Abstract
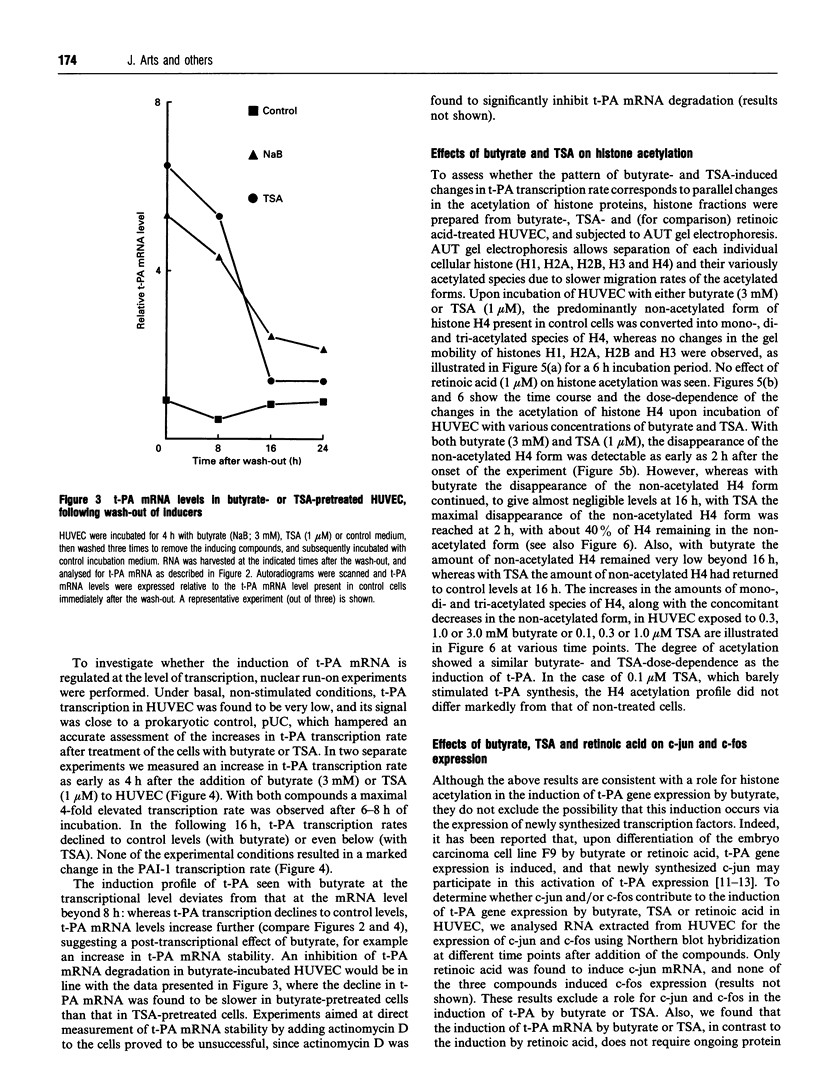
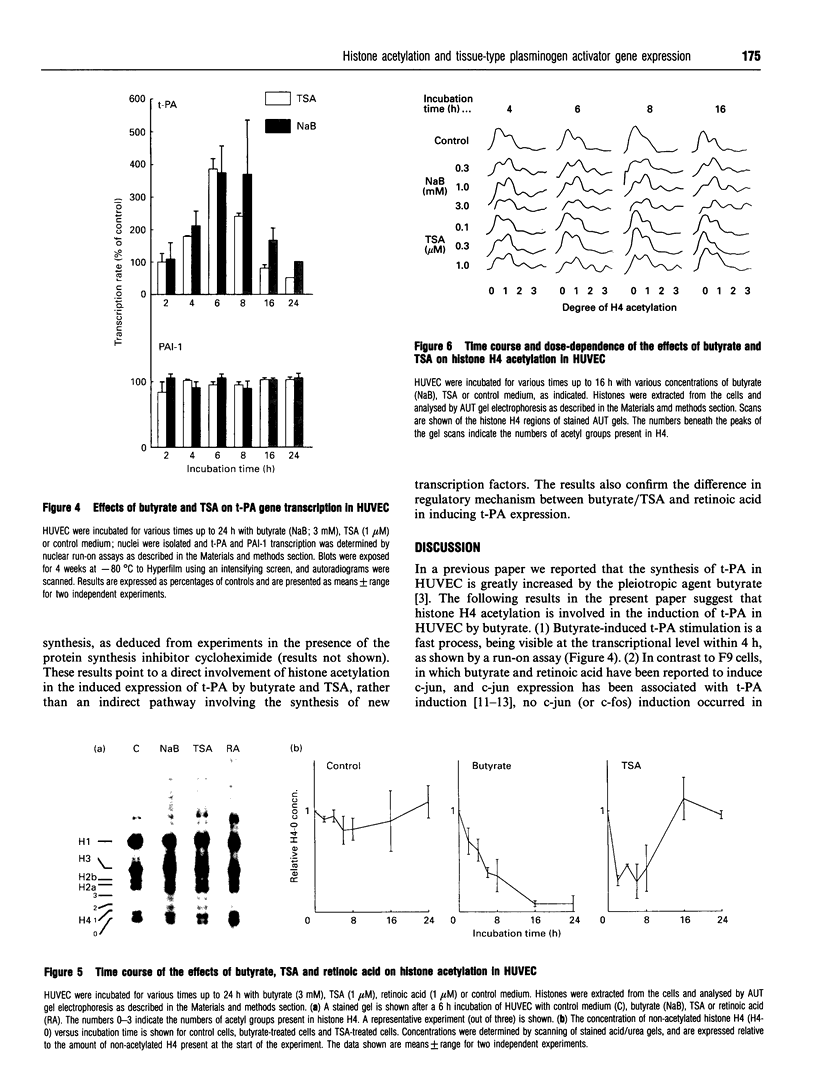
We have previously shown that the pleiotropic agent sodium butyrate strongly stimulates tissue-type plasminogen activator (t-PA) expression in human umbilical vein endothelial cells (HUVEC). Here we provide the following evidence that the butyrate-induced t-PA expression in HUVEC involves histone H4 acetylation. (1) t-PA induction by butyrate occurs at the transcriptional level and does not require new protein synthesis, indicating a direct effect. (2) t-PA induction by butyrate can be fully mimicked by a specific, structurally unrelated, histone deacetylase inhibitor, trichostatin A. (3) At optimally stimulatory conditions, a combination of butyrate and trichostatin A does not enhance t-PA production more than each of the compounds alone, indicating that both compounds act through a common regulatory mechanism. (4) Induction of t-PA transcription by butyrate and trichostatin A was found to be preceded by histone H4 acetylation; at suboptimal inducing concentrations of butyrate and trichostatin A, the degree of acetylation of histone H4 caused by each agent was similarly reduced. These results are consistent with a role for histone H4 acetylation in t-PA induction by butyrate in HUVEC.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckle R., Balmer M., Yenidunya A., Allan J. The promoter and enhancer of the inactive chicken beta-globin gene contains precisely positioned nucleosomes. Nucleic Acids Res. 1991 Mar 25;19(6):1219–1226. doi: 10.1093/nar/19.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P., Schoonjans L., Kieckens L., Ream B., Degen J., Bronson R., De Vos R., van den Oord J. J., Collen D., Mulligan R. C. Physiological consequences of loss of plasminogen activator gene function in mice. Nature. 1994 Mar 31;368(6470):419–424. doi: 10.1038/368419a0. [DOI] [PubMed] [Google Scholar]
- Chen T. A., Allfrey V. G. Rapid and reversible changes in nucleosome structure accompany the activation, repression, and superinduction of murine fibroblast protooncogenes c-fos and c-myc. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5252–5256. doi: 10.1073/pnas.84.15.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cousens L. S., Gallwitz D., Alberts B. M. Different accessibilities in chromatin to histone acetylase. J Biol Chem. 1979 Mar 10;254(5):1716–1723. [PubMed] [Google Scholar]
