced therapy; CD, Crohn’s disease; MOA, mechanism of action; SD, standard deviation; UC, ulcerative colitis.
Figure 1.
The proportion of patients with any use of AT during follow-up. Abbreviations: AT, advanced therapy; CD, Crohn’s disease; UC, ulcerative colitis.
Objective 2: Treatment Pathways Leading to AT Initiation Among Patients With CD and UC (Cohort 2)
Baseline patient demographic and clinical characteristics
In total, 2594 patients with CD were included in the analysis (Table 3). The mean age (± SD) was 40.7 ± 14.4 years, with a mean pre-AT period of 5.9 ± 3.2 years; 54.0% of patients were female. When stratified by the number of MOAs of conventional therapy used prior to AT initiation, those patients with 3 versus those with ≤2 MOAs were older (44.1 ± 14.1 years), had longer pre-AT periods (7.2 ± 3.2 years), and had received treatment for a mean 1107.9 ± 936.6 days before the index date.
Table 3.
Baseline characteristics among all patients with CD by prior number of MOAs (cohort 2).
| Characteristics among patients with CD | All CD patients N = 2594 |
≤1 MOA N = 1220 |
2 MOAs N = 1062 |
3 MOAs N = 312 |
P-value |
|---|---|---|---|---|---|
| Length of pre-AT period [years], mean ± SD | 5.9 ± 3.2 | 5.4 ± 3.1 | 6.1 ± 3.2 | 7.2 ± 3.2 | <.0001 |
| Age at the index date, mean ± SD | 40.7 ± 14.4 | 38.9 ± 14.2 | 41.9 ± 14.3 | 44.1 ± 14.1 | <.0001 |
| Female, n (%) | 1401 (54.0) | 648 (53.1) | 580 (54.6) | 173 (55.5) | .6671 |
| Last CD disease location category in the baseline, n (%) | .0554 | ||||
| Large intestine | 430 (17.1) | 184 (15.8) | 193 (18.5) | 53 (17.3) | |
| Small and large intestine | 532 (21.1) | 251 (21.5) | 211 (20.2) | 70 (22.8) | |
| Small intestine | 768 (30.5) | 374 (32.1) | 322 (30.8) | 72 (23.5) | |
| Unspecified | 787 (31.3) | 357 (30.6) | 318 (30.5) | 112 (36.5) | |
| Insurance, n (%) | .0533 | ||||
| Commercial | 2530 (97.5) | 1199 (98.3) | 1027 (96.7) | 304 (97.4) | |
| Medicare | 64 (2.5) | 21 (1.7) | 35 (3.3) | 8 (2.6) | |
| Baseline CCI, mean ± SD | 0.7 ± 1.2 | 0.7 ± 1.1 | 0.8 ± 1.3 | 0.9 ± 1.3 | .0015 |
| Number of treated days prior to index date, mean ± SD | 419.3 ± 677.1 | 120.9 ± 310.5 | 559.9 ± 700.7 | 1107.9 ± 936.6 | <.0001 |
| IBD disease complications, n (%) | |||||
| Fistula | 272 (10.5) | 153 (12.5) | 91 (8.6) | 28 (9.0) | .0055 |
| Intestinal abscess | 204 (7.9) | 107 (8.8) | 77 (7.3) | 20 (6.4) | .2411 |
| Intestinal stricture | 334 (12.9) | 167 (13.4) | 135 (12.7) | 32 (10.3) | .2655 |
| Perianal abscess | 168 (6.5) | 99 (8.1) | 56 (5.3) | 13 (4.2) | .0048 |
| Stoma | 23 (0.9) | 9 (0.7) | 10 (0.9) | 4 (1.3) | .5580 |
| EIMs, n (%) | 170 (6.6) | 75 (6.2) | 81 (7.6) | 14 (4.5) | .1053 |
| Baseline presence of IBD-related ED visit, n (%) | 472 (18.2) | 185 (15.2) | 226 (21.3) | 61 (19.6) | .0006 |
| Baseline presence of IBD-related hospitalization, n (%) | 461 (17.8) | 213 (17.5) | 199 (18.7) | 49 (15.7) | .4334 |
Abbreviations: AT, advanced therapy; CCI, Charlson Comorbidity Index; CD, Crohn’s disease; ED, emergency department; EIM, extraintestinal manifestation; IBD, inflammatory bowel disease; MOA, mechanism of action; SD, standard deviation.
