Abstract
Background
The COVID-19 pandemic and the ensuing implementation of control measures caused widespread societal disruption. These disruptions may also have affected community transmission and seasonal circulation patterns of endemic respiratory viruses.
Objective
We aimed to investigate the impact of COVID-19–related disruption on influenza-related emergency hospital admissions and deaths in Wales in the first 2 years of the pandemic.
Methods
A descriptive analysis of influenza activity was conducted using anonymized pathology, hospitalization, and mortality data from the Secure Anonymised Information Linkage Databank in Wales. The annual incidence of emergency hospitalizations and deaths with influenza-specific diagnosis codes between January 1, 2015, and December 31, 2021, was estimated. Case definitions of emergency hospitalization and death required laboratory confirmation with a polymerase chain reaction test. Trends of admissions and deaths were analyzed monthly and yearly. We conducted 2 sensitivity analyses by extending case definitions to include acute respiratory illnesses with a positive influenza test and by limiting admissions to those with influenza as the primary diagnosis. We also examined yearly influenza testing trends to understand changes in testing behavior during the pandemic.
Results
We studied a population of 3,235,883 Welsh residents in 2020 with a median age of 42.5 (IQR 22.9–61.0) years. Influenza testing in Wales increased notably in the last 2 months of 2020, and particularly in 2021 to 39,720 per 100,000 people, compared to the prepandemic levels (1343 in 2019). The percentage of influenza admissions matched to an influenza polymerase chain reaction test increased from 74.8% (1890/2526) in 2019 to 85.2% (98/115) in 2021. However, admissions with a positive test per 100,000 population decreased from 17.0 in 2019 to 2.7 and 0.6 in 2020 and 2021, respectively. Similarly, deaths due to influenza with a positive influenza test per 100,000 population decreased from 0.4 in 2019 to 0.0 in 2020 and 2021. Sensitivity analyses showed similar patterns of decreasing influenza admissions and deaths in the first 2 years of the COVID-19 pandemic.
Conclusions
Nonpharmaceutical interventions to control COVID-19 were associated with a substantial reduction in the transmission of the influenza virus, with associated substantial reductions in hospital cases and deaths observed. Beyond the pandemic context, consideration should be given to the role of nonpharmaceutical community-driven interventions to reduce the burden of influenza.
Introduction
COVID-19 has resulted in enormous national, regional, and global health and economic and societal impacts, with 24.9 million cumulative cases and over 232,112 deaths in the United Kingdom as of February 4, 2024 [1]. The absence of effective and safe antiviral treatments or vaccines in the early stages of the pandemic [2] led to a variety of nonpharmaceutical interventions (NPIs) worldwide, including social distancing, mask wearing, handwashing, self-isolation, border closure, and lockdowns [3].
A growing body of evidence suggests these NPIs might have also reduced the transmission of other respiratory pathogens, such as the influenza virus, that have similar routes of transmission of SARS-CoV-2. Variable degrees of reduction in the activity of the influenza virus during the COVID-19 pandemic have been reported globally. Evidence from temperate zones of the Southern Hemisphere showed that influenza virus activity during the first wave of the COVID-19 pandemic was lower than historical seasons, despite continued or even increased testing for influenza viruses in some countries [4,5]. Several studies from these regions have attributed the observed reduction of influenza virus activity to the implementation of NPIs for COVID-19, these coinciding with the peak of the influenza season [6-9]. Furthermore, in several African countries, where more school closures have been implemented at various levels during the pandemic, stronger implementation of school closures was associated with a 20% reduction in influenza positivity rate [10].
Surveillance reports in the Northern Hemisphere showed similar patterns of declining levels of influenza activity in the early stages of the COVID-19 pandemic [5], with studies estimating a 64% reduction in incidence in China and Singapore and a 44% reduction in transmissibility in Hong Kong [8,11,12]. In China, NPIs were associated with a substantial reduction in both the number of influenza-like illness cases and infection duration [13]. In South Korea, NPIs, such as school closures, travel restrictions, and face mask use, were associated with a 91%‐100% reduction in influenza incidence [14,15]. Furthermore, the influenza positivity rate declined in the first year of the pandemic in India and Saudi Arabia [16,17]. Similarly, among the UK Armed Forces population, NPIs in the first year of the COVID-19 pandemic have been negatively correlated with the incidence of influenza-like illness [18].
Given the substantial burden of influenza on health care systems and its potential to cause severe illnesses and deaths among high-risk groups, it is important to understand the impact of NPIs enacted during the COVID-19 pandemic on severe influenza outcomes. A few studies have examined this impact by analyzing the impact of these NPIs on influenza-related hospitalization and critical care [7,11,19-21,undefined,undefined], although data regarding the impact on influenza mortality are limited [22].
In this study, we sought to explore influenza activity in Wales, United Kingdom, during the first 2 years of the COVID-19 pandemic and the preceding 5 years. Specifically, we aimed to investigate the potential impact of the implementation of NPIs during the COVID-19 pandemic on severe influenza illness leading to emergency hospital admission or death.
Methods
Ethical Considerations
In this study, we only used fully anonymized data from the Secure Anonymised Information Linkage (SAIL) Databank [23-26]. The SAIL’s independent Information Governance Review Panel approved this study (project 0911). This study was exempted from human subject research ethics review, and the requirement for informed consent was waived because the study only used fully anonymized data.
Data Sources
We used anonymized population-scale, individual-level linked data from the SAIL Databank in Wales [23-26]. Within SAIL, we used the following Wales-wide data sources for population-level cohorts that have been set up for COVID-19 research [27]. Demographic data were extracted from the Welsh Demographic Service Dataset. We extracted data on laboratory tests for influenza from the Welsh Results Reports Service (WRRS) data set. The WRRS data set contains pathology records with increasing geographical coverage across Wales over the last decade. Hospital admission data were extracted from the Patient Episode Database for Wales, which include secondary care data from all National Health Service hospitals in Wales. We extracted mortality data from the Annual District Death Extract (ADDE) data set, which is based on the Office for National Statistics mortality register, in which cause of death data is regularly checked and validated [28]. All the above data sources were linked together using SAIL’s Anonymised Linkage Field.
Study Design, Population, and Case Definitions
This study follows a descriptive research design using data about the entire population of Wales. We extracted emergency hospital admissions and deaths due to influenza infection between January 1, 2015, and December 31, 2021. Since our case definitions involve laboratory confirmation, we determined the study’s start date based on our assessment of the quality and coverage of influenza testing data in the WRRS data set at the time of analysis.
