Abstract
At the start of the Zika virus (ZIKV) epidemic in 2015, ZIKV spread across South and Central America, and reached parts of the southern United States placing pregnant women at risk for fetal microcephaly, fetal loss, and other adverse pregnancy outcomes associated with congenital ZIKA syndrome (CZS). For this reason, testing of a safe and efficacious ZIKV vaccine remains a global health priority. Here we report that a single immunization with Ad26.M.Env ZIKV vaccine, when administered prior to conception, fully protects pregnant rhesus macaques from ZIKV viral RNA in blood and tissues with no adverse effects in dams and fetuses. Furthermore, vaccination prevents ZIKV distribution to fetal tissues including the brain. ZIKV associated neuropathology was absent in offspring of Ad26.M.Env vaccinated dams, although pathology was limited in fetuses from non-immunized, challenged dams. Vaccine efficacy is associated with induction of ZIKV neutralizing antibodies in pregnant rhesus macaques. These data suggest the feasibility of vaccine prevention of CZS in humans.
Subject terms: Vaccines, Virology
Introduction
In late 2015, an epidemic of fetal microcephaly in Brazil associated with high levels of circulating Zika virus (ZIKV), led to a global race to develop a ZIKV vaccine to protect women and unborn children from the potential devastating effects of congenital ZIKA syndrome (CZS)1–4. ZIKV, a member of the Flaviviridae family, was first identified in Uganda in 1954, and while sharing a genus with other viruses that cause significant human disease such as dengue, yellow fever, Japanese encephalitis, and West Nile viruses, had only been associated with asymptomatic to mild flu-like symptoms prior to reports of CZS in Brazil in late 2015. Similar to other medically important flaviviruses, ZIKV is primarily acquired by Aedes mosquitos, and can also be spread transplacentally in pregnant women5,6, in blood transfusions7, and through sexual contact8. Recently, genetic polymorphisms have been associated with development of CZS9.
The World Health Organization (WHO) declared an end to the ZIKV epidemic at the end of 201610, and ZIKV transmission is currently at low levels world-wide, however vaccine development for emergency deployment remains a high priority by the WHO11. ZIKV also remains a concern for individuals traveling to endemic areas and for individuals living in areas with continued transmission such as the Caribbean where over 15% of pregnant women continue to test positive for ZIKV12. CDC guidance still recommends that pregnant women, partners of pregnant women, or those considering pregnancy to delay travel to areas with ZIKV outbreaks and to consult with medical providers before traveling to ZIKV endemic regions13.
Current low levels of ZIKV world-wide limit the establishment of clinical trial sites and enrollment of participants in Phase III efficacy studies11,14. Ideally a ZIKV vaccine could be deployed in the event of a ZIKV resurgence and administered safely to pregnant women. Preclinical studies in mice and non-human primates (NHP) have shown that induction of neutralizing antibodies by a number of vaccine platforms is effective in preventing ZIKV acquisition15–18). A number of candidate ZIKV vaccines have completed safety studies in Phase I and II clinical trials19–22. The macaque model has been a useful model for studying dynamics of viral replication and shedding during ZIKV infection16,23–26. We and others have shown that the rhesus macaque model consistently reproduces features of ZIKV infection in pregnancy including prolonged ZIKV vRNA in blood and persistence of ZIKV vRNA in lymphoid tissues and the placenta23,26–33. Although fetal microcephaly has not been reported as a fetal outcome of ZIKV infection in monkeys, experimental ZIKV challenge of pregnant non-human primates has recapitulated other adverse outcomes of ZIKV exposure observed in human pregnancy including fetal loss, fetal cerebral calcifications, gliosis, and long-term developmental alterations in infant macaques30,34–36. As in humans, these events represent a small proportion of overall pregnancy outcomes. While DNA vaccination can protect against ZIKV vRNA in blood, break-through viral replication was seen in a subset of animals16 and DNA vaccination was only partially protective against vRNA in blood in pregnant macaques37. Attempts to treat pregnant monkeys with cocktails of neutralizing antibodies also failed to prevent ZIKV vRNA in blood and adverse fetal outcomes were observed including fetal loss27.
Adenoviruses are a wide-spread cause of common upper respiratory tract infections that are typically mild and self-limiting. Adenovirus vectored vaccines are non-replicating yet self-adjuvanting due to their potent induction of innate anti-viral responses that contribute to the induction of broad cellular and humoral immune responses after a single immunization38. Ad26 has emerged as a lead Ad vector platform due to low levels of pre-existing immunity and induction of durable neutralizing Ab responses39. A common correlate of protection for currently licensed flavivirus vaccines including the yellow fever, dengue, and west nile viruses is the induction of neutralizing antibodies against the envelope (Env) protein40. Ad26.M.Env is a monovalent recombinant Ad26-based ZIKV vaccine candidate that encodes for ZIKV membrane (M) protein, lacking the peptide precursor, and envelope (Env) antigens (amino acids 216–794 of the polyprotein) derived from the ZIKV strain BeH815744 that was shown to be immunogenic in animal models41). This construct has also been tested in a human Phase I study (Ad26.ZIKV.001) where it was shown to be well-tolerated and immunogenic22. In addition, it was shown to be protective against fetal demise in pregnant interferon alpha/beta receptor knock-out mice42), a highly susceptible model where ZIKV infection leads to high levels of ZIKV plasma vRNA levels and placental ZIKV vRNA and fetal loss43. We opted to test this vaccine for safety and efficacy in pregnant rhesus macaques that were ZIKV challenged during the critical equivalent of human first trimester pregnancy. Here we show that Ad26.M.Env prevents peripheral blood ZIKV vRNA and tissue vRNA in pregnant macaques and fetuses with no evidence of ZIKV-associated fetal pathology in rhesus monkeys.
Results
Ad26.M.Env vaccination induced potent anti-ZIKV neutralizing antibodies and prior vaccination did not impact conception in female macaques
Thirteen female macaques were immunized with 1011vp of Ad26.M.Env expressing both the ZIKV M protein transmembrane domain without the peptide precursor, and the envelope (Env) antigens (Ad26.M.Env) and were returned to the breeding colony 17 days post-vaccination (Fig. 1a). Dams were vaccinated a minimum of 2 months and a maximum of 7 months prior to challenge. An additional 13 females were selected that received no vaccination that were placed in the breeding colony at the same time as the vaccine group. No adverse events were reported in either vaccinated or non-vaccinated animals prior to ZIKV challenge. Some animals in both the vaccinated and non-vaccinated groups had reports of weight loss, diarrhea, and trauma that required clinical intervention, but need for clinical intervention was not linked to vaccination or ZIKV challenge. Vaccinated and non-vaccinated females were monitored clinically by complete red blood cell count (CBC). Animals generally maintained normal reference ranges for white blood cell count, red blood cell count, and total lymphocyte counts through-out the study period (Supplementary Fig. 1).
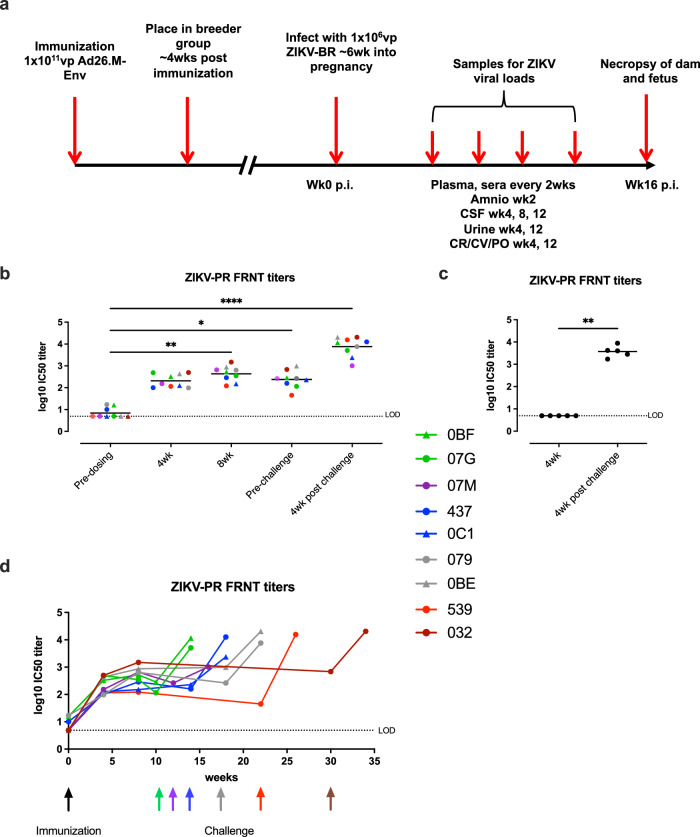
Fig. 1. Ad26.M.Env induces robust neutralizing antibodies in sera in female breeding macaques.