- Curran T., Peters G., Van Beveren C., Teich N. M., Verma I. M. FBJ murine osteosarcoma virus: identification and molecular cloning of biologically active proviral DNA. J Virol. 1982 Nov;44(2):674–682. doi: 10.1128/jvi.44.2.674-682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrin L. K., Mann R. K., Kayne P. S., Grunstein M. Yeast histone H4 N-terminal sequence is required for promoter activation in vivo. Cell. 1991 Jun 14;65(6):1023–1031. doi: 10.1016/0092-8674(91)90554-c. [DOI] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbes T. R., Clayton A. L., Thorne A. W., Crane-Robinson C. Core histone hyperacetylation co-maps with generalized DNase I sensitivity in the chicken beta-globin chromosomal domain. EMBO J. 1994 Apr 15;13(8):1823–1830. doi: 10.1002/j.1460-2075.1994.tb06451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbes T. R., Thorne A. W., Crane-Robinson C. A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J. 1988 May;7(5):1395–1402. doi: 10.1002/j.1460-2075.1988.tb02956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P., Turner B. M. The inactive X chromosome in female mammals is distinguished by a lack of histone H4 acetylation, a cytogenetic marker for gene expression. Cell. 1993 Jul 30;74(2):281–289. doi: 10.1016/0092-8674(93)90419-q. [DOI] [PubMed] [Google Scholar]
- Kooistra T., Schrauwen Y., Arts J., Emeis J. J. Regulation of endothelial cell t-PA synthesis and release. Int J Hematol. 1994 Jun;59(4):233–255. [PubMed] [Google Scholar]
- Kooistra T., van den Berg J., Töns A., Platenburg G., Rijken D. C., van den Berg E. Butyrate stimulates tissue-type plasminogen-activator synthesis in cultured human endothelial cells. Biochem J. 1987 Nov 1;247(3):605–612. doi: 10.1042/bj2470605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. Y., Hayes J. J., Pruss D., Wolffe A. P. A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell. 1993 Jan 15;72(1):73–84. doi: 10.1016/0092-8674(93)90051-q. [DOI] [PubMed] [Google Scholar]
- Maciag T., Cerundolo J., Ilsley S., Kelley P. R., Forand R. An endothelial cell growth factor from bovine hypothalamus: identification and partial characterization. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5674–5678. doi: 10.1073/pnas.76.11.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollinedo F., Gajate C., Tugores A., Flores I., Naranjo J. R. Differences in expression of transcription factor AP-1 in human promyelocytic HL-60 cells during differentiation towards macrophages versus granulocytes. Biochem J. 1993 Aug 15;294(Pt 1):137–144. doi: 10.1042/bj2940137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishina Y., Sumi T., Iwai S. A., Kosaka M., Nishimune Y. The induction of jun genes during the reversible changes induced with sodium butyrate on the differentiation of F9 cells. Exp Cell Res. 1993 Oct;208(2):492–497. doi: 10.1006/excr.1993.1271. [DOI] [PubMed] [Google Scholar]
- Riggs M. G., Whittaker R. G., Neumann J. R., Ingram V. M. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 1977 Aug 4;268(5619):462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- Souleimani A., Asselin C. Regulation of C-fos expression by sodium butyrate in the human colon carcinoma cell line Caco-2. Biochem Biophys Res Commun. 1993 May 28;193(1):330–336. doi: 10.1006/bbrc.1993.1628. [DOI] [PubMed] [Google Scholar]