Among the 2431 patients with UC included in this analysis, 47.4% (n = 1152) were female with a mean age of 42.7 ± 13.8 years and a mean pre-AT period of 6.0 ± 3.3 years (Table 4). When stratified by the number of MOAs of conventional therapy used prior to AT initiation, there was a significant (P < .0001) trend wherein those who had received 3 MOAs had the longest pre-AT periods (6.5 ± 3.3 years) and mean number of days treated prior to the index date (1135.7 ± 938.2). Information regarding the geographical region of patients with CD or UC can be found in Supplementary Table 1.
Table 4.
Baseline characteristics among all patients with UC by prior number of MOAs (cohort 2).
| Characteristics among patients with UC | All UC patients N = 2431 | ≤1 MOAN = 358 | 2 MOAsN = 1511 | 3 MOAs N = 562 | P-value |
|---|---|---|---|---|---|
| Length of pre-AT period [years], mean ± SD | 6.0 ± 3.3 | 5.3 ± 3.3 | 5.9 ± 3.2 | 6.5 ± 3.3 | <.0001 |
| Age at the index date, mean ± SD | 42.7 ± 13.8 | 42.7 ± 13.8 | 42.3 ± 13.9 | 43.8 ± 13.6 | .0771 |
| Female, n (%) | 1152 (47.4) | 162 (45.3) | 727 (48.1) | 263 (46.8) | .5905 |
| Last UC disease location categories in the baseline, n (%) | .0139 | ||||
| Left-sided | 348 (17.7) | 28 (10.7) | 237 (18.9) | 83 (18.4) | |
| Proctitis | 254 (12.9) | 44 (16.9) | 147 (11.7) | 63 (14.0) | |
| Proctosigmoiditis/rectosigmoiditis | 275 (14.0) | 34 (13.0) | 171 (13.6) | 70 (15.5) | |
| Universal colitis | 1092 (55.5) | 155 (59.4) | 702 (55.9) | 235 (52.1) | |
| Insurance, n (%) | .4575 | ||||
| Commercial | 2363 (97.2) | 349 (97.5) | 1472 (97.4) | 542 (96.4) | |
| Medicare | 68 (2.8) | 9 (2.5) | 39 (2.6) | 20 (3.6) | |
| Baseline CCI, mean ± SD | 0.7 ± 1.3 | 0.9 ± 1.8 | 0.6 ± 1.1 | 0.7 ± 1.25 | .0009 |
| Number of treated days prior to index date, mean ± SD | 685.0 ± 773.0 | 191.9 ± 401.3 | 634.2 ± 678.6 | 1135.7 ± 938.2 | <.0001 |
| EIMs, n (%) | 138 (5.7) | 27 (7.5) | 79 (5.2) | 32 (5.7) | .2353 |
| Baseline presence of IBD-related ED visit, n (%) | 322 (13.3) | 28 (7.8) | 213 (14.1) | 81 (14.4) | .0045 |
| Baseline presence of IBD-related hospitalization, n (%) | 326 (13.4) | 44 (12.3) | 224 (14.8) | 58 (10.3) | .0223 |
Abbreviations: AT, advanced therapy; CCI, Charlson Comorbidity Index; ED, emergency department; EIM, extraintestinal manifestation; IBD, inflammatory bowel disease; MOA, mechanism of action; SD, standard deviation; UC, ulcerative colitis.