a Macaques were vaccinated 17 days prior to introduction into breeding groups, monitored for pregnancy every two weeks and challenged with ZIKV-BR six weeks post-conception. ZIKV-PR neutralizing antibody responses in sera of dams (n = 9) immunized with 1011 vp Ad26.M.Env (b, d) or non-immunized control animals (n = 5) (c) determined by FRNT. d Colored connective lines represent the neutralization response in time. Neutralizing antibody titers are reported as the log10 of the inverse of the serum dilution that reduce the number of input virus by 50% (IC50). The mean responses per group are indicated with a horizontal line. The dashed line shows the lower limit of detection (LOD) defined as the log10 of one dilution below the start dilution of the samples (0.70 log10). Individual animals of group 1 (Ad26.M.Env immunized) are color coded to represent number of weeks between immunization and challenge; green (10 weeks); purple (12 weeks); blue (14 weeks); gray (18 weeks); red (22 weeks); brown (30 weeks). Black arrow indicates time of vaccination; colored arrows indicate time of challenge matched to inter-immunization and challenge interval color in figure legend. Amino amniocentesis, CSF cerebral spinal fluid, CR colorectal swab, CV cervical swab, PO oropharyngeal swab. b Friedman one-way ANOVA, followed by Dunn’s test for multiple comparisons (c) Mann–Whitney test; *p = /< 0.05; **p = /< 0.01; ***p = < 0.001. Samples below LOD were set to 0.70.
Females in all groups were monitored bi-weekly by ultrasound for pregnancy. Pregnant females in the Ad26.M.Env vaccine and no vaccine groups were infected with 1×106 vp of ZIKV via the subcutaneous route 6 weeks post-conception (10–30 weeks post-vaccination based on timing of confirmed pregnancy; Supplementary Table 1). Three pregnant non-vaccinated animals were not challenged to serve as normal pregnant controls. Plasma, sera, cerebral spinal fluid, urine, colorectal, cervical, and saliva samples were collected during pregnancy from all dams as indicated (Fig. 1a).
Neutralizing antibody titers were determined by both Immunospot focus reduction neutralization (FRNT) and by microneutralization assay (MN50 VNA). Before immunization, animals (OBF, 079 and 437) showed very low neutralization titers, whereas the other six animals showed no neutralization titers when assayed with FRNT (Fig. 1b). Four weeks after immunization, all animals developed a neutralizing response that was maintained or only marginally decreased 8 weeks after immunization and in pre-challenge serum which was obtained 10 to 30 weeks after immunization (Fig. 1b, d). Four weeks after challenge, all animals of the Ad26.M.Env immunized group had increased ZIKV neutralization titers (Fig. 1b). Serum of animals of the non-immunized control group were assayed pre- and post- challenge. Pre-challenge, no neutralization titers were detected with FRNT analysis, whereas ZIKV neutralization titers developed after challenge (Fig. 1c). These FRNT results were confirmed by the MN50 neutralization assay (Supplementary Fig. 2). Vaccinated animals (Group 1) had higher neutralizing Ab four weeks post-vaccination compared to non-vaccinated animals (p = 0.0010, Mann–Whitney test). Post-challenge neutralizing titers were comparable between Ad26.M.Env and non-vaccinated animals (Mann–Whitney test), consistent with minimal anamnestic antibody responses in vaccinated animals and rapid virus neutralization (Fig. 1b, c, Supplementary Fig. 2). Next, antibody responses against ZIKV NS1 protein were measured by ELISA. Ad26.M.Env does not contain a NS1 antigen. In accordance, Ad26.M.Env vaccinated or non-vaccinated animals had low (079) or undetectable NS1 binding antibody responses in pre-challenge samples. Four weeks after challenge, all 5 non-vaccinated dams developed high NS1-specific antibody titers (mean titer of 3.69 log10). Eight out of 9 dams that received Ad26.M.Env also developed NS1-specific titers after challenge although the group mean NS1 titer (1.75log10) was approximately 100-fold (2 log10) lower compared to the group mean NS1-titer in the non-immunized animals (p = 0.005, Mann–Whitney test; Supplementary Fig. 3).
Ad26.M.Env vaccination induced anti-Env cellular immune responses in macaques
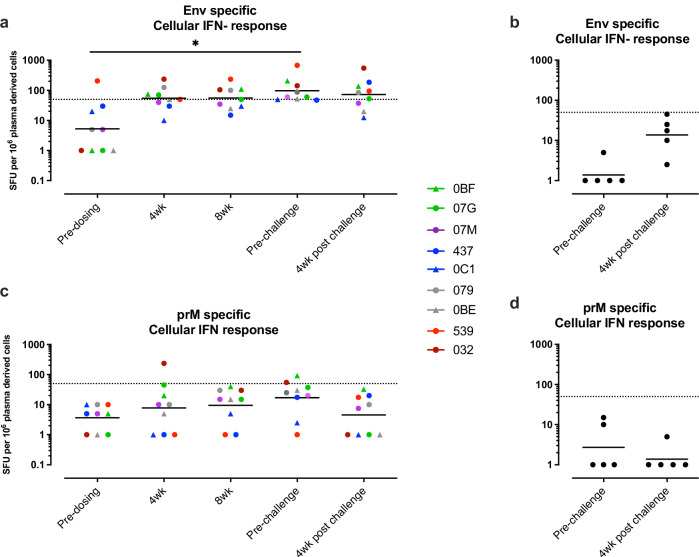
Env and prM directed cellular immune responses were measured by IFNγ ELISPOT on frozen PBMC’s isolated pre- immunization, post-immunization, pre-challenge, and post-challenge. Ad26.M.Env vaccination resulted in induction of ZIKV specific cellular responses (Fig. 2). The geometric mean Env-specific cellular immune responses in the group that received Ad26.M.Env was above the LOD of the assay, determined at 50 SFU per 106 PBMCs, at week 4 and 8 after immunization, and at the pre-challenge timepoint (geomean SFU 55.03, 55.92, and 97.41, respectively). The Env-specific cellular immune responses after immunization were higher when compared to the Env-specific cellular immune responses pre-immunization, or in non-immunized animals which were both below the limit of detection (Fig. 2a, b). Vaccinated animals had significantly higher anti-Env cellular immune responses pre-challenge (p = 0.0106) and post-challenge (p = 0.0470) compared to non-vaccinated animals (Mann–Whitney test; with data points below the LOD of 50 SFU set on LOD). The prM-specific cellular responses were generally low and geometric mean responses do not exceed the cut-off of 50 SFU per 106 PBMCs (Fig. 2c, d). Notably, Env and prM cellular responses did not increase after challenge as compared to the pre-challenge timepoint indicating a lack of anamnestic cellular responses in Ad26.M.Env vaccinated animals (Fig. 2a, c).
Fig. 2. Ad26.M.Env vaccine induces low level cellular immune anti-Env responses in pregnant macaques.
IFNγ ELISPOT responses in PBMCs of dams (n = 9) immunized with 1011 vp Ad26.M.Env (a, c) or non-immunized control animals (n = 5) (b, d). a, b Env-specific responses and (c, d) PrM specific responses are shown. The geometric mean response per group is indicated with a horizontal line. Responses above 50 SFU per 106 PBMCs indicated by the dotted line are considered positive. Individual animals of group 1 (Ad26.M.Env immunized) are color coded to represent number of weeks between immunization and challenge; green (10wk); purple (12 weeks); blue (14 weeks); gray (18 weeks); red (22 weeks); brown (30 weeks). a Friedman one-way ANOVA, followed by Dunn’s test for multiple comparisons; *p = /< 0.05; **p = /< 0.01; ***p = < 0.001. Samples below LOD were set to 50.
