Abstract
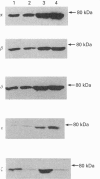
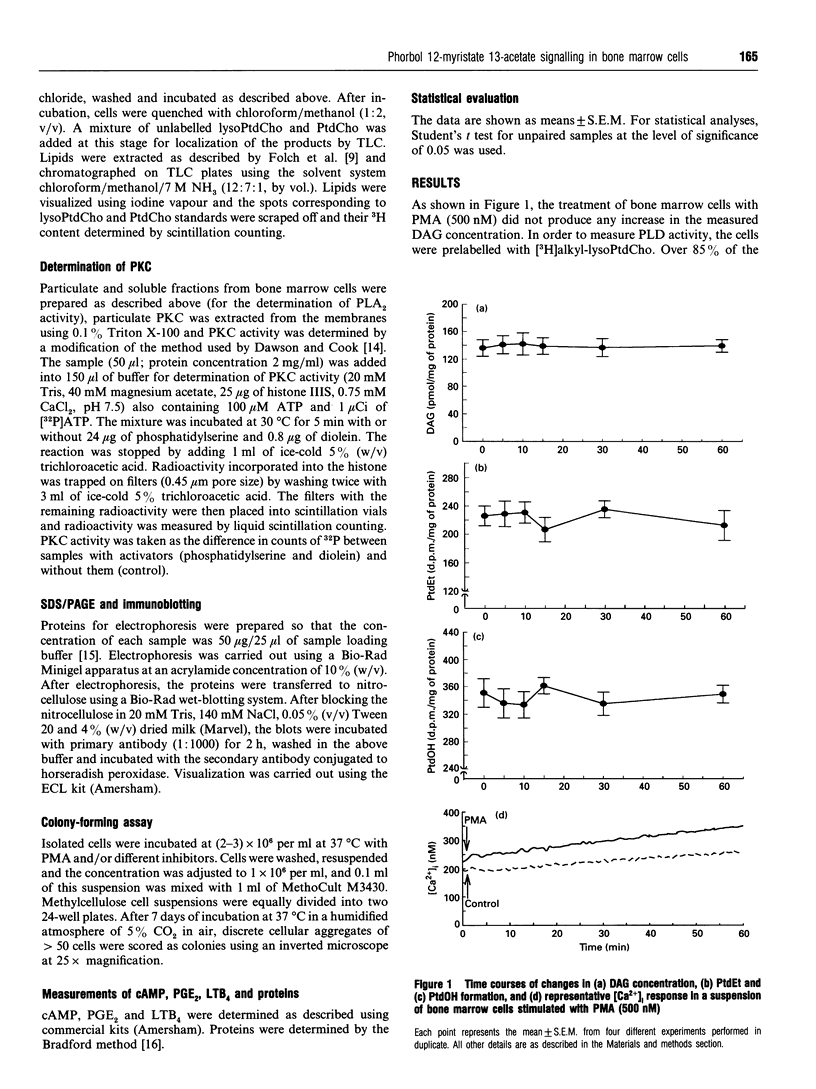
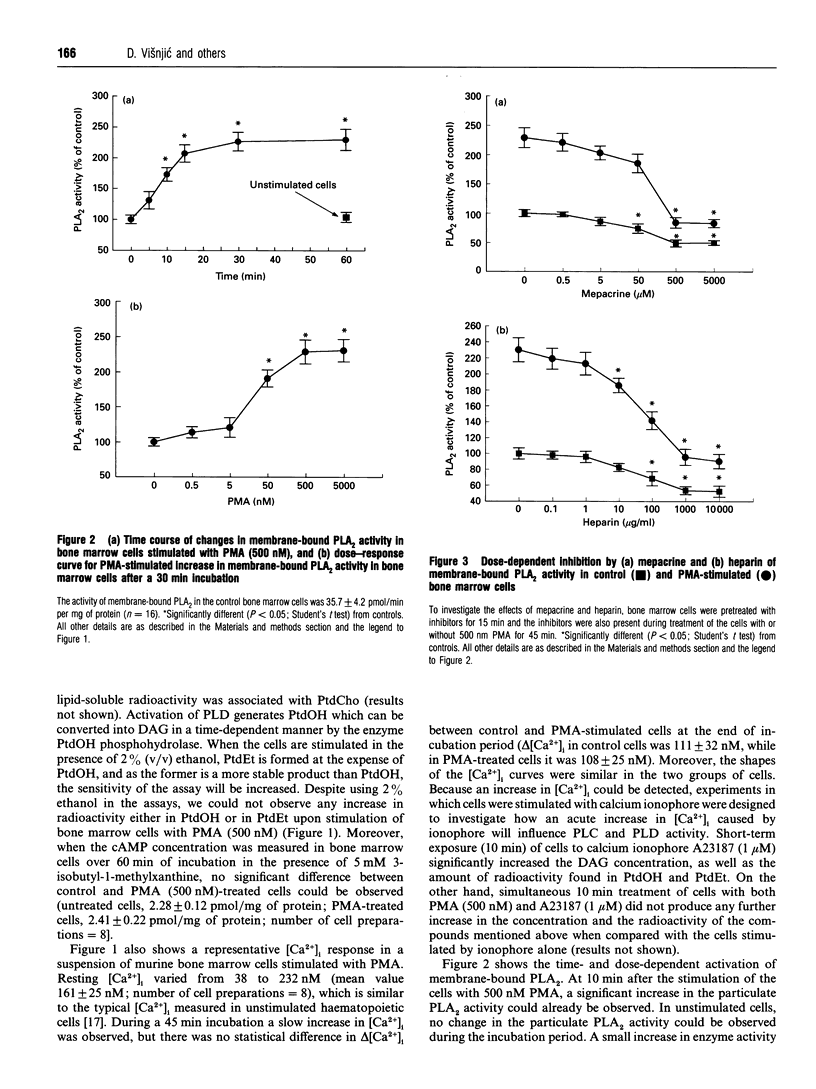
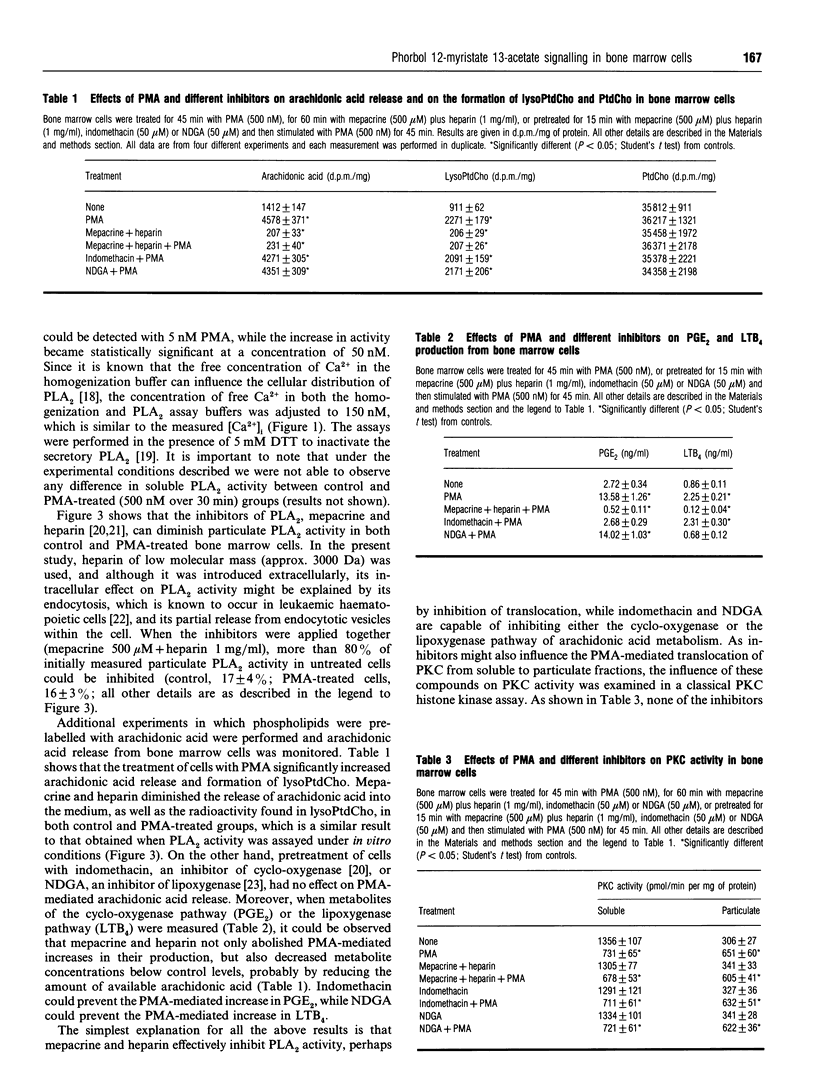
Phorbol 12-myristate 13-acetate (PMA)-mediated signalling was investigated in relation to the ability of murine (CBA) bone marrow cells to form colonies in vitro. Treatment of marrow cells with PMA did not influence the 1,2-diacylglycerol or cyclic AMP concentrations, the intracellular Ca2+ concentration or phospholipase D activity. PMA increased particulate phospholipase A2 (PLA2) activity, lysophosphatidylcholine formation and arachidonic acid release from bone marrow cells; these effects were abolished when cells were pretreated with the putative PLA2 inhibitors heparin and mepacrine. While indomethacin and nordihydroguaiaretic acid inhibited either the cyclo-oxygenase or lipoxygenase pathway of arachidonic acid metabolism, as measured by their products prostaglandin E2 and leukotriene B4, they did not influence PMA-mediated PLA2 activation or translocation of protein kinase C (PKC) from the soluble to the particulate fraction. Treatment of cells with PMA increased the amounts of membrane-bound alpha, beta, delta, epsilon and zeta isoforms of PKC in bone marrow cells. Pretreatment of cells with PLA2 inhibitors reduced the amount of membrane-bound PKC-zeta in unstimulated cells and diminished PMA-induced translocation of PKC-zeta to membranes without affecting other PKC isoforms. This effect could be overcome by exogenous addition of arachidonic acid, suggesting that PKC-zeta may operate downstream of the activated PLA2. On the other hand, wortmannin, an inhibitor of phosphatidylinositol 3-kinase, did not influence the amount of PKC-zeta associated with particulate fractions in control cells and could not abolish the PMA-mediated translocation of this isoform. Short-term exposure (45 min) of bone marrow cells to PMA, phorbol 12,13-dibutyrate or arachidonic acid increased the number of colonies formed over 7 days in a methylcellulose-based culture in vitro. The effects of PMA, but not those of arachidonic acid, could be prevented by putative PLA2 inhibitors. This suggests that PMA-mediated activation of conventional PKCs and novel PKCs leads to PLA2 activation which, by releasing arachidonic acid from phospholipids, activates PKC-zeta. This signalling pathway appears to be mitogenic for bone marrow cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bashey A., Healy L., Marshall C. J. Proliferative but not nonproliferative responses to granulocyte colony-stimulating factor are associated with rapid activation of the p21ras/MAP kinase signalling pathway. Blood. 1994 Feb 15;83(4):949–957. [PubMed] [Google Scholar]