We defined an emergency influenza hospital admission based on the following criteria: having influenza (J09-J11) recorded in any diagnosis position using the International Statistical Classification of Diseases, Tenth Revision (ICD-10); the route to hospital was via emergency department, bed bureau, referral from a general practitioner or consultant for immediate admission, or National Health Service Direct [29]; and a positive result for influenza A or B virus using a polymerase chain reaction (PCR) test, collected within 7 days before or after admission date.
A death due to influenza was defined as a death record in the ADDE data set with an ICD-10 code of influenza (J09-J11) as the underlying cause of death and a positive result for influenza A or B virus using a PCR test, collected in the last 28 days before death.
Multimedia Appendix 1 contains a list of ICD-10 codes used to define influenza and influenza-related illness.
Analysis
We present the incidence of influenza tests and hospital admissions and deaths due to influenza over the period from January 2015 to December 2021, with the denominator being the entire population of Wales. We present the trends of influenza tests per month throughout the study period. We then present the trends of admissions and deaths both per month and per year by the result of the matching influenza test, if any. For each year, we calculated the percentage of influenza admissions and deaths with a matching influenza test including those that satisfied our case definitions, that is, with a positive influenza test.
Two sensitivity analyses were then performed: in the first, we extended the diagnosis code set to include influenza-related respiratory illnesses with acute presentations, in addition to influenza, in line with approaches used elsewhere [30-32]. In the second sensitivity analysis, emergency admissions were limited to those with an influenza-specific code as the primary diagnosis.
The analysis was performed in R (version 4.1.3; R Foundation for Statistical Computing). Records with low-quality linkage or a missing linkage field were excluded from the analysis. Appropriate disclosure control checks were carried out to maintain privacy protection and avoid patient identification in line with SAIL’s information governance policy. These include replacing counts smaller than 5 with 0.
Reporting
The STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) and the RECORD (Reporting of Studies Conducted Using Observational Routinely-Collected Health Data) statements were followed in the reporting of this study [33,34].
Results
The study is based on the entire population of Wales. A total of 3,235,883 had a residential address in Wales in mid-2020, the population was 50.7% (1,641,970/3,235,883) female, and the median age was 42.5 (IQR 22.9–61.0) years.
Influenza Tests
The number of influenza PCR tests per 100,000 people slowly increased from 461.3 in 2015 to 1343 in 2019. However, these tests increased notably in the last 2 months of 2020, raising the total in that year to 3237. In 2021, tests increased substantially to 39,720 per 100,000 people (Figure 1 and Table 1).
Figure 1. Number of polymerase chain reaction tests for influenza A and B in Wales per month from 2015 to 2021. The dashed vertical line represents the date on which the COVID-19 pandemic was declared. Influenza test records were extracted from the Welsh Results Reports Service data source.
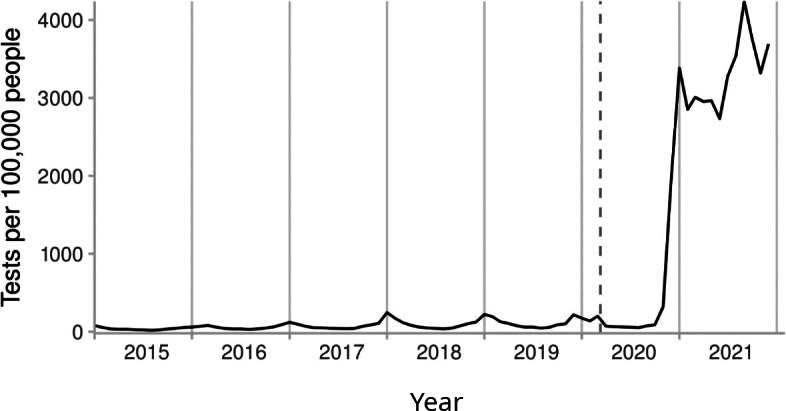
Table 1. Number of polymerase chain reaction tests for influenza A and B in Wales per year from 2015 to 2021. Influenza test records were extracted from the Welsh Results Reports Service data source.
| Year | Population, n | Tests, n | Tests per 100,000 population, n |
| 2015 | 3,202,489 | 14,773 | 461.3 |
| 2016 | 3,210,914 | 20,890 | 650.6 |
| 2017 | 3,219,095 | 26,414 | 820.5 |
| 2018 | 3,226,596 | 37,158 | 1151.6 |
| 2019 | 3,234,939 | 43,445 | 1343.0 |
| 2020 | 3,235,883 | 104,605 | 3232.7 |
| 2021 | 3,243,287 | 1,288,234 | 39,720.0 |
Admissions
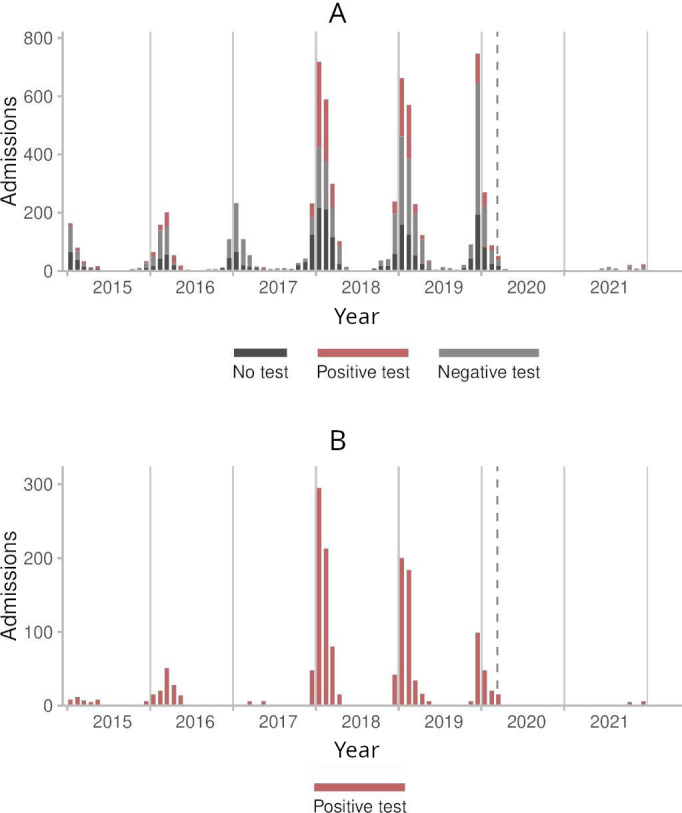
Before the COVID-19 pandemic, the annual incidence rates per 100,000 population of admissions with influenza-specific diagnosis codes ranged from 12.0 in 2015 to 78.1 in 2019. This decreased to 13.5 and 3.5 in 2020 and 2021, respectively (Table 2). The percentage of those admissions that could be matched to an influenza PCR test increased from 56.7% (217/383) in 2015 to 74.8% (1890/2526) in 2019 and reached 85.2% (98/115) in 2021. There were no marked changes in test positivity among those admissions during the pandemic. However, the number of admissions with a positive test per 100,000 people decreased from 17.0 in 2019 to 2.7 and 0.6 in 2020 and 2021, respectively. Figure 2 shows the monthly trend of admissions with influenza-specific diagnosis codes over the study period.