Ad26.M.Env vaccinated pregnant females were completely protected against ZIKV vRNA in blood and tissues
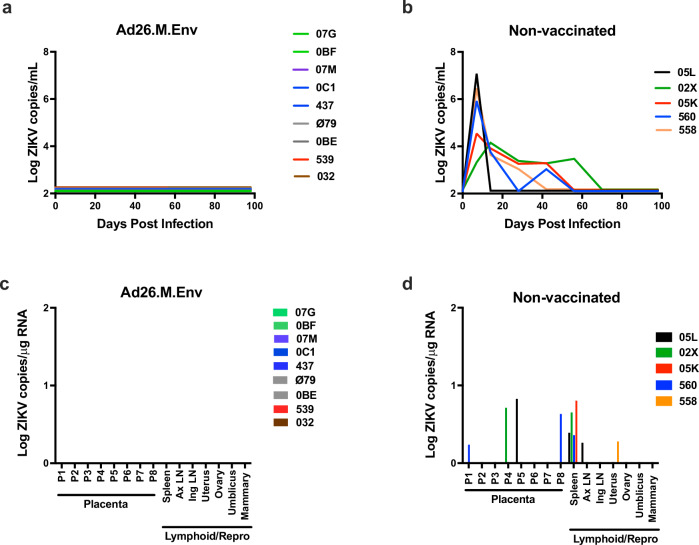
Pregnant females in the Group 1 (Ad26.M.Env vaccinated) and Group 2 (non-vaccinated) were infected with 1x103 PFU (1×106 vp) Zika virus from the 2015 Brazilian epidemic at 6 weeks post-conception (being 10 to 30 weeks post vaccination). Vaccinated dams had no detectable virus in plasma post-ZIKV challenge even though animals were challenged from 10 to 30 weeks after vaccination, depending on the timepoint of conceiving (Fig. 3a). In contrast, non-vaccinated, challenged pregnant macaques all had detectable viral load, with a mean peak vRNA of 5.5 log10 on day 7 post-challenge (Fig. 3b) consistent with peak vRNA reported for ZIKV infected non-pregnant and pregnant macaques23,25,26,28,32,36,37,44,45. On average, non-vaccinated, challenged animals had detectable virus for 42 days with a range of 7 to 56 days consistent with previous reports that pregnancy prolongs ZIKV viremia in rhesus monkeys. All colorectal, vaginal, saliva, and amniocentesis samples were negative for ZIKV vRNA for all ZIKV-challenged dams irrespective of vaccination status.
Fig. 3. Ad26.M.Env vaccination prevents viral replication in blood and dissemination of ZIKV to tissues in pregnant dams.
Pregnant Ad26.M.Env vaccinated (n = 9) and non- vaccinated (n = 5) macaques were challenged with 1×106 vp of ZIKV at 6 weeks following conception and sera was monitored longitudinally for 100 days following challenge. ZIKV log10 copies per mL of sera from (a) Ad26.M.Env vaccinated dams and (b) non-vaccinated dams were determined by qRT-PCR and depicted as log10 ZIKV copies/mL sera at days 0, 7, 14, 28, 42, 56, 70, 84, and 98. The limit of detection of this assay was 100 copies/mL sera (2Log10). a Individual animals of group 1 (Ad26.M.Env immunized) are color coded to represent number of weeks between immunization and challenge; green (10wk); purple (12 weeks); blue (14 weeks); gray (18 weeks); red (22 weeks); brown (30 weeks). Lymphoid and reproductive tissues were collected from dams at time of Cesarian section (C-section). C-sections were performed 10–14 days prior to estimated full-term delivery dates (~21–23 weeks pregnancy) at approximately 16 weeks following ZIKV challenge. Viral RNA was determined by RT-PCR for (c) Ad26.M.Env vaccinated and (d) non-vaccinated dams on tissues collected at necropsy. AxLN axillary lymph node, IngLN inguinal LN.
After confirmation of pregnancy, dams were monitored monthly for fetal biometric analyses including measurements of biparietal diameter, occipitofrontal length, head circumference, and femur length by ultrasonography. No abnormalities were noted in fetal biometric parameters in all study groups (Supplementary Fig. 4) and fetal brain weights and brain:fetal body weight ratios at necropsy were similar across groups (Supplementary Fig. 5).
Dams had scheduled Cesarian sections (C-section) and euthanasia when fetuses were term, approximately 2 weeks prior to estimated delivery date. Maternal tissues previously shown to have detectable virus throughout pregnancy were collected for evaluation by RT-PCR for ZIKV vRNA33). None of the nine Ad26.M.Env vaccinated dams had detectable vRNA in any tissues surveyed (Fig. 3c). All non-vaccinated, challenged dams had detectable vRNA in at least one of the analyzed tissues. 4/5 animals showed positive vRNA in maternal spleen, consistent with previous reports23,33,34), and one dam had vRNA detected in the axillary LN. One dam had detectable vRNA in the uterus, and 3/5 dams had virus detectable in the placenta (Fig. 3d). Placental pathology was evaluated for dams in all groups by both a veterinary pathologist and a human gynecological pathologist specializing in placental histopathology. Histopathological placental findings in all groups were typical of near-term/term placentas in macaques with evidence of maternal thrombosis and infarction in all groups (Supplementary Fig. 5, Table 1)46. Fetal: placental ratios were within the expected limits for term fetuses and did not vary significantly between groups (Supplementary Fig. 5).
Neonates born to Ad26.M.Env vaccinated dams were negative for ZIKV vRNA and had no ZIKV-associated histopathological abnormalities
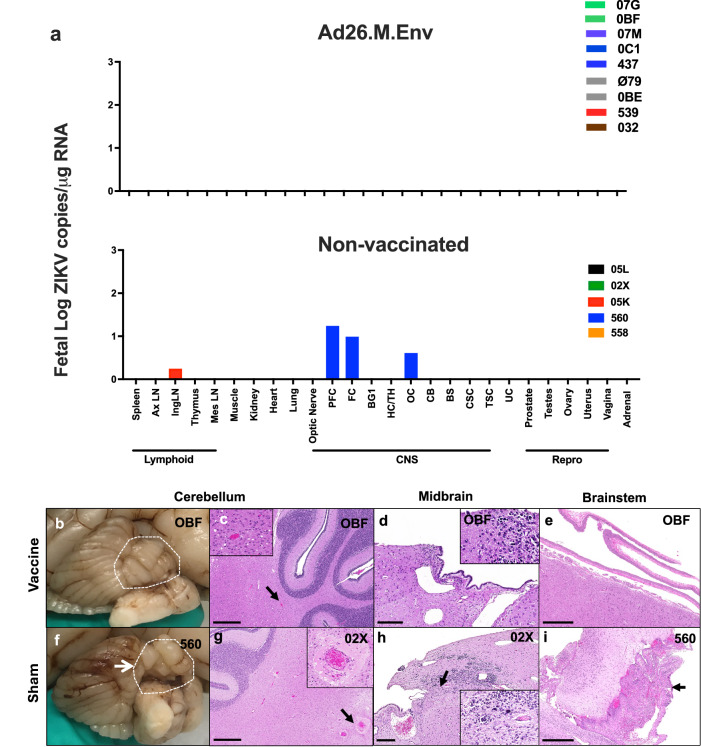
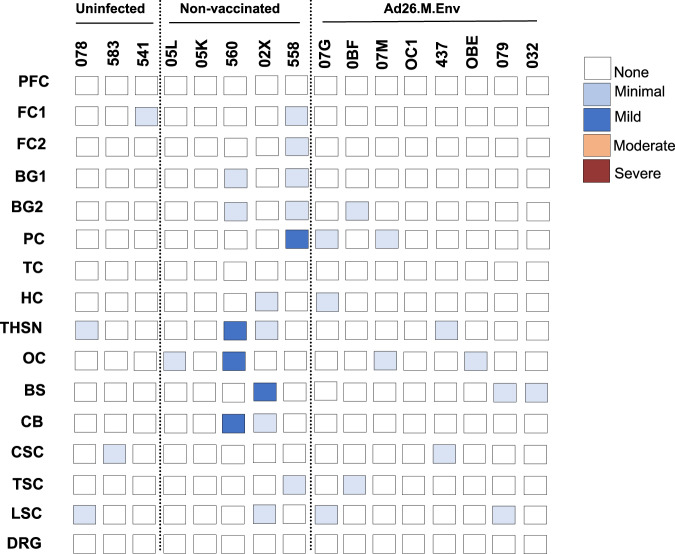
Following C-section and euthanasia, fetuses were inspected for gross abnormalities and fetal tissues collected for histopathology and evaluation of vRNA. Fetuses of vaccinated dams had no evidence of viral replication in tissues (Fig. 4a, upper) while 2/5 fetuses from non-vaccinated, challenged dams had detectable virus in tissues, one of which (Fetus 560) had extensive detection of ZIKV in the brain (Fig. 4a, lower). Histopathologic evaluation of brain from fetuses born to Ad26.M.Env vaccinated dams showed no evidence of previously reported ZIKV neuropathology including microcalcifications and perivascular edema (Fig. 4b–e, Fig. 5). Fetuses from non-vaccinated, challenged dams had a myriad of abnormal findings (Table 1) including a gross cerebellar malformation (Fig. 4f), asymmetry of the left parietal lobe (Supplementary Fig.6, Table 1), and a gross dystrophic calcification on the liver (Table 1), focal edema (Fig. 4g), microcalcification (Fig. 4h), and meningeal proliferation (Fig. 4i). However, the fetuses from the non-vaccinated, challenged dams overall had fewer histopathological findings than previously reported (Fig.5)33. Tissues that were positive for ZIKV by qPCR were evaluated by IHC and RNAscope ISH, but virus was not detected within lesions therefore gross and histological abnormalities in fetuses from non-vaccinated, challenged dams could not be definitively linked to previous or on-going ZIKV viral replication.