Treatment pathways prior to AT initiation
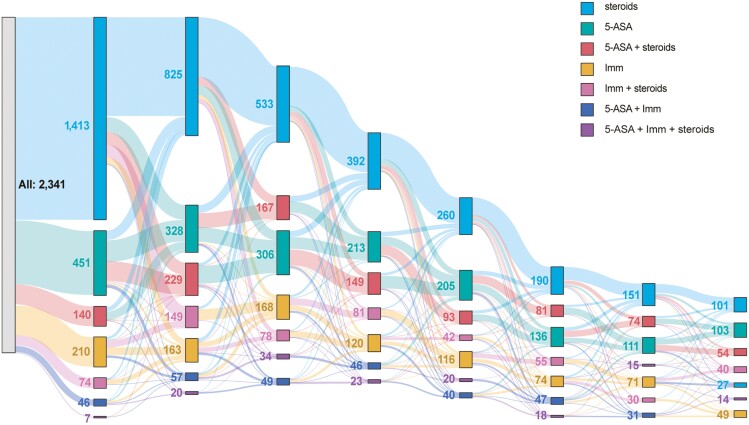
Among patients with CD, the mean number of conventional therapy episodes was 4.0 ± 4.3 (Table 5; Figure 2), with 39.5% (n = 1024) having ≥4 episodes; only 9.8% (n = 253) had not received CT. Most patients (82.2%) had ≥1 corticosteroid episode prior to AT initiation, with 46.5% (n = 991) reporting ≥4 corticosteroid episodes, with a mean of 121.0 ± 279.7 days on corticosteroids. The mean number of MOAs tried prior to AT initiation was 1.6 ± 0.8. Approximately one-third had received corticosteroids only (34.2%) or had received 5-ASA with corticosteroids (28.2%) prior to AT initiation.
Table 5.
Characterization of Conventional Therapy Pathways Before AT Initiation in Patients With CD and UC (Cohort 2)
| CD (N = 2594) | UC (N = 2431) | |
|---|---|---|
| Length of pre-AT period [years], mean ± SD | 5.9 ± 3.2 | 6.0 ± 3.3 |
| Overall number of days on conventional therapy, mean ± SD | 419.3 ± 677.1 | 685.0 ± 773.0 |
| Number of episodes of conventional therapy before AT initiation | ||
| Mean ± SD | 4.0 ± 4.3 | 5.9 ± 5.0 |
| 0 episodes, n (%) | 253 (9.8) | 60 (2.5) |
| 1 episode, n (%) | 570 (22.0) | 239 (9.8) |
| 2 episodes, n (%) | 436 (16.8) | 320 (13.2) |
| 3 episodes, n (%) | 311 (12.0) | 314 (12.9) |
| 4 + episodes, n (%) | 1024 (39.5) | 1498 (61.6) |
| Patients with at least 1 corticosteroid episode before AT initiation,an (%) | 2132 (82.2) | 2224 (91.5) |
| Overall number of days on coticosteroids,a mean ± SD | 121.0 ± 279.7 | 137.8 ± 209.0 |
| Number of corticosteroid episodes before AT initiation among corticosteroid users | ||
| Mean ± SD | 4.6 (4.3) | 6.3 (5.0) |
| 1 episode, n (%) | 447 (21.0) | 179 (8.1) |
| 2 episodes, n (%) | 397 (18.6) | 286 (12.9) |
| 3 episodes, n (%) | 297 (13.9) | 294 (13.2) |
| 4 + episodes, n (%) | 991 (46.5) | 1465 (65.9) |
| Number of MOAs of conventional therapy before AT initiation, mean ± SD | 1.6 ± 0.8 | 2.1 ± 0.7 |
| Categorical number of MOAs of conventional therapy before AT initiation, b n (%) | ||
| 1 MOA | ||
| 5-ASA only | 85 (3.6) | 96 (4.1) |
| Immunomodulators | 82 (3.5) | 9 (0.4) |
| Corticosteroidsa | 800 (34.2) | 193 (8.1) |
| 2 MOAs | ||
| 5-ASA and immunomodulators | 42 (1.8) | 42 (1.8) |
| Corticosteroidsa and immunomodulators | 360 (15.4) | 91 (3.8) |