- Tang S. J., Ko L. W., Lee Y. H., Wang F. F. Induction of fos and sis proto-oncogenes and genes of the extracellular matrix proteins during butyrate induced glioma differentiation. Biochim Biophys Acta. 1990 Jan 30;1048(1):59–65. doi: 10.1016/0167-4781(90)90022-t. [DOI] [PubMed] [Google Scholar]
- Tichonicky L., Kruh J., Defer N. Sodium butyrate inhibits c-myc and stimulates c-fos expression in all the steps of the cell-cycle in hepatoma tissue cultured cells. Biol Cell. 1990;69(1):65–67. doi: 10.1016/0248-4900(90)90329-2. [DOI] [PubMed] [Google Scholar]
- Twisk J., Lehmann E. M., Princen H. M. Differential feedback regulation of cholesterol 7 alpha-hydroxylase mRNA and transcriptional activity by rat bile acids in primary monolayer cultures of rat hepatocytes. Biochem J. 1993 Mar 15;290(Pt 3):685–691. doi: 10.1042/bj2900685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman J. L., Roeder R. G. Binding of transcription factor TFIID to the major late promoter during in vitro nucleosome assembly potentiates subsequent initiation by RNA polymerase II. Cell. 1987 Nov 20;51(4):613–622. doi: 10.1016/0092-8674(87)90130-9. [DOI] [PubMed] [Google Scholar]
- Yang-Yen H. F., Chiu R., Karin M. Elevation of AP1 activity during F9 cell differentiation is due to increased c-jun transcription. New Biol. 1990 Apr;2(4):351–361. [PubMed] [Google Scholar]
- Yoshida M., Kijima M., Akita M., Beppu T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem. 1990 Oct 5;265(28):17174–17179. [PubMed] [Google Scholar]
- de Groot R. P., Kruyt F. A., van der Saag P. T., Kruijer W. Ectopic expression of c-jun leads to differentiation of P19 embryonal carcinoma cells. EMBO J. 1990 Jun;9(6):1831–1837. doi: 10.1002/j.1460-2075.1990.tb08308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hinsbergh V. W., Havekes L., Emeis J. J., van Corven E., Scheffer M. Low density lipoprotein metabolism by endothelial cells from human umbilical cord arteries and veins. Arteriosclerosis. 1983 Nov-Dec;3(6):547–559. doi: 10.1161/01.atv.3.6.547. [DOI] [PubMed] [Google Scholar]
- van Zonneveld A. J., Chang G. T., van den Berg J., Kooistra T., Verheijen J. H., Pannekoek H., Kluft C. Quantification of tissue-type plasminogen activator (t-PA) mRNA in human endothelial-cell cultures by hybridization with a t-PA cDNA probe. Biochem J. 1986 Apr 15;235(2):385–390. doi: 10.1042/bj2350385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg E. A., Sprengers E. D., Jaye M., Burgess W., Maciag T., van Hinsbergh V. W. Regulation of plasminogen activator inhibitor-1 mRNA in human endothelial cells. Thromb Haemost. 1988 Aug 30;60(1):63–67. [PubMed] [Google Scholar]