Fig. 4. Ad26.M.Env vaccination prevents ZIKV dissemination and tissue pathology in fetuses following ZIKV challenge of pregnant dams.
Necropsy and collection of fetal tissues were performed on fetuses following euthanasia after term Cesarian delivery. Each dam had only a single fetus. a RT-PCR was performed on fetal tissues in fetuses from Ad26.M.Env vaccinated and ZIKV-challenged dams (upper) and non-vaccinated and ZIKV-challenged dams (lower). Formalin-fixed gross specimens and histopathological changes in fetuses from Ad26.M.Env vaccinated and ZIKV challenged dams (b–e) as compared to non-vaccinated and ZIKV challenged dams (f–i) showing normal cerebellar folio (b; dotted white line), vasculature within cerebellum (c), midbrain progenitor cells (d), and meninges (e) as compared to cerebellar dysplasia (f, dotted white line), perivascular edema of cerebellar vessel (g), microcalcification within midbrain progenitor cells (h), and unusual thickening of the meninges (i) in macaque infants from non-vaccinated dams. AxLN axillary lymph node, IngLN inguinal LN, Mes LN mesenteric lymph node, PFC prefrontal cortex, FC frontal cortex, BG1 basal ganglia section 1, HC/TH hippocampus, thalamus, OC occipital lobe; CB cerebellum, BS brain stem, CSC cervical spinal cord, TSC thoracic spinal cord, UC umbilical cord. Scale bars = 200 uM (d, h); 500 uM (c, e, g, i).
Fig. 5. Histopathologic scoring for neuropathology in macaque infants.
Summary of neuropathologic lesions in the CNS (see also Table 1). Scoring system is defined as previously described in ref. 33. PFC prefrontal cortex, FC frontal cortex, BG basal ganglia, TH thalamus, SN substantia nigra, HC hippocampus, OC occipital cortex, PC parietal cortex, TC temporal cortex, CB cerebellum, BS brain stem, CSC cervical spinal cord, TSC thoracic spinal cord, LSC lumbosacral spinal cord, DRG dorsal root ganglia.
Table 1.
Summary of gross and histopathological findings in dam and neonate tissues
| Animal ID | Condition | Group | Sex | Gross Findings | Histopathology-Fetus | Histopathology-Placenta |
|---|---|---|---|---|---|---|
| 78 | Control | 3 | F | NSF | Hemorrhage in lateral ventricle | moderate calcifications |
| 583 | Control | 3 | M | NSF | Cortical microcalcification | NSF |
| 541 | Control | 3 | M | NSF | Increased meningeal cellularity | NSF |
| 05K | ZIKV | 2 | F | Hard, white lesion noted in liver | Cortical/neuroprogenitor dysplasia (mild); focal gliosis | placental thinning |
| 560 | ZIKV | 2 | F | absent occipital gyrus (L); focal proliferation of neuropil on the right cerebellar lateral hemisphere | Cortical/neuroprogenitor dysplasia (mild); Cortical and periventricular microcalcification; multifocal microhemorrhage; mild neuropil vacuolation/rarefication; multifocal meningeal proliferation; increased meningeal cellularity | NSF |
| 05L | ZIKV | 2 | M | Dilated lateral ventricle upon examination of fixed specimens; asymmetry L parietal lobe | Cortical microcalcification | thrombosis maternal vessel |
| 02X | ZIKV | 2 | M | Absent gyrus in parietal cortex; cloudy CSF | Microcalcification within neuroprogenitor clusters, perivascular edema, and necrosis; mild neuropil vacuolation/rarefication; focal gliosis | NSF |
| 558 | ZIKV | 2 | M | Enlarged ventricle noted upon examination of fixed specimens | Cortical/neuroprogenitor dysplasia (mild); cortical microcalcification; hemorrhage in lateral ventricle; multifocal gliosis; increased meningeal cellularity; multifocal spinal cord microhemorrhage | Moderate infarction/necrosis chorionic plate; thrombosis maternal vessel |
| 07G | Vaccinated, ZIKV | 1 | M | NSF | Cortical/neuroprogenitor dysplasia (mild); mild neuropil vacuolation/rarefication; focal gliosis | thrombosis maternal vessel |
| 0BF | Vaccinated, ZIKV | 1 | M | NSF | NSF | Mild abruption; thrombosis maternal vessel |
| 437 | Vaccinated, ZIKV | 1 | M | NSF | NSF | Mild infarction/necrosis chorionic plate; placental thinning; thrombosis maternal vessel |
| 07M | Vaccinated, ZIKV | 1 | F | NSF | Cortical microcalcification; multifocal microhemorrhage; mild neuropil vacuolation/rarefication | NSF |
| OC1 | Vaccinated, ZIKV | 1 | F | NSF | NSF | NSF |
| OBE | Vaccinated, ZIKV | 1 | F | NSF | NSF | Not evaluated; Vaginal delivery infant |
| 79 | Vaccinated, ZIKV | 1 | F | NSF | NSF | NSF |
| 32 | Vaccinated, ZIKV | 1 | F | NSF | Mild neuropil vacuolation/rarefication | Mild infarction/necrosis chorionic plate; thrombosis maternal vessels |
Discussion
ZIKV viral infections world-wide resulted in a pandemic of fetal malformations and fetal loss in pregnant women in 2016. The repercussions of this devasting disease will continue to manifest itself as cohorts of exposed women and children continue to be followed. Although massive ZIKV exposure across endemic regions of South America has likely resulted in elevated neutralizing antibodies in exposed populations, waning of immunity over time will likely lead to cyclical re-emergence of ZIKV as seen with previous outbreaks in Malaysia. The United States is at risk for the re-emergence of a future ZIKV pandemic since the Aedes aegypti mosquito vector is endemic to the southern United States. The lack of exposure of the US population to ZIKV during the 2016 epidemic makes US individuals vulnerable to future ZIKV epidemics. Development of a well-tolerated and safe vaccine that can be used pre- and perinatally is critical to protecting naïve individuals during future ZIKV outbreaks.