- Batlle E., Fabre M., García de Herreros A. Antipeptide antibodies directed against the C-terminus of protein kinase C zeta (PKC zeta) react with a Ca(2+)- and TPA-sensitive PKC in HT-29 human intestinal epithelial cells. FEBS Lett. 1994 May 16;344(2-3):161–165. doi: 10.1016/0014-5793(94)00379-3. [DOI] [PubMed] [Google Scholar]
- Berra E., Diaz-Meco M. T., Dominguez I., Municio M. M., Sanz L., Lozano J., Chapkin R. S., Moscat J. Protein kinase C zeta isoform is critical for mitogenic signal transduction. Cell. 1993 Aug 13;74(3):555–563. doi: 10.1016/0092-8674(93)80056-k. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Blackwell G. J., Duncombe W. G., Flower R. J., Parsons M. F., Vane J. R. The distribution and metabolism of arachidonic acid in rabbit platelets during aggregation and its modification by drugs. Br J Pharmacol. 1977 Feb;59(2):353–366. doi: 10.1111/j.1476-5381.1977.tb07500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Capdevila J., Gil L., Orellana M., Marnett L. J., Mason J. I., Yadagiri P., Falck J. R. Inhibitors of cytochrome P-450-dependent arachidonic acid metabolism. Arch Biochem Biophys. 1988 Mar;261(2):257–263. doi: 10.1016/0003-9861(88)90340-2. [DOI] [PubMed] [Google Scholar]
- Carroll M. P., May W. S. Protein kinase C-mediated serine phosphorylation directly activates Raf-1 in murine hematopoietic cells. J Biol Chem. 1994 Jan 14;269(2):1249–1256. [PubMed] [Google Scholar]
- Channon J. Y., Leslie C. C. A calcium-dependent mechanism for associating a soluble arachidonoyl-hydrolyzing phospholipase A2 with membrane in the macrophage cell line RAW 264.7. J Biol Chem. 1990 Apr 5;265(10):5409–5413. [PubMed] [Google Scholar]
- Clark J. D., Lin L. L., Kriz R. W., Ramesha C. S., Sultzman L. A., Lin A. Y., Milona N., Knopf J. L. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell. 1991 Jun 14;65(6):1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Karnowsky M. J. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977 Feb 17;265(5595):625–626. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- Dawson W. D., Cook J. S. Parallel changes in amino acid transport and protein kinase C localization in LLC-PK1 cells treated with TPA or diradylglycerols. J Cell Physiol. 1987 Jul;132(1):104–110. doi: 10.1002/jcp.1041320114. [DOI] [PubMed] [Google Scholar]
- Dennis E. A. Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem. 1994 May 6;269(18):13057–13060. [PubMed] [Google Scholar]