Table 2. Annual incidence of emergency admissions with influenza-specific International Statistical Classification of Diseases, Tenth Revision (ICD-10) codes at any diagnosis position, including those with influenza polymerase chain reaction test.a.
| Year | Population | Admissions with influenza-specific ICD-10 codes at any diagnosis position | |||
| Total, n (per 100,000 population) | With influenza test | ||||
| Total, n (%) | Positive, n (per 100,000 population)b | Positivity (%)c | |||
| 2015 | 3,202,489 | 383 (12.0) | 217 (56.7) | 46 (1.4) | 21.2 |
| 2016 | 3,210,914 | 652 (20.3) | 446 (68.4) | 129 (4.0) | 28.9 |
| 2017 | 3,219,095 | 784 (24.4) | 465 (59.3) | 70 (2.2) | 15.1 |
| 2018 | 3,226,596 | 2078 (64.4) | 1395 (67.1) | 658 (20.4) | 47.2 |
| 2019 | 3,234,939 | 2526 (78.1) | 1890 (74.8) | 551 (17.0) | 29.2 |
| 2020 | 3,235,883 | 436 (13.5) | 299 (68.6) | 87 (2.7) | 29.1 |
| 2021 | 3,243,287 | 115 (3.5) | 98 (85.2) | 19 (0.6) | 19.4 |
For each year between 2015 and 2021, the table includes the population estimate for Wales, the total number of admissions with influenza-specific ICD-10 codes, the number of those admissions that match an influenza test collected within 7 days before or after admission date, the number of those admissions with positive tests, and test positivity. Admission records were extracted from the Patient Episode Database for Wales. Influenza test records were extracted from the Welsh Results Reports Service data source.
This column represents the case definition: emergency admission with an influenza-specific ICD-10 code at any diagnosis position and a positive influenza test.
Positivity % is the percentage of emergency admissions with influenza-specific ICD-10 codes and an influenza test where the test was positive.
Figure 2. Monthly counts of admissions with influenza-specific codes at any diagnosis position in Wales before and during the COVID-19 pandemic. Counts smaller than 5 are suppressed. (A) Stacked bar chart showing the counts of all admissions per influenza polymerase chain reaction status (no test, positive test, or negative test). (B) Bar chart showing the counts of admissions with positive tests only. The dashed vertical line represents the date on which the COVID-19 pandemic was declared. Admission records were extracted from the Patient Episode Database for Wales. Influenza test records were extracted from the Welsh Results Reports Service data source.
Deaths
Before the pandemic, the annual incidence rates per 100,000 population of deaths with influenza-specific ICD-10 codes as the underlying cause ranged from 0.6 (2015) to 2.3 (2018), before decreasing to 0.7 and 0.0 deaths in 2020 and 2021, respectively (Table 3). The percentage of those deaths that could be matched to an influenza PCR test ranged before the pandemic between 72.7% (16/22) in 2016 and 77.3% (58/75) in 2018 before decreasing to 60.9% (14/23) in 2020. The percentage of tests with positive results ranged between 0% and 56.9% (33/58) before the pandemic. With laboratory confirmation of influenza, the incidence rate ranged between 0.0 to 1.0 deaths per 100,000 people before the pandemic, whereas no deaths with positive tests were identified in either 2020 or 2021.
Table 3. Annual incidence of deaths with influenza as the underlying cause, including those with influenza polymerase chain reaction test.a.
| Year | Population | Deaths with influenza as the underlying cause | |||
| Total, n (per 100,000 population) | With influenza test | ||||
| Total, n (%) | Positive, n (per 100,000 population)b | Positivity (%)c | |||
| 2015 | 3,202,489 | 20 (0.6) | 15 (75) | 0 (0)d | 0 |
| 2016 | 3,210,914 | 22 (0.7) | 16 (72.7) | 5 (0.2) | 31.3 |
| 2017 | 3,219,095 | 26 (0.8) | 19 (73.1) | 0 (0.0) | 0 |
| 2018 | 3,226,596 | 75 (2.3) | 58 (77.3) | 33 (1.0) | 56.9 |
| 2019 | 3,234,939 | 52 (1.6) | 40 (76.9) | 12 (0.4) | 30 |
| 2020 | 3,235,883 | 23 (0.7) | 14 (60.9) | 0 (0) | 0 |
| 2021 | 3,243,287 | 0 (0) | 0 (0) | 0 (0) | —e |
For each year between 2015 and 2021, the table includes the population estimate for Wales, the total number of deaths with influenza as the underlying cause, the number of those deaths that match an influenza test collected in the last 28 days before death, the number of those deaths with positive tests, and test positivity. Death records were extracted from the Annual District Death Extract data set. Influenza test records were extracted from the Welsh Results Reports Service data source.
This column represents our case definition: death due to influenza as the underlying cause with a positive influenza test.
Positivity % is the percentage of deaths with influenza as the underlying cause and an influenza test where the test was positive.
Counts withfewer less than 5 are presentedreported as 0.
Not available.
The sensitivity analyses in Multimedia Appendix 2 demonstrate similar trends of decreasing laboratory-confirmed influenza admissions and deaths during the COVID-19 pandemic using the wider case definitions (ie, by extending the diagnosis code set to include influenza-related acute respiratory illnesses) as well as the trends of laboratory-confirmed admissions with a primary diagnosis of influenza.
Discussion
Principal Findings
This national analysis has demonstrated a substantial reduction in the incidence of influenza and influenza-associated hospitalizations and deaths during the first 2 years of the COVID-19 pandemic compared with prepandemic levels. This reduction was also seen in our sensitivity analyses using wider case definitions of laboratory-confirmed influenza hospitalizations and deaths. We also observed a surge in influenza testing toward the end of 2020 and throughout the second year of the pandemic.