The Ad26.M.Env (Ad26.ZIKV.001) vaccine was tested in a Phase I clinical trial and all regimens tested were well tolerated, with no safety concerns identified. Vaccination induced robust ZIKV neutralizing titers that were of similar magnitude to those induced in macaques in this study22. In addition, transfer of immune sera from vaccinated study participants to mice protected mice against ZIKV vRNA in blood22. Viral vectored vaccines that are immunogenic with a single immunization remain an attractive option for use in resource-poor settings where vaccine access may be limited. The Ad26.CoV2.S vaccine construct was widely deployed during the COVID-19 pandemic and was protective against severe disease and variant infection after a single or prime-boost immunization47,48. In addition, Ad26.CoV2.S showed durable neutralizing antibody responses which may offer some advantages over similar mRNA constructs in resource-limited settings or during outbreak scenarios49. Currently an Ad26 Ebola vaccine (Ad26.ZEBOV) is licensed and recommended as a childhood vaccine in Ebola endemic regions50. Although, adenoviral based vaccines used to combat COVID-19 were associated with immune-mediated thrombocytopenia and thrombosis, the mechanisms for these AE have not been fully elucidated, and more work is required to determine if these AE are related to the interplay between the Adenovirus based vaccine and the COVID-19 spike protein, or to the Adenovirus vaccine platform itself and can be avoided by improvements in vaccine platform. mRNA based vaccines against ZIKV have also been shown to be immunogenic and protective in NHP challenge studies, with variable efficacy in inducing neutralizing antibody responses in people18,20. Nevertheless, adenoviral constructs remain one of the most studied vaccine vectors and will likely continue to be an important construct for global use.
Here we show that an Ad26.M.Env vaccine is safe and efficacious against preventing ZIKV vRNA in sera and tissue of pregnant rhesus macaques. Antibody titers induced by A26.M.Env ZIKV vaccination in macaques were of similar magnitude as those induced by vaccination in a Phase I clinical trial in people22 and to titers induced by the inactivated Zika virus vaccine (ZPIV)19. While current low levels of ZIKV worldwide will impede the ability to conduct prevention of infection studies in people, studies of ZIKV vaccine protection in non-human primates provide critical data that can be extrapolated for immunobridging studies as has been done with Ebola vaccines51,52. Vaccine induced neutralizing antibodies against ZIKV have been shown to be sufficient for protection in mice that have undergone CD4+ and CD8 + T cell depletions15. Similar studies have not been performed to confirm that Ab titers alone are sufficient for maternal protection during pregnancy. Notably, Ad26.M.Env also induced robust anti-Env CD4+ and CD8 + T cell responses. Further work is necessary to determine if cellular immune responses offer improved quality of protection during pregnancy. In both this study and the Phase I clinical trial with Ad26.ZIKV.00122) no anti-PrM cellular immune responses were observed. This may be the consequence of limited antigen size or immunodominance of the Env epitope.
This study had several limitations that prevented us from assessing the full potential of the ZIKV Ad26.M.Env in protecting pregnant dams. In our effort to capture fetal neuropathology, we allowed the pregnancies to continue to term which limited our ability to study ZIKV associated placental pathology which has been well-described in the macaque and marmoset models of ZIKV infection during pregnancy53. Similarly, we did not sacrifice fetuses at timepoints where unvaccinated dams remained viremic in plasma, which limited our ability to more rigorously determine if vaccination protects against virus in the placenta and fetal brain during periods of robust systemic viral replication. In addition, fewer neuropathological findings were detected in fetuses from non-vaccinated, challenged dams in the current study as compared to our previous report33 highlighting the need for studies with large numbers of animals to capture the full spectrum of fetal outcomes. Such large-scale studies are typically not possible due to restrictions on the use of NHP for research and limitations in animal availability. Likewise, we observed two gross anomalies in the fetal brains of non-vaccinated, ZIKV challenged animals, but given the limited evaluation of fetal macaque brains at term in normal colony macaques, we cannot rule out that the gross lesions observed represent normal variation in macaque brains despite their absence in the vaccinated group. Furthermore, questions remain regarding the potential role for previous infection with other related viruses such as Dengue fever virus (DENV) in predisposing to ZIKV-induced development of CZS54. Recent studies in both pregnant macaques and marmosets have shown evidence of enhanced neuropathology and placental pathology in previously DENV exposed, ZIKV infected animals55,56. Future studies on ZIKV vaccine efficacy in DENV pre-exposed dams may be warranted. Lastly, we were unable to follow a cohort of infants longitudinally in this study to determine whether vaccination of dams prevented neurocognitive deficits in offspring57).
All non-vaccinated dams in this study had persistent virus detected in lymphoid organs at term, supporting that ZIKV infection during pregnancy can have severe consequences for pregnant women in the absence of clinical signs. Here we show that vaccination with Ad26.M.Env prevents persistent ZIKV replication in lymphoid organs, placenta and fetal tissue including brain in the non-human primate model with a single immunization. Future studies evaluating Ad26.M.Env protection during the acutely viremic phase in pregnant macaques would extend these findings. These data combined with the Phase I safety and immunogenicity data in people suggests that the Ad26.M.Env is likely to be efficacious in preventing maternal ZIKV infection.
Methods
Experimental model and subject details
Outbred, healthy Indian-origin female rhesus monkeys (Macaca mulatta) were housed at Alphagenesis, Yemassee, SC. Animals selected for this study were research naive. Upon arrival and standard quarantine procedures, animals were tested for tuberculosis (TB) at least three times at intervals of two weeks. Animals were also screened for Herpes B, Simian retrovirus (SRV), Simian Immunodeficiency Virus (SIV), Simian T-cell Leukemia Virus (STLV), and Measles. All animals were Herpes B, SRV, SIV, and STLV negative. Study animals were selected based on age. Females were all aged 4- 8 years old with similar age and weight distribution per study group. The study protocol was reviewed and approved by the Alphagenesis Institutional Care and Use Committee (IACUC). All experiments conformed to regulatory standards outlined by the American Veterinary Medical Association (AVMA) and American Association of Laboratory Animal Medicine (AALAM).
Breeding, immunization, and ZIKV challenge
Seven weeks prior to immunization, females were removed from their breeding group. For Group 1 (Ad26.M.Env vaccinated, ZIKV-challenge), nine dams that were not pregnant according to ultrasound were intramuscularly immunized with 1×1011 vp Ad26.M.Env. The nine immunized dams were reintroduced to their breeding groups 17 days later. All animals were provided enrichment according to recommended guidelines. Dams were monitored for pregnancy every 2 weeks by ultrasound until confirmed pregnant. After confirmed pregnancy, dams were monitored by ultrasound every 4 weeks. For Group 1, all nine dams had confirmed pregnancy. For Group 2 (non-vaccinated, ZIKV-challenge controls), 7 dams were included in the study, of which 5 were confirmed pregnant. Pregnant control dams (Group 3, no vaccination, no ZIKV-challenge) were included from the breeding colony and were of similar age and source as vaccinated and non-vaccinated, challenged animals (Groups 1&2).
Approximately six weeks after calculated date of conception (based on ultrasound results), equivalent to human 1st trimester of pregnancy, dams from groups 1 and 2 were challenged with 1 × 106 vp (103 PFU) of ZIKV-BR via the subcutaneous route. During pregnancy, blood and PBMCs were isolated for immunogenicity readouts. Plasma, cerebrospinal fluid (CFS), urine, colorectal biopsies, inguinal/axillary lymph node (LN) biopsies, rectal, vaginal and saliva secretion were taken to monitor ZIKV vRNA. At approximately week 21–23 of pregnancy ( ≈ week 16 following challenge) fetuses of all groups were delivered by C- section except for one (OBE) from the vaccine group that was born naturally due to earlier than predicted delivery. One pregnancy was lost in the vaccine group due to culture confirmed staphylococcal placentitis (539) and this dam/infant pair was removed from the study. Reproductive failure or preterm delivery is significant among primates, and the observations in this study were within the historical control range for the testing facility (AGI), and within expected outcomes for pregnancies in rhesus monkeys.
Five live male infants and three live female infants were delivered among the vaccinated, challenged group. Three live male infants and two live female infants were delivered to the non-vaccinated, challenged group. Two live males and one live female infant were delivered to the control group (non-vaccinated, non-ZIKV challenged; Table 1). Dams and fetuses were euthanized for post-mortem gross pathology, histopathology, and virologic assessments.
Method details
Ad26.M.Env
Complete details about the construction and production of the Ad26.M.Env construct are available15,16,22. Briefly, the vaccine was produced on the human PER.C61 cell line and purified and characterized as described previously in ref. 58. Ad26 particle concentrations were determined by optical density at 260 nm and viral infectivity by TCID50 assay. All vaccine preparations were tested for bioburden and endotoxin levels (MicroSafe, Millipore, Leiden, The Netherlands) and have passed pre-set release criteria for animal experiments.