| 5-ASA and corticosteroidsa | 660 (28.2) | 1378 (58.1) |
| 3 MOAs | ||
| 5-ASA and immunomodulators and corticosteroids | 312 (13.3) | 562 (23.7) |
| Number of days on conventional therapy before AT initiation by number of MOAs, a mean ± SD | ||
| 1 MOA | ||
| 5-ASA only | 470.7 ± 629.9 | 458.3 ± 615.9 |
| Immunomodulators | 505.3 ± 662.5 | 554.8 ± 527.7 |
| Corticosteroidsa | 82.5 ± 163.0 | 102.0 ± 196.9 |
| 2 MOAs | ||
| 5-ASA and immunomodulators | 1090.17 ± 989.9 | 1041.1 ± 993.8 |
| Corticosteroidsa and immunomodulators | 513.1 ± 663.4 | 622.5 ± 587.6 |
| 5-ASA and corticosteroidsa | 551.7 ± 686.0 | 622.6 ± 669.2 |
| 3 MOAs | ||
| 5-ASA and immunomodulators and corticosteroidsa | 1107.9 ± 936.6 | 1135.7 ± 938.2 |
aIncludes systemic oral or injectable corticosteroids only (ie, betamethasone, budesonide, cortisone, desoxycorticosterone, dexamethasone, hydrocortisone, methylprednisolone, paramethasone, prednisolone, prednisone, and triamcinolone.
bAmong patients with at least 1 course (defined as the addition of a new therapy, a switch to a different therapy, or re-start of the same therapy after ≥60 days) of conventional therapy.
Abbreviations: ASA, aminosalicylic acid; AT, advanced therapy; CD, Crohn’s disease; MOA, mechanism of action; SD, standard deviation; UC, ulcerative colitis.
Figure 2.
Sankey Diagram of conventional treatment pathways before AT initiation in CD—The road to AT. Abbreviations: ASA, aminosalicylic acid; CD, Crohn’s disease; Imm, immunomodulator.
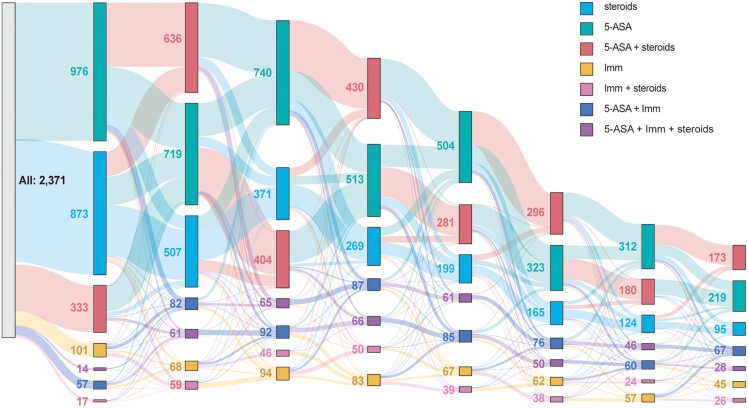
Among patients with UC, the mean number of conventional therapy episodes was 5.9 ± 5.0, with 61.6% (n = 1498) having had ≥4 episodes (Table 5; Figure 3). Most patients (91.5%) had ≥ 1 corticosteroid episode prior to AT initiation, with 65.9% (n = 1465) having ≥4 steroid episodes. The mean number of days on corticosteroids was 137.8 ± 209.0 days. On average, patients with UC had received 2.1 ± 0.7 MOAs prior to AT initiation. More than half of patients (58.1%; n = 1378) received 5-ASA with corticosteroids and 23.7% had received all 3 MOAs (ie, 5-ASA, corticosteroids, and immunomodulators) prior to AT initiation.
Figure 3.
Sankey Diagram of conventional treatment pathways before AT initiation in UC—The road to AT. Abbreviations: ASA, aminosalicylic acid; Imm, immunomodulator; UC, ulcerative colitis.