Interpretation
The significant reductions in influenza admissions and deaths in Wales that we observed during the COVID-19 pandemic are unlikely to be attributed to natural fluctuations in influenza activity over the years. Following the 2009‐2010 H1N1 pandemic, Public Health Wales influenza surveillance reports showed a surge in general practitioner consultations for influenza-like illness, peaking in the 2010‐2011 season, then decreasing to low to medium intensity in the subsequent years. A notable increase occurred in 2017‐2018, followed by a less severe 2018‐2019 season and a shorter 2019‐2020 season [35]. However, the significantly reduced influenza activity during the first 2 years of the COVID-19 pandemic was unlikely to be a mere continuation of the preceding downward trend, and it was even lower than the multiyear low- to medium-intensity activity prior to the 2017‐2018 surge. Instead, it is more plausible that this reduction was mainly due to diminished influenza virus circulation due to the widespread implementation of NPIs. This interpretation is corroborated by the resurgence of influenza activity following the lifting of public health restrictions in early 2022, with the influenza 2022‐2023 season exhibiting levels of activity comparable to those observed in the prepandemic 2019‐2020 season [36]. In the rest of the United Kingdom, broadly similar patterns were observed, as influenza activity was diminished during the period of COVID-19 restrictions and resurged in the 2022‐2023 season after the restrictions were lifted [37]. The reduction in the incidence of influenza during COVID-19–related NPIs can be explained by the similar pathways of infection between the 2 viral diseases [8].
In addition to the impact of COVID-19–related NPIs, influenza immunization might have contributed to the reduction we observed in severe influenza outcomes. Public Health Wales has reported that influenza vaccine uptake rates in people 65 years or older in Wales have increased from 65.7% and 67.1% in 2018/2019 and 2019/2020 to 76.5% in 2020/2021 [38].
The decline in influenza activity during the pandemic is unlikely due to underdetection, given that we observed a substantial rise in influenza testing during the 2020‐2021 winter season compared to nonpandemic years. This surge in testing could be largely explained by the use of respiratory multiplex testing and serious concerns about a possible concurrent rise in influenza and COVID-19 cases and the potential severity of coinfection [39].
There is a significant overlap between the population groups susceptible to severe illness from influenza and COVID-19 [40,41]. It is possible that high COVID-19 mortality has led to a reduction in the influenza-susceptible population, particularly older people and people with chronic health conditions, which might have contributed to the reduction in influenza severe outcomes during the pandemic.
Comparison With Prior Work
Several reports have suggested that the pandemic has had direct effects on the circulation of influenza virus in the population. Koutsakos et al [42] noted a considerable global reduction in influenza cases for both influenza A and B viruses in their study. They also reported a globally disappearing influenza B/Yamagata lineage, suggesting that the lineage may have become extinct during the pandemic. A later analysis of the World Health Organization-FluNet database confirmed low levels of B/Yamagata lineage being detected during the COVID-19 pandemic up to March 2023 [43]. It may also be argued that the B/Yamagata lineage may have already been at a low prevalence at the beginning of the pandemic due to the previous history of its inherent volatility, lower reproductive strength, slower growth phase, and shorter transmission chains [42]. The rapid decline of the B/Yamagata lineage throughout the COVID-19 pandemic has made experts suggest a more pronounced vulnerability and breakdown of the lineage, especially with the widespread movement restrictions and social distancing conditions [42,44].
In a systematic review of studies from 15 countries, Fricke et al [45] further suggested that NPIs targeted at SARS-CoV-2 transmission reduced influenza burden in many settings, with recommendations for low-threshold NPIs in influenza prevention programs. In South Korea, NPIs were widely implemented to mitigate the spread of COVID-19, with a decrease in hospitalizations for influenza, pneumonia, asthma, and chronic obstructive pulmonary disease reported during the intervention [46]. In China, also with strict NPIs aimed at controlling COVID-19, almost a two-thirds reduction in the incidence rate of seasonal influenza was reported in 2020 [8,47]. Another study analyzed ED visits, hospitalizations, and ICU admissions in 4 hospitals in France during the 2020 influenza season, the first wave of COVID-19, and lockdown period [48] and found a surge in influenza emergency department visits that heralded the influenza season, followed by a mild decline in cases during the first wave, and thereafter by a significant decrease during and after lockdown. As observed in this study, these reports all further align with findings in our previous studies in Scotland and Wales [49,50]. We reported that UK-wide lockdowns were associated with about 40% and 50% reductions in severe asthma and chronic obstructive pulmonary disease exacerbations, respectively, across both Scotland and Wales, with no corresponding increase in deaths [49,50].
Implications for Public Health Policy
Influenza has been a major cause of morbidity and mortality as well as a significant economic burden in the United Kingdom and globally [51-53]. Our findings indicate that the widespread implementation of NPIs has also impacted influenza circulation, which is consistent with global data. This suggests that, beyond the COVID-19 context, strategic NPIs used at scale could potentially reduce influenza incidence and improve outcomes.
While strict measures, such as lockdowns, are most unlikely to be politically or socially acceptable nor feasible in the long term, other NPIs, such as regular handwashing, wearing of face masks where needed, and reducing social contact or even self-isolation when potentially infected, may be more acceptable, particularly if focused on and around the main influenza season. In addition to NPIs, vaccination is one of the most effective preventive measures against influenza, and there is considerable merit in drives to promote and maintain high levels of influenza vaccine uptake, as this was certainly suboptimal in Wales [54]. The aforementioned measures should be coupled with innovative, comprehensive public health education strategies, such as ground-up, community-driven approaches, to increase public awareness about the importance of these preventive measures, how to correctly implement them, and to challenge misconceptions about influenza [55,56]. However, the feasibility, acceptability, and effectiveness of NPIs can vary significantly between countries due to factors, such as population density, political systems, and sociocultural factors [47,57-59,undefined,undefined].
The potential impact of COVID-19–related NPIs on influenza perhaps extends to other common respiratory infections [8,46]. For example, Song et al [60] clearly noted in a 2020 study of about 75,000 children in the United States that COVID-19 public health interventions were highly effective in preventing seasonal respiratory viral infections, such as respiratory syncytial virus, human parainfluenza virus, and human metapneumovirus, besides influenza. A decline in circulation of other respiratory pathogens, such as rhinovirus, respiratory enterovirus, and Streptococcus pneumoniae, have been also reported at the onset of the pandemic, when the strictest NPIs were implemented, before rebounding to varying degrees later [22].
Careful consideration should be given to a likely interaction between the influenza virus and SARS-CoV-2, especially when the winter approaches or in the event of another viral respiratory outbreak. Studies have suggested that coinfection could have a significant impact on disease burden and mortality. Stowe et al [61] noted that coinfection with the influenza virus had about 6 times higher risk of death than those with neither influenza nor SARS-CoV-2, whereas Swets et al [62] reported that coinfection with the influenza virus increased the probability of invasive mechanical ventilation by about 4 times and doubled in-hospital mortality.