ZIKV challenge stock preparation
ZIKV-BR (Brazil ZKV2015) was propagated in Vero cells (World Health Organization, NICSC-011038011038) that were maintained in EMEM media supplemented with 10%FBS, 6mM L-glutamine and 1x pen/strep. Cells were passaged twice a week and incubated at 37 °C, 10% CO2.
Ultrasonography
Ultrasounds were performed every 2–4 weeks in the ZIKV-infected pregnant rhesus monkeys as well as in 3 uninfected pregnant rhesus monkeys in the same breeding facility. Animals were sedated with Telazol (5 mg/kg), and a GE Logic E with an 8CRS Micro-convex transducer (FOV 132, 3.6–10 MHz) was used for multiparameter biometric measurements, including biparietal diameter (BPD), occipitofrontal diameter (OFD), head circumference (HC), crown-rump length (CRL), abdominal circumference (AC), and femur length (FL).
Amniocentesis
Animals were sedated with Telazol HCL (4–7 mg/kg IM). The area on the abdomen was clipped and sterilely prepped with triple alternating applications of betadine and alcohol. Using sterile technique, a 22-gauge 3.34-inch needle on a 3-cc syringe was inserted into the ventral abdomen to the amniotic sac with ultrasound guidance. 2 cc of amniotic fluid was collected and frozen immediately.
RT-PCR
RT-PCR assays were utilized to monitor viral loads in plasma, CSF, lymph node biopsies, colorectal biopsies, colorectal weck samples, and urine longitudinally every 2–4 weeks as indicated in the experimental design (see Fig. 1a) and amniotic fluid collected by amniocentesis at day 14 post-ZIKV infection, and from tissues collected at necropsy, essentially as previously described in refs. 15,16,26,33. RNA was extracted with a QIAcube HT (Qiagen, Germany). Liquid samples were extracted using the Qiacube 96 Cador pathogen HT, and tissue samples were lysed in Qiazol, using the Tissuelyser II (Qiagen, Germany), chloroform treated and extracted with the Qiacube 96 RNeasy HT kit. The wildtype ZIKV BeH815744 Cap gene was utilized as a standard. RNA standards were generated using the AmpliCap-Max T 7 High Yield Message Maker Kit (Cell Script) and purified with RNA clean and concentrator kit (Zymo Research, CA, USA). RNA quality and concentration was assessed by the BIDMC Molecular Core Facility. Log dilutions of the RNA standard were reverse transcribed and included with each RT-PCR assay. Viral loads were calculated as virus particles (VP) per microgram of total RNA as measured on the NanoDrop (Thermo Scientific, Waltham, MA, USA) or as VP per million cells, as shown in Figs. 3 and 4. Assay sensitivity was >100 copies/mL, >100 copies per million cells, and >3 copies/mg total RNA.
Neutralization Assays
ZIKV-specific neutralizing antibodies were measured by fold reduction neutralization (FRNT) and microneutralization (MN) assays, as previously described in refs. 15,16,26,33. For FRNT assay Vero cells were seeded at a concentration of 2 × 104 cells/well in 96-well plates 24 h prior to the assay initiation. Heat inactivated serum samples were serially diluted prior to being mixed and incubated with input virus ZIKV-PR (PRVABC59) for 1 hour at 37 °C. Cell-seeded 96-well plates were infected with 100 μL of the virus/serum mixtures for 1 hour before the addition of overlay media. Each serum dilution was tested in triplicate wells. Approximately 24 h after infection, ZIKV foci were detected using an antiflavivirus detection antibody, a horseradish peroxidase (HRP)-conjugated secondary antibody and True-Blue peroxidase substrate. ZIKV foci were visualized and counted using an ImmunoSpot analyzer and software. Each assay run included virus input and media-only control wells, as well as negative and positive control serum samples. Neutralizing antibody titers were reported as the inverse of the serum dilution estimated to reduce the number of input virus by 50% (FRNT50) as shown in Fig. 1.
Microneutralization assays were performed at WRAIR. Serum samples were serially diluted three-fold in 96-well micro-plates, in a total volume of 100 uL. 102 PFU ZIKV-PR (PRVABC59) in a total volume of 100uL was added and incubated at 35 °C for 2 h. Serum/virus mixtures were then transferred to microtiter plates containing confluent Vero cell monolayers (World Health Organization, NICSC-011038011038). After incubation for 4 days, cells were fixed with absolute ethanol: methanol for 1 h at –20 °C and washed three times with PBS. The pan-flavivirus monoclonal antibody 6B6-C1 conjugated to HRP (6B6-C1 was a gift from JT Roehrig, CDC) was then added to each well, incubated at 35 °C for 2 h, and washed with PBS. Plates were washed, developed with 3,3’,5,5’–tetramethylbenzidine (TMB) for 50 min at room temperature, stopped with 1:25 phosphoric acid, and absorbance was read at 450 nm. For a valid assay, the average absorbance at 450 nm of three non-infected control wells had to be % 0.5, and virus-only control wells had to be R 0.9. Normalized absorbance values were calculated, the MN50 titer was determined by a log mid-point linear regression model. The MN50 titer was calculated as the reciprocal of the serum dilution that neutralized R 50% of ZIKV, and seropositivity was defined as a titer R10, with the maximum measurable titer 7290, as shown in Supplementary Fig. 2.
Tissue Collection and Histopathology
Within 14 days of estimated term gestation (26 weeks), dams and fetuses were euthanized with intravenous sodium pentobarbital, and delivery was by caesarian section. Complete necropsies were performed by a veterinarian (A.J.M) on fetuses immediately following euthanasia, utilizing standard necropsy procedures with standard sterile surgical grade necropsy instruments and dissection blades. Briefly, peripheral lymphoid tissues were collected, followed by the gastrointestinal tract and abdominal organs. The pleural cavity was opened and the tongue, pharynx, trachea, esophagus, heart, and lungs (“pluck”) were removed en masse. Reproductive organs were collected, followed by brain, spinal cord, and eyes. Ruskin-Liston bone cutting forceps were used to expose the spinal cord to the level of the cauda equina. Limited necropsies were performed on dams for tissues previously shown to harbor viral RNA including reproductive organs, lymphoid tissues, spleen, and placenta. Fresh tissues were collected utilizing sterile blades for viral RT-PCR in RNAlater (Ambion). Frozen tissue for histopathology was prepared by trimming tissue, placing tissue samples into cryomolds with optimal cutting temperature medium (OCT, Tissue-Tek), and flash freezing on-site. Additional tissues were fixed in 10% neutral buffered formalin (NBF) for histopathology. Formalin-fixed tissues were trimmed, processed, and embedded in paraffin, sectioned, and stained with hematoxylin and eosin, and evaluated independently by two blinded veterinary pathologists (A.J.M., R.B.). Placenta was evalualated by a blinded gynecologic pathologist (J.L.H).
Immunohistochemistry and in situ hybridization
Immunohistochemistry and in situ hybridization (RNAscopeTM) were performed as previously described in ref. 33. Briefly, tissue sections were deparaffinized in xylene and rehydrated through graded ethanol solutions to distilled water. Endogenous peroxidase activity was blocked by incubation with 3% hydrogen peroxide followed by heat induced epitope retrieval (HIER) in citrate buffer (Vector Labs) using a slide steamer (IHC World). Tissues were treated for nonspecific protein binding (Protein Block, DAKO) followed by application of mouse-anti ZIKV envelope (BioFront Technologies; BF-1176-56, 1:200) for 30 min at room temperature. A biotin-free polymer-based alkaline phosphatase kit with Permanent Red was used to detect antigen-antibody complexes (Polink-1 AP, Golden Bridge International Labs; #D18-18). In situ detection of ZIKV RNA was performed using RNAscope (ACDBio) technology. The ZIKV Asian probe (formerly O4, #468361) and red detection kit were used according to the manufacturer’s instructions.