Discussion
This study demonstrated that, over a mean 2.3-year follow-up, 65% of patients newly diagnosed with CD or UC initiated a 5-ASA, immunomodulator, systemic corticosteroids, or AT after diagnosis; the majority of patients (≥77%) initiated corticosteroid monotherapy. Overall, 14% of patients with CD, and 6% of those with UC, had any use of an AT during the follow-up period. Among the larger cohort of CD/UC patients, the pathway to AT initiation included an average of ≥4 episodes of conventional therapy. Likewise, most patients (CD: 82%; UC: 92%) received at least 1 steroid course prior to AT initiation; on average, patients cycled through 4–6 steroid courses. Most patients had received ≥2 MOAs of conventional therapy prior to initiating an AT. Of those receiving all 3 MOAs, the mean time to AT initiation was >1100 days.
A previous report using claims data from 2008 to 2016 detailed treatment pathways among newly diagnosed CD (N = 16 260) and UC (N = 28 119) patients in the United States. Among patients with CD, 40% of patients initiated corticosteroid monotherapy as a first-line treatment; 35% initiated 5-ASAs. Conversely, 61% of patients with UC initiated 5-ASAs as a first-line treatment, with only 25% initiating corticosteroid monotherapy. Overall, <5% of patients with CD, and <1% of patients with UC, initiated a biologic therapy. During the follow-up period (median of 3–3.5 years), 81% of patients with CD and 94% of patients with UC did not initiate biologic treatment.14 In the time since that report, treatment guidelines for the management of CD and UC have evolved. Currently, guidelines recommend initiating an AT in patients with moderate to severe CD or UC in order to induce early remission and prevent further organ damage.8–10,15,16 Recent data from the PROFILE study confirm, at least in CD, that early initiation of AT is a highly effective strategy12 and highlights the importance of this treatment approach.
The results of the current study differ from the previous report. Indeed, this analysis demonstrated that approximately 65% of newly diagnosed patients with CD and UC initiated a systemic disease-related treatment following diagnosis. Despite current treatment recommendations, which promote early use of ATs in patients with more severe disease,8,10 80.4% of patients initiated corticosteroid monotherapy. Only 14.4% of patients with CD and 5.9% of patients with UC had any AT use during the follow-up period (a mean of 2.3 years). While one could argue that the previously published work showing low utilization of ATs from 2008 to 2016 was during a time of transition of the treatment paradigm in IBD toward a more intensive treatment approach,14 the time period covered in this analysis (ie, 2017–2021) aligns with established literature and guidelines recommending early utilization of ATs,9,15,16 thus, the marginal improvement in the use of ATs demonstrated in this study is disappointing. However, given the inability to easily evaluate disease severity for the cohort of patients in this analysis, we are unable to state an appropriate goal for utilization of ATs in this patient cohort. Yet, the proportion of patients with both CD and UC who experienced disease-related complications, corticosteroid use, emergency department visits, and hospitalizations (Tables 1, 3, and 4) implies a cohort of patients with more moderate-to-severe disease. While treatment guidelines do suggest initiation of corticosteroids to assist in the induction of remission/response, they are not intended to be continued for maintenance of remission due to high risk of adverse effects.15,16 Thus, we would expect significantly higher utilization of ATs than that observed in this analysis.
Among the larger study population which included all eligible patients initiating an AT, this study showed that patients cycle through multiple different conventional pathway episodes and MOAs prior to AT initiation. Indeed, patients in this study had an average of ≥4 episodes of conventional treatment prior to AT initiation. Moreover, most patients (>82%) had a least 1 episode of corticosteroids and ≥79% of patients received 2 or more corticosteroid episodes. Most patients tried more than one MOA before initiating an AT, with corticosteroids and immunomodulators being the most common. These data highlight a troubling trend of treatment switching and delay of AT initiation in patients not responding to CT. These treatment patterns have been reported to lead to suboptimal treatment (as indicated by dose escalation, chronic corticosteroid use, nonadherence, and/or ≥2 emergency department visits) and increased healthcare resource utilization. In a study of patients with CD, 73% of patients receiving conventional therapy presented with ≥1 indicator of suboptimal treatment. Those with ≥4 indicators of suboptimal treatment who remained on a conventional therapy had nearly 4 times higher healthcare costs than those responding to treatment (ie, no indicators), which was driven by increased inpatient and outpatient visits.17 Altogether these data suggest that regular monitoring of disease activity and timely switch to ATs may benefit patients with CD or UC who are not responding to CT. Ideally, a predictive model could be developed for which patients will most need early AT. A recent article described a machine learning-based approach that developed an algorithm that was capable of identifying patients who are currently inadequately treated with traditional therapies and who may benefit from biological therapy18; more work is needed in this area.