- Dethlefsen S. M., Shepro D., D'Amore P. A. Arachidonic acid metabolites in bFGF-, PDGF-, and serum-stimulated vascular cell growth. Exp Cell Res. 1994 Jun;212(2):262–273. doi: 10.1006/excr.1994.1142. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Spooncer E. Growth and differentiation in the hemopoietic system. Annu Rev Cell Biol. 1987;3:423–441. doi: 10.1146/annurev.cb.03.110187.002231. [DOI] [PubMed] [Google Scholar]
- Diaz-Meco M. T., Dominguez I., Sanz L., Dent P., Lozano J., Municio M. M., Berra E., Hay R. T., Sturgill T. W., Moscat J. zeta PKC induces phosphorylation and inactivation of I kappa B-alpha in vitro. EMBO J. 1994 Jun 15;13(12):2842–2848. doi: 10.1002/j.1460-2075.1994.tb06578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diccianni M. B., Mistry M. J., Hug K., Harmony J. A. Inhibition of phospholipase A2 by heparin. Biochim Biophys Acta. 1990 Oct 1;1046(3):242–248. doi: 10.1016/0005-2760(90)90237-r. [DOI] [PubMed] [Google Scholar]
- Durstin M., Durstin S., Molski T. F., Becker E. L., Sha'afi R. I. Cytoplasmic phospholipase A2 translocates to membrane fraction in human neutrophils activated by stimuli that phosphorylate mitogen-activated protein kinase. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3142–3146. doi: 10.1073/pnas.91.8.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Favre C. J., Lew D. P., Krause K. H. Rapid heparin-sensitive Ca2+ release following Ca(2+)-ATPase inhibition in intact HL-60 granulocytes. Evidence for Ins(1,4,5)P3-dependent Ca2+ cycling across the membrane of Ca2+ stores. Biochem J. 1994 Aug 15;302(Pt 1):155–162. doi: 10.1042/bj3020155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hug H., Sarre T. F. Protein kinase C isoenzymes: divergence in signal transduction? Biochem J. 1993 Apr 15;291(Pt 2):329–343. doi: 10.1042/bj2910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharbanda S., Saleem A., Emoto Y., Stone R., Rapp U., Kufe D. Activation of Raf-1 and mitogen-activated protein kinases during monocytic differentiation of human myeloid leukemia cells. J Biol Chem. 1994 Jan 14;269(2):872–878. [PubMed] [Google Scholar]
- Kishimoto T., Taga T., Akira S. Cytokine signal transduction. Cell. 1994 Jan 28;76(2):253–262. doi: 10.1016/0092-8674(94)90333-6. [DOI] [PubMed] [Google Scholar]