ELISPOT
ZIKV-specific cellular immune responses were assessed by IFN-γ ELISPOT assays shown in Fig. 2 using pools of over- lapping 15-amino-acid peptides covering the prM and Env proteins (JPT, Berlin, Germany), essentially as we previously described in ref. 16. 96-well multiscreen plates (Millipore, MA, USA) were coated overnight with 100 mL/well of 5 mg/ml anti-human interferon-g (BD Biosciences, CA, USA; BD #554699) in endotoxin-free Dulbecco’s PBS (D-PBS). The plates were then washed three times with D-PBS containing 0.25% Tween 20 (D-PBS-Tween), blocked for 1–4 h with D-PBS containing 5% FBS at 37 °C, and incubated with 2 mg/ml of each peptide and 2 × 105 monkey PBMC in triplicate in 100 mL reaction mixture volumes. Following an 18–24 h incubation at 37 °C, the plates were washed nine times with PBS-Tween and incubated for 3 min with distilled water. The plates were then incubated with 1 mg/ml biotinylated anti-human interferon-g (U-Cytech Biosciences, UT, NETH) for 2 h at room temperature, washed six times with PBS-Tween, and incubated for 2 h with streptavidin-alkaline phosphatase (Southern Biotechnology Associates, AL, USA). Following five washes with PBS-Tween and one with PBS, the plates were developed with nitroblue tetrazolium-5- bromo-4-chloro-3-indolyl-phosphate chromogen (Pierce, IL, USA), stopped by washing with tap water, air-dried, and read using an ELISPOT reader (Cellular Technology Ltd., OH, USA). The numbers of spot-forming cells (SFU) per 106 cells were calculated. The medium background levels were typically < 15 SFU per 106 cells. SFU per 106 PBMCs of unstimulated PBMCs was subtracted from specific responses of corresponding individual macaques. Specific responses that were at or below zero after background subtraction were set to 1.
ELISA
Monkey ZIKV NS1 ELISA kits (Alpha Diagnostic International, TX, USA) were used to determine endpoint binding antibody titers using a modified protocol16. 96-well plates coated with ZIKV NS1 protein (RV-403310-1 Alpha Diagnostics) were first equilibrated at room temperature with 300 ml of kit working wash buffer for 5 min. 6 ml of monkey serum was added to the top row, and 3-fold serial dilutions were tested in the remaining rows. Serum samples were incubated at room temperature for 1 hr, and plates washed 4 times. 100 mL of anti-monkey IgG HRP-conjugate working solution was then added to each well and incubated for 30 min at room temperature. Plates were washed 5 times, developed for 15 min at room temperature with 100 ml of TMB substrate, and stopped by the addition of 100 ml of stop solution. Plates were analyzed at 450 nm/550 nm on a VersaMax microplate reader using Softmax Pro 6.0 software (Molecular Devices, CA, USA). ELISA endpoint titers were defined as the highest reciprocal serum dilution that yielded an absorbance > 2-fold over background values and plotted as Log10 endpoint titer.
Supplementary information
Acknowledgements
We acknowledge Abishek Chandrashekar and Brianna Altimonti for scientific discussions and technical support. We acknowledge funding from Janssen, the Ragon Institute of MGH, MIT, and Harvard, and NIH NIAID K08 135098-01A1.
Author contributions
DHB, RZ, LF, FC conceptualized and designed the study; WJR, MJF, AJM, AC, and FC supervised and conducted the NHP studies; AJM, RB, and JLH performed the pathological analyses. PA supervised all virologic assays. RAB performed the neutralization assays. FC, ENB, LF, and AJM curated and analyzed the data; AJM wrote the paper; FC, LF, RZ, and DHB reviewed and edited the manuscript.
Data availability
All data generated and analyzed in this study are available from the Lead Contact upon reasonable request.
Competing interests
LF, RZ, and FC are or were employees of Janssen Vaccines & Prevention B.V. DHB and PA are co-inventors on the patent PCT/US2017/036900 (Compositions and methods for preventing and treating ZIKA virus infection). All other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Amanda J. Martinot, Email: amanda.martinot@tufts.edu
Dan H. Barouch, Email: dbarouch@bidmc.harvard.edu
Supplementary information
The online version contains supplementary material available at 10.1038/s41541-024-00927-8.
References
- 1.Brasil, P. et al. Zika virus infection in pregnant women in Rio de Janeiro. N. Engl. J. Med.375, 2321–2334 (2016). 10.1056/NEJMoa1602412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Driggers, R. W. et al. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N. Engl. J. Med.374, 2142–2151 (2016). 10.1056/NEJMoa1601824 [DOI] [PubMed] [Google Scholar]
- 3.Hoen, B. et al. Pregnancy outcomes after ZIKV infection in french territories in the Americas. N. Engl. J. Med.378, 985–994 (2018). 10.1056/NEJMoa1709481 [DOI] [PubMed] [Google Scholar]
- 4.Haby, M. M., Pinart, M., Elias, V. & Reveiz, L. Prevalence of asymptomatic Zika virus infection: a systematic review. Bull. World Health Organ.96, 402–413D (2018). 10.2471/BLT.17.201541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langerak, T. et al. Transplacental Zika virus transmission in ex vivo perfused human placentas. Plos Negl. Trop. D.16, e0010359 (2022). 10.1371/journal.pntd.0010359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adibi, J. J., Marques, E. T. A. Jr, Cartus, A. & Beigi, R. H. Teratogenic effects of the Zika virus and the role of the placenta. Lancet387, 1587–1590 (2016). 10.1016/S0140-6736(16)00650-4 [DOI] [PubMed] [Google Scholar]
- 7.Vasquez, A. M., Sapiano, M. R. P., Basavaraju, S. V., Kuehnert, M. J. & Rivera-Garcia, B. Survey of blood collection centers and implementation of guidance for prevention of transfusion-transmitted Zika virus. Infect. — Puerto Rico, 2016. Mmwr Morbidity Mortal. Wkly Rep.65, 375–378 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Oster, A. M. et al. Update: interim guidance for prevention of sexual transmission of Zika virus — United States, 2016. Morbidity Mortal. Wkly Rep.65, 323–325 (2016). 10.15585/mmwr.mm6512e3 [DOI] [PubMed] [Google Scholar]
- 9.Santos, C. N. O. et al. Association between genetic variants in TREM1, CXCL10, IL4, CXCL8 and TLR7 genes with the occurrence of congenital Zika syndrome and severe microcephaly. Sci. Rep.13, 3466 (2023). 10.1038/s41598-023-30342-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. Situation Report: Zika Virus, Microcephaly and Guillain Barre Syndrome 3 November, 2016. (WHO< 2016).
- 11.Vannice, K. S. et al. Demonstrating vaccine effectiveness during a waning epidemic: A WHO/NIH meeting report on approaches to development and licensure of Zika vaccine candidates. Vaccine37, 863–868 (2019). 10.1016/j.vaccine.2018.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christie, C. D. C., Lue, A. M. & Melbourne-Chambers, R. H. Dengue, chikungunya and zika arbovirus infections in Caribbean children. Curr. Opin. Pediatr.35, 155–165 (2023). 10.1097/MOP.0000000000001229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CDC. CDC Traveler’s Health:Zika. https://wwwnc.cdc.gov/travel/diseases/zika.