The data from this analysis also suggest that significant knowledge gaps regarding best treatment practices may exist for providers. Indeed, a recent, prospective study determined that, among 197 eligible providers who completed the web-based survey, approximately one-third felt they needed a better understanding of how to care for patients with prior malignancy, for patients who were pregnant or considering becoming pregnant, and for elderly patients, as well as treatment options, including newly approved therapies (eg, Janus kinase inhibitors).19 More than half of providers did not participate in shared decision-making with their patients and cited time limits as the primary reason. Most providers wished to earn continuing medical education credits but preferred in-person educational settings.19 Studies have also demonstrated that patients also desire to gain a better understanding of their disease, including the risks and benefits of treatment, how to cope with their disease, and how to advocate for themselves.20,21
The strengths of this study are that the MarketScan database is based on a closed system that captures a relatively comprehensive set of medical and pharmacy claims incurred during the eligibility period of a patient. Likewise, this study benefits from the large sample size of the MarketScan database, which comprises a diverse group of individuals distributed across all geographic regions of the United States. Limitations of this study are that the claims database cannot capture disease severity, provide rationale for changes in therapy, or ensure patients actually take the medication as prescribed. Likewise, this study is subject to other data limitations inherent to insurance claims data, such as data entry errors or coding limitations. For example, the data presented here demonstrate high rates of complications based on diagnosis codes used in claims. However, data regarding follow-up and/or confirmatory diagnostics or event outcomes are unavailable, therefore rates of these events may be overestimated. Moreover, based on claims, some UC patients had complications suggestive of CD; however, the number of patients is quite small so it is unlikely that it significantly influenced the results. The impact of surgery was not studied as part of this analysis but could be a focus of future work. This study does not examine the impact of health insurance policies on access to ATs. Many health insurance companies may also require patients to step through multiple CTs prior to receiving ATs. Likewise, patients in the UC cohort tended to be older, which may impact the likelihood of providers prescribing and/or patients seeking advanced therapies due to misconceptions regarding safety. The results may not be generalizable to other populations, such as individuals with noncommercial health insurance or no health insurance. Likewise, no data on patient race and/or ethnicity were collected, thus further limiting the generalizability of the findings.
Conclusions
Despite significant progress in treatments, patients newly diagnosed with CD and UC continue to receive CT, especially corticosteroids, as their first treatment. Moreover, this study showed that patients cycle through multiple CTs, with an average of >4.5 courses of corticosteroids over a substantial period of time, before initiating an AT. These practice patterns are not in line with modern treatment recommendations. Better dissemination of treatment guidelines, as well as the removal of treatment barriers (eg, cost, needs for prior authorization, etc.) and increased allotment of time providers, may spend with patients, may help to effectively update current practice patterns. Likewise, clinician and patient education regarding risks associated with ineffective therapy, such as disease progression and complications, could facilitate the improvement of current practice patterns.
Supplementary Material
Acknowledgments
Medical writing services are provided by Samantha Francis Stuart, PhD, of Fishawack Facilitate Ltd, part of Avalere Health, and funded by AbbVie.
Contributor Information
Corey A Siegel, Inflammatory Bowel Disease Center, Section of Gastroenterology and Hepatology, Dartmouth Hitchcock Medical Center, Lebanon, NH, USA.
Dolly Sharma, Department of Health Economics and Outcomes Research, AbbVie, Inc, North Chicago, IL, USA.