The significant decrease in exposure to influenza during the COVID-19 pandemic could potentially diminish population immunity and increase the severity of future influenza epidemics [43]. Therefore, it is important to closely monitor influenza activity and its circulating subtypes and strengthen vaccine uptake.
Limitations
Unlike our previous studies [49,50], we did not directly model the impact of NPIs on influenza admissions and deaths. Rather, we descriptively analyzed the available data and presented our estimates as such, which have implications for statistical inference and study interpretations. In addition, we did not evaluate the potential effect of influenza vaccination on hospitalizations and deaths. Our analysis did not account for influenza strains or weather factors, which might have influenced the changes in influenza circulation patterns and associated hospitalizations and deaths during the pandemic. We also did not examine whether the incidence of influenza severe illness might have been affected by COVID-19–related mortality displacement or a potential reduction of influenza-vulnerable population due to high COVID-19 mortality. Therefore, we are unable to infer a causal relationship between NPIs and influenza emergency admissions and deaths. However, this causal relationship is highly plausible, and our findings, based on 5-year prepandemic and 2-year pandemic nationwide data, are consistent with an abundance of evidence from around the world that NPIs targeted at SARS-CoV-2 transmission might have also reduced influenza burden as well.
Our case definitions of influenza admissions and deaths prioritized specificity over sensitivity by requiring influenza-specific ICD-10 codes besides laboratory confirmation. Influenza-specific diagnosis codes have been used elsewhere to identify influenza hospitalizations [63,64]. However, although these diagnosis codes have been shown to have high specificity to identify laboratory-confirmed influenza hospitalizations, they have only moderate sensitivity [65-68]. In addition, the version of the WRRS data set from which we extracted influenza testing data might have incomplete geographical coverage. Nonetheless, we are more interested in trends than counts, and our sensitivity analysis has indeed demonstrated similar trends of laboratory-confirmed influenza hospitalizations and deaths before and during the COVID-19 pandemic using liberal case definitions. In these case definitions, we extended the diagnosis code set to include a wide range of non–COVID-19 acute respiratory illnesses. This ensured the capture of a broader range of admissions and deaths due to influenza and influenza-related exacerbation of respiratory conditions, including cases that lacked an influenza diagnosis code. Since an influenza swab is often collected when there is a reasonable clinical suspicion of influenza, laboratory confirmation of influenza controls for the wide list of acute respiratory illnesses and keeps the case definition reasonably specific to influenza. This liberal approach has been adopted by the World Health Organization and other studies [30,32,65,69,70].
Conclusions
Our national, population-based data show substantial reductions in influenza-associated hospitalization and mortality in Wales over the first 2 years of the pandemic compared with prepandemic levels. These reductions are unlikely to reflect changes in testing behavior. Rather, these most likely reflect a decrease in circulation and exposure to the influenza virus associated with the widespread adoption of a range of NPIs targeted at COVID-19.
The abundant evidence about the impact of large-scale NPIs on influenza could redefine our approach to influenza control in nonpandemic contexts. A comprehensive approach to influenza control that strategically incorporates NPIs, innovative public health education approaches, and effective vaccination programs could potentially lead to sustained reductions in influenza incidence and associated morbidity and mortality. Given the likely challenges of implementing NPIs outside the pandemic context, research and policy should focus on understanding the barriers to their implementation and piloting proactive, community-led public health strategies to reduce the burden of influenza.
supplementary material
Acknowledgments
This study makes use of anonymized data provided by patients and collected by the National Health Service (NHS) as part of their care and support. These data are held in the Secure Anonymised Information Linkage (SAIL) Databank. The authors would like to acknowledge all the data providers who make anonymized data available for research. SAIL is not responsible for the interpretation of these data. This work was supported by the Con-COV team funded by the Medical Research Council (grant MR/V028367/1). This work was supported by Health Data Research UK, which receives its funding from HDR UK Ltd (HDR-9006), funded by the UK Medical Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, Department of Health and Social Care (England), Chief Scientist Office of the Scottish Government Health and Social Care Directorates, Health and Social Care Research and Development Division (Welsh Government), Public Health Agency (Northern Ireland), British Heart Foundation, and the Wellcome Trust. This work was supported by the Administrative Data Research (ADR) Wales program of work. The ADR Wales program of work is aligned to the priority themes as identified in the Welsh Government’s national strategy: Prosperity for All. ADR Wales brings together data science experts at Swansea University Medical School, staff from the Wales Institute of Social and Economic Research, Data and Methods at Cardiff University, and specialist teams within the Welsh Government to develop new evidence, which supports Prosperity for All by using the SAIL Databank at Swansea University, to link and analyze anonymized data. ADR Wales is part of the Economic and Social Research Council (part of UK Research and Innovation), funded by ADR UK (grant ES/S007393/1). This work was supported by the Wales COVID-19 Evidence Centre, funded by Health and Care Research Wales. The authors wish to acknowledge the collaborative partnership that enabled acquisition and access to the deidentified data, which led to this output. The collaboration was led by the Swansea University Health Data Research UK team under the direction of the Welsh Government Technical Advisory Cell and includes the following groups and organizations: the SAIL Databank, ADR Wales, Digital Health and Care Wales (formerly NHS Wales Informatics Service), Public Health Wales, NHS Shared Services, and the Welsh Ambulance Service Trust. All research conducted has been completed under the permission and approval of the SAIL independent Information Governance Review Panel (project 0911). This study was funded by the Medical Research Council (MR/R008345/1) with the support of BREATHE—The Health Data Research Hub for Respiratory Health (MC_PC_19004), which is funded through the UK Research and Innovation Industrial Strategy Challenge Fund and delivered through Health Data Research UK. The funders played no role in the study design, data collection, analysis, interpretation, manuscript writing, or decision to publish.
Abbreviations
- ADDE
Annual District Death Extract
- ICD-10
International Statistical Classification of Diseases, Tenth Revision
- NPI
nonpharmaceutical intervention
- PCR
polymerase chain reaction
- RECORD
Reporting of Studies Conducted Using Observational Routinely-Collected Health Data
- SAIL
Secure Anonymised Information Linkage
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- WRRS
Welsh Results Reports Service
Footnotes
Data Availability: The anonymized person-level data sets analyzed in this study are not publicly available due to the strict information governance policy of the Secure Anonymised Information Linkage Databank. Requests to access these data should be submitted to the Secure Anonymised Information Linkage Databank.
Authors’ Contributions: AS conceived the study. CR designed the study. EV and MA prepared case definitions and code sets. MA extracted the data, conducted data analyses, and wrote up the Methods and Results. SS supported the data extraction. DA, EV, and MA wrote the first draft. GAD, RAL, AS, and IR reviewed the final draft and checked for important intellectual content. MA, SS, AA, and RAL had full access to all the underlying data. All authors critically reviewed and approved the final version of the manuscript.