- 14.Lunardelli, V. A. S., Apostolico, J. D. S., Fernandes, E. R. & Rosa, D. S. Zika virus—an update on the current efforts for vaccine development. Hum. Vacc. Immunother.17, 1–5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larocca, R. A. et al. Vaccine protection against Zika virus from Brazil. Nature536, 474–478 (2016). 10.1038/nature18952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbink, P. et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. SCIENCE353, 1129–1132 (2016). 10.1126/science.aah6157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richner, J. M. et al. Modified mRNA vaccines protect against Zika virus infection. Cell168, 1114–1125.e10 (2017). 10.1016/j.cell.2017.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bollman, B. et al. An optimized messenger RNA vaccine candidate protects non-human primates from Zika virus infection. npj Vaccines8, 58 (2023). 10.1038/s41541-023-00656-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stephenson, K. E. et al. Safety and immunogenicity of a Zika purified inactivated virus vaccine given via standard, accelerated, or shortened schedules: a single-centre, double-blind, sequential-group, randomised, placebo-controlled, phase 1 trial. Lancet Infect. Dis.20, 1061–1070 (2020). 10.1016/S1473-3099(20)30085-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Essink, B. et al. The safety and immunogenicity of two Zika virus mRNA vaccine candidates in healthy flavivirus baseline seropositive and seronegative adults: the results of two randomised, placebo-controlled, dose-ranging, phase 1 clinical trials. Lancet Infect. Dis.23, 621–633 (2023). 10.1016/S1473-3099(22)00764-2 [DOI] [PubMed] [Google Scholar]
- 21.Modjarrad, K. et al. Preliminary aggregate safety and immunogenicity results from three trials of a purified inactivated Zika virus vaccine candidate: phase 1, randomised, double-blind, placebo-controlled clinical trials. Lancet391, 563–571 (2018). 10.1016/S0140-6736(17)33106-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salisch, N. C. et al. A double-blind, randomized, placebo-controlled phase 1 study of Ad26.ZIKV.001, an Ad26-vectored anti-Zika virus vaccine. Ann. Intern. Med.174, 585–594 (2021). 10.7326/M20-5306 [DOI] [PubMed] [Google Scholar]
- 23.Dudley, D. M. et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat. Commun.7, 12204 (2016). 10.1038/ncomms12204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coffey, L. L. et al. Zika virus tissue and blood compartmentalization in acute infection of rhesus macaques. PLoS ONE12, e0171148–15 (2017). 10.1371/journal.pone.0171148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osuna, C. E. et al. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat. Med.22, 1448–1455 (2016). 10.1038/nm.4206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aid, M. et al. Zika virus persistence in the central nervous system and lymph nodes of rhesus monkeys. Cell169, 610–620.e14 (2017). 10.1016/j.cell.2017.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magnani, D. M. et al. Fetal demise and failed antibody therapy during Zika virus infection of pregnant macaques. Nat. Commun. 9, 1624 (2018) 10.1038/s41467-018-04056-4. [DOI] [PMC free article] [PubMed]
- 28.Raasch, L. E. et al. Fetal loss in pregnant rhesus macaques infected with high-dose African-lineage Zika virus. Plos Negl. Trop. D.16, e0010623 (2022). 10.1371/journal.pntd.0010623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ausderau, K. et al. Neonatal development in prenatally Zika virus-exposed infant macaques with dengue immunity. Viruses13, 1878 (2021). 10.3390/v13091878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohr, E. L. et al. Ocular and uteroplacental pathology in a macaque pregnancy with congenital Zika virus infection. PLoS ONE13, e0190617–e0190628 (2018). 10.1371/journal.pone.0190617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crooks, C. M. et al. Previous exposure to dengue virus is associated with increased Zika virus burden at the maternal-fetal interface in rhesus macaques. Plos Negl. Trop. D.15, e0009641 (2021). 10.1371/journal.pntd.0009641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirsch, A. J. et al. Zika virus infection in pregnant rhesus macaques causes placental dysfunction and immunopathology. Nat. Commun.9, 263 (2018). 10.1038/s41467-017-02499-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinot, A. J. et al. Fetal neuropathology in Zika virus-infected pregnant female rhesus monkeys. Cell173, 1111–1122.e10 (2018). 10.1016/j.cell.2018.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dudley, D. M. et al. Miscarriage and stillbirth following maternal Zika virus infection in nonhuman primates. Nat. Med.24, 1104–1107 (2018). 10.1038/s41591-018-0088-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waldorf, K. M. A. et al. Congenital Zika virus infection as a silent pathology with loss of neurogenic output in the fetal brain. Nat. Med.24, 368–374 (2018). 10.1038/nm.4485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen, S. M. et al. Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques. PLoS Pathog.13, e1006378–22 (2017). 10.1371/journal.ppat.1006378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rompay, K. K. A. V. et al. DNA vaccination before conception protects Zika virus-exposed pregnant macaques against prolonged viremia and improves fetal outcomes. Sci. Transl. Med.11, eaay2736 (2019). 10.1126/scitranslmed.aay2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gebre, M. S. et al. Novel approaches for vaccine development. Cell184, 1589–1603 (2021). 10.1016/j.cell.2021.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barouch, D. H. et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine29, 5203–5209 (2011). 10.1016/j.vaccine.2011.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barouch, D. H., Thomas, S. J. & Michael, N. L. Prospects for a Zika Virus Vaccine. Immunity46, 176–182 (2017). 10.1016/j.immuni.2017.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cox, F. et al. Adenoviral vector type 26 encoding Zika virus (ZIKV) M-Env antigen induces humoral and cellular immune responses and protects mice and nonhuman primates against ZIKV challenge. PLoS ONE13, e0202820–19 (2018). 10.1371/journal.pone.0202820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larocca, R. A. et al. Adenovirus vector-based vaccines confer maternal-fetal protection against Zika virus challenge in pregnant IFN-αβR−/− mice. Cell Host Microbe26, 591–600.e4 (2019). 10.1016/j.chom.2019.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miner, J. J. et al. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell165, 1081–1091 (2016). 10.1016/j.cell.2016.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waldorf, K., Stencel-Baerenwald, J. E. & Kapur, R. P. Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat. Med.22, 1256–1259 (2016). [DOI] [PMC free article] [PubMed]
- 45.Coffey, L. L. et al. Intraamniotic Zika virus inoculation of pregnant rhesus macaques produces fetal neurologic disease. Nat. Commun.9, 2414 (2018). 10.1038/s41467-018-04777-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cline, J. M. et al. The placenta in toxicology. Part III: Pathologic assessment of the placenta. Toxicol. Pathol.42, 339–344 (2014). 10.1177/0192623313482207 [DOI] [PubMed] [Google Scholar]
- 47.Sadoff, J. et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N. Engl. J. Med.384, 2187–2201 (2021). 10.1056/NEJMoa2101544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hardt, K. et al. Efficacy, safety, and immunogenicity of a booster regimen of Ad26.COV2.S vaccine against COVID-19 (ENSEMBLE2): results of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Infect. Dis.22, 1703–1715 (2022). 10.1016/S1473-3099(22)00506-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prather, A. A. et al. Predictors of long-term neutralizing antibody titers following COVID-19 vaccination by three vaccine types: the BOOST study. Sci. Rep.13, 6505 (2023). 10.1038/s41598-023-33320-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manno, D. et al. Safety and immunogenicity of an Ad26.ZEBOV booster dose in children previously vaccinated with the two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen: an open-label, non-randomised, phase 2 trial. Lancet Infect. Dis.23, 352–360 (2023). 10.1016/S1473-3099(22)00594-1 [DOI] [PubMed] [Google Scholar]
- 51.Bockstal, V. et al. Non-human primate to human immunobridging demonstrates a protective effect of Ad26.ZEBOV, MVA-BN-Filo vaccine against Ebola. npj Vaccines7, 156 (2022). 10.1038/s41541-022-00564-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roozendaal, R. et al. Nonhuman primate to human immunobridging to infer the protective effect of an Ebola virus vaccine candidate. npj Vaccines5, 112 (2020). 10.1038/s41541-020-00261-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim, I.-J. et al. Protective efficacy of a Zika purified inactivated virus vaccine candidate during pregnancy in marmosets. npj Vaccines9, 35 (2024). 10.1038/s41541-024-00824-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carvalho, M. S., Freitas, L. P., Cruz, O. G., Brasil, P. & Bastos, L. S. Association of past dengue fever epidemics with the risk of Zika microcephaly at the population level in Brazil. Sci. Rep.10, 1752 (2020). 10.1038/s41598-020-58407-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saron, W. A. A. et al. Exacerbated Zika virus–induced neuropathology and microcephaly in fetuses of dengue-immune nonhuman primates. Sci. Transl. Med.15, eadd2420 (2023). 10.1126/scitranslmed.add2420 [DOI] [PubMed] [Google Scholar]
- 56.Kim, I.-J. et al. Impact of prior dengue virus infection on Zika virus infection during pregnancy in marmosets. Sci. Transl. Med.15, eabq6517 (2023). 10.1126/scitranslmed.abq6517 [DOI] [PubMed] [Google Scholar]
- 57.Raper, J. et al. Long-term alterations in brain and behavior after postnatal Zika virus infection in infant macaques. Nat. Commun.11, 2534 (2020). 10.1038/s41467-020-16320-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abbink, P. et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol.81, 4654–4663 (2007). 10.1128/JVI.02696-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and analyzed in this study are available from the Lead Contact upon reasonable request.