Jenny Griffith, Department of Health Economics and Outcomes Research, AbbVie, Inc, North Chicago, IL, USA.
Quynhchau Doan, Department of Health Economics and Outcomes Research, AbbVie, Inc, North Chicago, IL, USA.
Si Xuan, Department of Health Economics and Outcomes Research, AbbVie, Inc, North Chicago, IL, USA.
Lisa Malter, Division of Gastroenterology, Department of Medicine, New York University Langone Medical Center, New York, NY, USA.
Author contributions
All authors contributed substantially to the study conception and design, interpretation of data, and drafting and revising the manuscript for intellectual content. S Xuan contributed to acquisition and analysis of data.
Funding
AbbVie funded the study. AbbVie participated in the study design, research, interpretation of the data, review, and approval. All authors had access to the relevant data and participated in the drafting, review, and approval of the abstract. No honoraria or payments were made for authorship.
Conflict of Interest
C.A.S. has received consultant/advisory board fees from AbbVie, BMS, Fresnius, Lilly, Janssen, Pfizer, ProciseDx, Prometheus, and Takeda; speaker fees for CME activities for AbbVie, Celgene, Janssen, Pfizer, Takeda; grant support from AbbVie, Janssen, Pfizer, Takeda. D.S., J.G., and S.X. are employees of AbbVie and may own stock or stock options. Q.D. is a former employee of AbbVie and may own stock or stock options. L.M. has received medical education grants from AbbVie, Janssen, Pfizer, and Takeda and served on an advisory board for AbbVie, BMS, Celltrion, Janssen, Merck, Pfizer, and Takeda. She has served as a consultant for AbbVie. She also holds the position of Associate Editor for Crohn’s & Colitis 360 and has been recused from reviewing or making decisions for the manuscript.
Ethical Statement
This study used de-identified data and thus did not require ethics board approval.
Data Availability
The data reported in this study are available from the corresponding author upon reasonable request.
References
- 1. Norton C, Czuber-Dochan W, Bassett P, et al. Assessing fatigue in inflammatory bowel disease: comparison of three fatigue scales. Aliment Pharmacol Ther. 2015;42(2):203-211. doi: 10.1111/apt.13255 [DOI] [PubMed] [Google Scholar]
- 2. Schreiner P, Rossel JB, Biedermann L, et al. ; Swiss IBD Cohort Study Group. Fatigue in inflammatory bowel disease and its impact on daily activities. Aliment Pharmacol Ther. 2021;53(1):138-149. doi: 10.1111/apt.16145 [DOI] [PubMed] [Google Scholar]
- 3. Knowles SR, Graff LA, Wilding H, Hewitt C, Keefer L, Mikocka-Walus A.. Quality of life in inflammatory bowel disease: a systematic review and meta-analyses-Part I. Inflamm Bowel Dis. 2018;24(4):742-751. doi: 10.1093/ibd/izx100 [DOI] [PubMed] [Google Scholar]
- 4. Knowles SR, Keefer L, Wilding H, Hewitt C, Graff LA, Mikocka-Walus A.. Quality of life in inflammatory bowel disease: a systematic review and meta-analyses-Part II. Inflamm Bowel Dis. 2018;24(5):966-976. doi: 10.1093/ibd/izy015 [DOI] [PubMed] [Google Scholar]
- 5. Terlizzi EP, Dahlhamer JM, Xu F, Wheaton AG, Greenlund KJ.. Health Care Utilization Among U.S. Adults With Inflammatory Bowel Disease, 2015–2016. US Department of Health and Human Services; Centers for Disease Control and Prevention; National Center for Health Statistics; 2021. Report No.: 152. [PubMed] [Google Scholar]
- 6. Ghiani M, Naessens D, Takacs P, Myers D, Bokemeyer B, Wilke T.. Long-term cost and complications of surgery in patients with ulcerative colitis: a claims data analysis. Int J Colorectal Dis. 2021;36(4):831-840. doi: 10.1007/s00384-021-03876-z [DOI] [PubMed] [Google Scholar]
- 7. Mehta F. Economic implications of inflammatory bowel disease and its management. Am J Manag Care. 2016;22:S51-S60. [PubMed] [Google Scholar]