- Kochs G., Hummel R., Meyer D., Hug H., Marmé D., Sarre T. F. Activation and substrate specificity of the human protein kinase C alpha and zeta isoenzymes. Eur J Biochem. 1993 Sep 1;216(2):597–606. doi: 10.1111/j.1432-1033.1993.tb18179.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liebermann D., Hoffman-Liebermann B., Sachs L. Regulation of gene expression by tumor promoters. II. Control of cell shape and developmental programs for macrophages and granulocytes in human myeloid leukemic cells. Int J Cancer. 1981 Sep 15;28(3):285–291. doi: 10.1002/ijc.2910280306. [DOI] [PubMed] [Google Scholar]
- Lin L. L., Lin A. Y., Knopf J. L. Cytosolic phospholipase A2 is coupled to hormonally regulated release of arachidonic acid. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6147–6151. doi: 10.1073/pnas.89.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L. L., Wartmann M., Lin A. Y., Knopf J. L., Seth A., Davis R. J. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993 Jan 29;72(2):269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Lin L. L., Kharbanda S., Knopf J., Kufe D. Macrophage colony stimulating factor activates phosphatidylcholine hydrolysis by cytoplasmic phospholipase A2. EMBO J. 1992 Dec;11(13):4917–4922. doi: 10.1002/j.1460-2075.1992.tb05598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi H., Brewer K. A., Exton J. H. Activation of the zeta isozyme of protein kinase C by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1993 Jan 5;268(1):13–16. [PubMed] [Google Scholar]
- Nakanishi H., Exton J. H. Purification and characterization of the zeta isoform of protein kinase C from bovine kidney. J Biol Chem. 1992 Aug 15;267(23):16347–16354. [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Ogawa M. Differentiation and proliferation of hematopoietic stem cells. Blood. 1993 Jun 1;81(11):2844–2853. [PubMed] [Google Scholar]
- Okada T., Kawano Y., Sakakibara T., Hazeki O., Ui M. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. Studies with a selective inhibitor wortmannin. J Biol Chem. 1994 Feb 4;269(5):3568–3573. [PubMed] [Google Scholar]
- Olivier A. R., Parker P. J. Expression and characterization of protein kinase C-delta. Eur J Biochem. 1991 Sep 15;200(3):805–810. doi: 10.1111/j.1432-1033.1991.tb16248.x. [DOI] [PubMed] [Google Scholar]
- Ottlinger M. E., Pukac L. A., Karnovsky M. J. Heparin inhibits mitogen-activated protein kinase activation in intact rat vascular smooth muscle cells. J Biol Chem. 1993 Sep 15;268(26):19173–19176. [PubMed] [Google Scholar]