Contributor Information
Mohammad Alsallakh, Email: m.a.alsallakh@swansea.ac.uk.
Davies Adeloye, Email: D.Adeloye@tees.ac.uk.
Eleftheria Vasileiou, Email: eleftheria.vasileiou@ed.ac.uk.
Shanya Sivakumaran, Email: Shanya.Sivakumaran@wales.nhs.uk.
Ashley Akbari, Email: A.Akbari@Swansea.ac.uk.
Ronan A Lyons, Email: R.A.Lyons@Swansea.ac.uk.
Chris Robertson, Email: chris.robertson@strath.ac.uk.
Igor Rudan, Email: igor.rudan@ed.ac.uk.
Gwyneth A Davies, Email: Gwyneth.Davies@Swansea.ac.uk.
Aziz Sheikh, Email: aziz.sheikh@ed.ac.uk.
References
- 1.Coronavirus disease (COVID-19) pandemic. World Health Organization. 2022. [06-07-2024]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 URL. Accessed.
- 2.Treatment and pharmaceutical prophylaxis of COVID-19. European Centre for Disease Prevention and Control. 2022. [06-07-2024]. https://www.ecdc.europa.eu/en/covid-19/latest-evidence/treatment URL. Accessed.
- 3.Prime Minister’s statement on coronavirus (COVID-19): 23 March 2020. GOV.UK. Mar 23, 2020. [06-07-2024]. https://www.gov.uk/government/speeches/pm-address-to-the-nation-on-coronavirus-23-march-2020 URL. Accessed.
- 4.WHO Regional Office for the Western Pacific Influenza situation updates 2020. World Health Organization. 2020. [06-07-2024]. https://iris.who.int/handle/10665/330699 URL. Accessed.
- 5.Influenza update N°374. World Health Organization. Aug 17, 2020. [06-07-2024]. https://www.who.int/publications/m/item/influenza-update-n-374 URL. Accessed.
- 6.Marriott D, Beresford R, Mirdad F, et al. Concomitant marked decline in prevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and other respiratory viruses among symptomatic patients following public health interventions in Australia: data from St Vincent’s Hospital and associated screening clinics. Clin Infect Dis. 2021 May 18;72(10):e649–e651. doi: 10.1093/cid/ciaa1256. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vásquez-Hoyos P, Diaz-Rubio F, Monteverde-Fernandez N, et al. Reduced PICU respiratory admissions during COVID-19. Arch Dis Child. 2021 Aug;106(8):808–811. doi: 10.1136/archdischild-2020-320469. doi. [DOI] [PubMed] [Google Scholar]
- 8.Lei H, Xu M, Wang X, et al. Nonpharmaceutical interventions used to control COVID-19 reduced seasonal influenza transmission in China. J Infect Dis. 2020 Nov 9;222(11):1780–1783. doi: 10.1093/infdis/jiaa570. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Itaya T, Furuse Y, Jindai K. Does COVID-19 infection impact on the trend of seasonal influenza infection? 11 countries and regions, from 2014 to 2020. Int J Infect Dis. 2020 Aug;97:78–80. doi: 10.1016/j.ijid.2020.05.088. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gharpure R, Olsen SJ, Davis WW. Quantified impacts of non-pharmaceutical interventions on influenza circulation during the COVID-19 pandemic in 13 African countries, 2020-2022. Influenza Other Respir Viruses. 2024 Jan;18(1):e13241. doi: 10.1111/irv.13241. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowling BJ, Ali ST, Ng TWY, et al. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study. Lancet Public Health. 2020 May;5(5):e279–e288. doi: 10.1016/S2468-2667(20)30090-6. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soo RJJ, Chiew CJ, Ma S, Pung R, Lee V. Decreased influenza incidence under COVID-19 control measures, Singapore. Emerg Infect Dis. 2020 Aug;26(8):1933–1935. doi: 10.3201/eid2608.201229. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Peng Y, Chen Z, et al. Impact of non-pharmaceutical interventions during COVID-19 on future influenza trends in Mainland China. BMC Infect Dis. 2023 Sep 27;23(1):632. doi: 10.1186/s12879-023-08594-1. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim HK, Min KD, Cho SI. Analysis of the effectiveness of non-pharmaceutical interventions on influenza during the coronavirus disease 2019 pandemic by time-series forecasting. BMC Infect Dis. 2023 Oct 24;23(1):717. doi: 10.1186/s12879-023-08640-y. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JH, Kim HY, Lee M. Respiratory syncytial virus outbreak without influenza in the second year of the coronavirus disease 2019 pandemic: a national sentinel surveillance in Korea, 2021-2022 season. J Korean Med Sci. 2022 Aug;37(34) doi: 10.3346/jkms.2022.37.e258. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain A, Mahesh S, Prakash O, Khan DN, Verma AK, Rastogi Y. Effect of COVID-19 pandemic on influenza; observation of a tertiary level virology laboratory. Virusdisease. 2024 Mar;35(1):27–33. doi: 10.1007/s13337-024-00860-3. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bin Saleh K, Badreldin HA, Alqahtani T, et al. Assessing the influence of COVID-19 on influenza prevalence: a multicenter time series study. J Infect Public Health. 2023 Dec;16(12):1989–1993. doi: 10.1016/j.jiph.2023.09.018. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 18.Otieno G, Rawlings NN. Impact of non-pharmaceutical interventions targeted at the COVID-19 pandemic on the incidence of influenza-like illness in the UK Armed Forces. PLoS One. 2022 Dec 1;17(12):e0270438. doi: 10.1371/journal.pone.0270438. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan KS, Liang FW, Tang HJ, Toh HS, Yu WL. Collateral benefits on other respiratory infections during fighting COVID-19. Med Clin (Barc) 2020 Sep 25;155(6):249–253. doi: 10.1016/j.medcli.2020.05.026. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee HH, Lin SH. Effects of COVID-19 prevention measures on other common infections, Taiwan. Emerg Infect Dis. 2020 Oct;26(10):2509–2511. doi: 10.3201/eid2610.203193. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee H, Lee H, Song KH, et al. Impact of public health interventions on seasonal influenza activity during the COVID-19 outbreak in Korea. Clin Infect Dis. 2021 Jul 1;73(1):e132–e140. doi: 10.1093/cid/ciaa672. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chow EJ, Uyeki TM, Chu HY. The effects of the COVID-19 pandemic on community respiratory virus activity. Nat Rev Microbiol. 2023 Mar;21(3):195–210. doi: 10.1038/s41579-022-00807-9. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ford DV, Jones KH, Verplancke JP, et al. The SAIL Databank: building a national architecture for e-health research and evaluation. BMC Health Serv Res. 2009 Sep 4;9:157. doi: 10.1186/1472-6963-9-157. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones KH, Ford DV, Jones C, et al. A case study of the Secure Anonymous Information Linkage (SAIL) gateway: a privacy-protecting remote access system for health-related research and evaluation. J Biomed Inform. 2014 Aug;50(100):196–204. doi: 10.1016/j.jbi.2014.01.003. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyons RA, Jones KH, John G, et al. The SAIL Databank: linking multiple health and social care datasets. BMC Med Inform Decis Mak. 2009 Jan 16;9:3. doi: 10.1186/1472-6947-9-3. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodgers SE, Lyons RA, Dsilva R, et al. Residential Anonymous Linking Fields (RALFs): a novel information infrastructure to study the interaction between the environment and individuals’ health. J Public Health. 2009 Dec 1;31(4):582–588. doi: 10.1093/pubmed/fdp041. doi. [DOI] [PubMed] [Google Scholar]
- 27.Lyons J, Akbari A, Torabi F, et al. Understanding and responding to COVID-19 in Wales: protocol for a privacy-protecting data platform for enhanced epidemiology and evaluation of interventions. BMJ Open. 2020 Oct 21;10(10):e043010. doi: 10.1136/bmjopen-2020-043010. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coronavirus (COVID-19) infection survey: methods and further information. Office for National Statistics. 2022. [06-07-2024]. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/methodologies/covid19infectionsurveypilotmethodsandfurtherinformation URL. Accessed.