- 8. Nakase H, Uchino M, Shinzaki S, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol. 2021;56(6):489-526. doi: 10.1007/s00535-021-01784-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Turner D, Ricciuto A, Lewis A, et al. ; International Organization for the Study of IBD. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570-1583. doi: 10.1053/j.gastro.2020.12.031 [DOI] [PubMed] [Google Scholar]
- 10. Okobi OE, Udoete IO, Fasehun OO, et al. A review of four practice guidelines of inflammatory bowel disease. Cureus. 2021;13(8):e16859. doi: 10.7759/cureus.16859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. D’Haens G, Baert F, van Assche G, et al. ; Belgian Inflammatory Bowel Disease Research Group. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet. 2008;371(9613):660-667. doi: 10.1016/S0140-6736(08)60304-9 [DOI] [PubMed] [Google Scholar]
- 12. Noor NM, Lee JC, Bond S, et al. ; PROFILE Study Group. A biomarker-stratified comparison of top-down versus accelerated step-up treatment strategies for patients with newly diagnosed Crohn’s disease (PROFILE): a multicentre, open-label randomised controlled trial. Lancet Gastroenterol Hepatol. 2024;9(5):415-427. doi: 10.1016/S2468-1253(24)00034-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Law CCY, Tkachuk B, Lieto S, et al. Early biologic treatment decreases risk of surgery in Crohn’s disease but not in ulcerative colitis: systematic review and meta-analysis. Inflamm Bowel Dis. 2023;30(7):1080-1086. doi: 10.1093/ibd/izad149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Siegel CA, Yang F, Eslava S, Cai Z.. Treatment pathways leading to biologic therapies for ulcerative colitis and Crohn’s disease in the United States. Clin Transl Gastroenterol. 2020;11(2):e00128. doi: 10.14309/ctg.0000000000000128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feuerstein JD, Ho EY, Shmidt E, et al. ; American Gastroenterological Association Institute Clinical Guidelines Committee. AGA Clinical Practice Guidelines on the medical management of moderate to severe luminal and perianal fistulizing Crohn’s Disease. Gastroenterology. 2021;160(7):2496-2508. doi: 10.1053/j.gastro.2021.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feuerstein JD, Isaacs KL, Schneider Y, Siddique SM, Falck-Ytter Y, Singh S; AGA Institute Clinical Guidelines Committee. AGA Clinical Practice Guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology. 2020;158(5):1450-1461. doi: 10.1053/j.gastro.2020.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pilon D, Ding Z, Muser E, et al. Indicators of suboptimal treatment and associated healthcare costs among patients with Crohn’s Disease initiated on biologic or conventional agents. Crohns Colitis 360. 2022;4(3):otac021. doi: 10.1093/crocol/otac021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scholer D, Kostev K, Peters M, et al. Machine learning can predict the probability of biologic therapy in patients with Inflammatory Bowel Disease. J Clin Med. 2022;11(15):4586. doi: 10.3390/jcm11154586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malter L, Jain A, Cohen BL, et al. Identifying IBD providers’ knowledge gaps using a Prospective Web-based Survey. Inflamm Bowel Dis. 2020;26(9):1445-1450. doi: 10.1093/ibd/izaa032 [DOI] [PubMed] [Google Scholar]
- 20. Khalil C, Van Deen W, Dupuy T, Bonthala N, Almario C, Spiegel B.. Developing patient-centered inflammatory bowel disease-related educational videos optimized for social media: qualitative research study. JMIR Med Educ. 2020;6(2):e21639. doi: 10.2196/21639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Krauthammer A, Harel T, Zevit N, Shouval DS, Shamir R, Weiss B.. Knowledge of disease and self-management of adolescents with inflammatory bowel diseases. Acta Paediatr. 2020;109(10):2119-2124. doi: 10.1111/apa.15211 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data reported in this study are available from the corresponding author upon reasonable request.