- Pukac L. A., Castellot J. J., Jr, Wright T. C., Jr, Caleb B. L., Karnovsky M. J. Heparin inhibits c-fos and c-myc mRNA expression in vascular smooth muscle cells. Cell Regul. 1990 Apr;1(5):435–443. doi: 10.1091/mbc.1.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner N., Bunge R. P., Glaser L. A neuronal cell surface heparan sulfate proteoglycan is required for dorsal root ganglion neuron stimulation of Schwann cell proliferation. J Cell Biol. 1985 Sep;101(3):744–754. doi: 10.1083/jcb.101.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly C. F., Fritze L. M., Rosenberg R. D. Antiproliferative effects of heparin on vascular smooth muscle cells are reversed by epidermal growth factor. J Cell Physiol. 1987 May;131(2):149–157. doi: 10.1002/jcp.1041310203. [DOI] [PubMed] [Google Scholar]
- Selbie L. A., Schmitz-Peiffer C., Sheng Y., Biden T. J. Molecular cloning and characterization of PKC iota, an atypical isoform of protein kinase C derived from insulin-secreting cells. J Biol Chem. 1993 Nov 15;268(32):24296–24302. [PubMed] [Google Scholar]
- Sieber F., Stuart R. K., Spivak J. L. Tumor-promoting phorbol esters stimulate myelopoiesis and suppress erythropoiesis in cultures of mouse bone marrow cells. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4402–4406. doi: 10.1073/pnas.78.7.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart R. K., Hamilton J. A. Tumor-promoting phorbol esters stimulate hematopoietic colony formation in vitro. Science. 1980 Apr 25;208(4442):402–404. doi: 10.1126/science.6245446. [DOI] [PubMed] [Google Scholar]
- Stutchfield J., Cockcroft S. Correlation between secretion and phospholipase D activation in differentiated HL60 cells. Biochem J. 1993 Aug 1;293(Pt 3):649–655. doi: 10.1042/bj2930649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamiya-Koizumi K., Umekawa H., Yoshida S., Ishihara H., Kojima K. A novel phospholipase A2 associated with nuclear matrix: stimulation of the activity and modulation of the Ca2+ dependency by polyphosphoinositides. Biochim Biophys Acta. 1989 Apr 3;1002(2):182–188. doi: 10.1016/0005-2760(89)90285-3. [DOI] [PubMed] [Google Scholar]
- Thornton S. C., Mueller S. N., Levine E. M. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983 Nov 11;222(4624):623–625. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- Whetton A. D., Heyworth C. M., Nicholls S. E., Evans C. A., Lord J. M., Dexter T. M., Owen-Lynch P. J. Cytokine-mediated protein kinase C activation is a signal for lineage determination in bipotential granulocyte macrophage colony-forming cells. J Cell Biol. 1994 May;125(3):651–659. doi: 10.1083/jcb.125.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G., Wooten M. W., Coleman E. S. Regulation of atypical zeta-protein kinase C in cellular signaling. Exp Cell Res. 1994 Sep;214(1):1–11. doi: 10.1006/excr.1994.1227. [DOI] [PubMed] [Google Scholar]