- 29.Admission method. NHS Wales Data Dictionary. 2022. [06-07-2024]. http://www.datadictionary.wales.nhs.uk/index.html#!WordDocuments/admissionmethod.htm URL. Accessed.
- 30.Sotomayor V, Fasce RA, Vergara N, De la Fuente F, Loayza S, Palekar R. Estimating the burden of influenza-associated hospitalizations and deaths in Chile during 2012-2014. Influenza Other Respir Viruses. 2018 Jan;12(1):138–145. doi: 10.1111/irv.12502. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Protocol for hospital-based, test-negative case–control studies to measure seasonal influenza vaccine effectiveness against influenza laboratory-confirmed SARI hospitalisation among the elderly across the European Union and European Economic Area member states. I-MOVE. Dec 5, 2019. [08-08-2024]. https://www.imoveflu.org/wp-content/uploads/2019/12/05-dec-2019_Final_generic_protocol_hospital-based_case_control_elderly_v3.pdf URL. Accessed.
- 32.A manual for estimating disease burden associated with seasonal influenza. World Health Organization. Sep 9, 2015. [06-07-2024]. https://www.who.int/publications/i/item/9789241549301 URL. Accessed.
- 33.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014 Dec;12(12):1495–1499. doi: 10.1016/j.ijsu.2014.07.013. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 34.Benchimol EI, Smeeth L, Guttmann A, et al. The Reporting of Studies Conducted Using Observational Routinely-Collected Health Data (RECORD) statement. PLoS Med. 2015 Oct 6;12(10):e1001885. doi: 10.1371/journal.pmed.1001885. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seasonal influenza in Wales 2019/20. Public Health Wales. 2019. [06-07-2024]. https://phw.nhs.wales/topics/immunisation-and-vaccines/fluvaccine/annual-influenza-surveillance-and-influenza-vaccination-uptake-reports/seasonal-influenza-in-wales-201920/ URL. Accessed.
- 36.Influenza surveillance report for 2024 week. Public Health Wales. 2024. [06-07-2024]. https://www2.nphs.wales.nhs.uk/CommunitySurveillanceDocs.nsf/3dc04669c9e1eaa880257062003b246b/daa484f5cf93b69c80258aca0057d6d2/FILE/PHW%20Influenza%20Surveillance%20Report%20for%202024%20week%207.pdf URL. Accessed.
- 37.Surveillance of influenza and other seasonal respiratory viruses in the UK, winter 2022 to 2023. UK Health Security Agency. 2023. [06-07-2024]. https://www.gov.uk/government/statistics/annual-flu-reports/surveillance-of-influenza-and-other-seasonal-respiratory-viruses-in-the-uk-winter-2022-to-2023 URL. Accessed.
- 38.Annual influenza surveillance and influenza vaccination uptake reports. Public Health Wales. 2022. [06-07-2024]. https://phw.nhs.wales/topics/immunisation-and-vaccines/fluvaccine/annual-influenza-surveillance-and-influenza-vaccination-uptake-reports/ URL. Accessed.
- 39.Iacobucci G. Covid-19: risk of death more than doubled in people who also had flu, English data show. BMJ. 2020 Sep;:m3720. doi: 10.1136/bmj.m3720. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 40.Mertz D, Kim TH, Johnstone J, et al. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ. 2013 Aug 23;347:f5061. doi: 10.1136/bmj.f5061. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis. 2021 Aug 21;21(1):855. doi: 10.1186/s12879-021-06536-3. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koutsakos M, Wheatley AK, Laurie K, Kent SJ, Rockman S. Influenza lineage extinction during the COVID-19 pandemic? Nat Rev Microbiol. 2021 Dec;19(12):741–742. doi: 10.1038/s41579-021-00642-4. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng L, Lin Y, Yang J, Fang K, Wu J, Zheng M. Global variability of influenza activity and virus subtype circulation from 2011 to 2023. BMJ Open Respir Res. 2023 Jul;10(1):e001638. doi: 10.1136/bmjresp-2023-001638. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grohskopf LA, Alyanak E, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2021-22 influenza season. MMWR Recomm Rep. 2021 Aug 27;70(5):1–28. doi: 10.15585/mmwr.rr7005a1. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fricke LM, Glöckner S, Dreier M, Lange B. Impact of non-pharmaceutical interventions targeted at COVID-19 pandemic on influenza burden—a systematic review. J Infect. 2021 Jan;82(1):1–35. doi: 10.1016/j.jinf.2020.11.039. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huh K, Kim YE, Ji W, et al. Decrease in hospital admissions for respiratory diseases during the COVID-19 pandemic: a nationwide claims study. Thorax. 2021 Sep;76(9):939–941. doi: 10.1136/thoraxjnl-2020-216526. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 47.Zhang W, Wu Y, Wen B, et al. Non-pharmaceutical interventions for COVID-19 reduced the incidence of infectious diseases: a controlled interrupted time-series study. Infect Dis Poverty. 2023 Mar 9;12(1):15. doi: 10.1186/s40249-023-01066-3. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Casalino E, Choquet C, Bouzid D, et al. Analysis of emergency department visits and hospital activity during influenza season, COVID-19 epidemic, and lockdown periods in view of managing a future disaster risk: a multicenter observational study. Int J Environ Res Public Health. 2020 Nov 10;17(22):8302. doi: 10.3390/ijerph17228302. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davies GA, Alsallakh MA, Sivakumaran S, et al. Impact of COVID-19 lockdown on emergency asthma admissions and deaths: national interrupted time series analyses for Scotland and Wales. Thorax. 2021 Sep;76(9):867–873. doi: 10.1136/thoraxjnl-2020-216380. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 50.Alsallakh MA, Sivakumaran S, Kennedy S, et al. Impact of COVID-19 lockdown on the incidence and mortality of acute exacerbations of chronic obstructive pulmonary disease: national interrupted time series analyses for Scotland and Wales. BMC Med. 2021 May 17;19(1):124. doi: 10.1186/s12916-021-02000-w. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matias G, Taylor RJ, Haguinet F, Schuck-Paim C, Lustig RL, Fleming DM. Modelling estimates of age-specific influenza-related hospitalisation and mortality in the United Kingdom. BMC Public Health. 2016 Jun 8;16:481. doi: 10.1186/s12889-016-3128-4. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.GBD 2017 Influenza Collaborators Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2019 Jan;7(1):69–89. doi: 10.1016/S2213-2600(18)30496-X. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Federici C, Cavazza M, Costa F, Jommi C. Health care costs of influenza-related episodes in high income countries: a systematic review. PLoS One. 2018 Sep 7;13(9):e0202787. doi: 10.1371/journal.pone.0202787. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oakley S, Bouchet J, Costello P, Parker J. Influenza vaccine uptake among at-risk adults (aged 16-64 years) in the UK: a retrospective database analysis. BMC Public Health. 2021 Sep 24;21(1):1734. doi: 10.1186/s12889-021-11736-2. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonnevie E, Rosenberg SD, Kummeth C, Goldbarg J, Wartella E, Smyser J. Using social media influencers to increase knowledge and positive attitudes toward the flu vaccine. PLoS One. 2020 Oct 16;15(10):e0240828. doi: 10.1371/journal.pone.0240828. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haldane V, Jung AS, De Foo C, et al. Strengthening the basics: public health responses to prevent the next pandemic. BMJ. 2021 Nov 28;375:e067510. doi: 10.1136/bmj-2021-067510. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qiu Z, Cao Z, Zou M, et al. The effectiveness of governmental nonpharmaceutical interventions against COVID-19 at controlling seasonal influenza transmission: an ecological study. BMC Infect Dis. 2022 Apr 4;22(1):331. doi: 10.1186/s12879-022-07317-2. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Polonsky JA, Bhatia S, Fraser K, et al. Feasibility, acceptability, and effectiveness of non-pharmaceutical interventions against infectious diseases among crisis-affected populations: a scoping review. Infect Dis Poverty. 2022 Jan 28;11(1):14. doi: 10.1186/s40249-022-00935-7. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Solomon H, Thea DM, Galea S, Sabin LL, Lucey DR, Hamer DH. Adherence to and enforcement of non-pharmaceutical interventions (NPIs) for COVID-19 prevention in Nigeria, Rwanda, and Zambia: a mixed-methods analysis. PLOS Glob Public Health. 2022 Sep 8;2(9):e0000389. doi: 10.1371/journal.pgph.0000389. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song X, Delaney M, Shah RK, Campos JM, Wessel DL, DeBiasi RL. Common seasonal respiratory viral infections in children before and during the coronavirus disease 2019 (COVID-19) pandemic. Infect Control Hosp Epidemiol. 2022 Oct;43(10):1454–1458. doi: 10.1017/ice.2021.430. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 61.Stowe J, Tessier E, Zhao H, et al. Interactions between SARS-CoV-2 and influenza, and the impact of coinfection on disease severity: a test-negative design. Int J Epidemiol. 2021 Aug 30;50(4):1124–1133. doi: 10.1093/ije/dyab081. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swets MC, Russell CD, Harrison EM, et al. SARS-CoV-2 co-infection with influenza viruses, respiratory syncytial virus, or adenoviruses. Lancet. 2022 Apr 16;399(10334):1463–1464. doi: 10.1016/S0140-6736(22)00383-X. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pivette M, Nicolay N, de Lauzun V, Hubert B. Characteristics of hospitalizations with an influenza diagnosis, France, 2012-2013 to 2016-2017 influenza seasons. Influenza Other Respir Viruses. 2020 May;14(3):340–348. doi: 10.1111/irv.12719. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hobbs JL, Whelan M, Winter AL, Murti M, Hohenadel K. Getting a grippe on severity: a retrospective comparison of influenza-related hospitalizations and deaths captured in reportable disease and administrative data sources in Ontario, Canada. BMC Public Health. 2019 May 14;19(1):567. doi: 10.1186/s12889-019-6924-9. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004 Sep 15;292(11):1333–1340. doi: 10.1001/jama.292.11.1333. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 66.Hamilton MA, Calzavara A, Emerson SD, et al. Validating International Classification of Disease 10th revision algorithms for identifying influenza and respiratory syncytial virus hospitalizations. PLoS One. 2021 Jan 7;16(1):e0244746. doi: 10.1371/journal.pone.0244746. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feemster KA, Leckerman KH, Middleton M, et al. Use of administrative data for the identificationof laboratory-confirmed influenza infection: the validity of influenza-specific ICD-9 codes. J Pediatric Infect Dis Soc. 2013 Mar;2(1):63–66. doi: 10.1093/jpids/pis052. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 68.Keren R, Wheeler A, Coffin SE, Zaoutis T, Hodinka R, Heydon K. ICD-9 codes for identifying influenza hospitalizations in children. Emerg Infect Dis. 2006 Oct;12(10):1603–1604. doi: 10.3201/eid1210.051525. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chavez D, Gonzales-Armayo V, Mendoza E, et al. Estimation of influenza and respiratory syncytial virus hospitalizations using sentinel surveillance data—La Paz, Bolivia. 2012-2017. Influenza Other Respir Viruses. 2019 Sep;13(5):477–483. doi: 10.1111/irv.12663. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Palekar RS, Rolfes MA, Arriola CS, et al. Burden of influenza-associated respiratory hospitalizations in the Americas, 2010-2015. PLoS One. 2019 Sep 6;14(9):e0221479. doi: 10.1371/journal.pone.0221479. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


