Abstract
Glycogen storage disease type 1a (GSD-1a) is a rare congenital disease. Recently, life expectancy with GSD-1a has been improved by its early diagnosis and management. Complications of diabetes with GSD-1a are extremely rare. The optimal treatment for glucose control using this disease combination remains unclear. The existence of GSD-1a and diabetes can cause both hypoglycemia and hyperglycemia, making glucose control especially problematic. In the present report, α-glucosidase inhibitor (α-GI) and dipeptidyl peptidase-4 (DPP-4) inhibitors improved hyperglycemia without symptoms of hypoglycemia in a patient with diabetes and GSD-1a using intermittent continuous glucose monitoring (isCGM).
Keywords: glycogen storage disease type 1a, diabetes, hypoglycemia, dipeptidyl peptidase-4 inhibitor, α-glucosidase inhibitor, intermittently scanned continuous glucose monitoring
Introduction
Glycogen storage disease (GSD) type 1a (GSD-1a) is a rare congenital glycogen metabolism disorder caused by deficiency of glucose 6-phosphatase (G6PC) (1). This enzyme is essential for the hydrolyze glucose-6 phosphate (G6P) to glucose, which is an essential process in gluconeogenesis. Glycogen deposits in the liver, kidney, and intestine that are not converted to glucose result in hepatomegaly, chronic kidney disorders, and diarrhea in GSD-1a (2). Dyslipidemia and hyperuricemia have also been observed as concomitant metabolic disorders. Among the clinical symptoms of GSD-1a, hypoglycemia, which occurs in early childhood, is the most important symptom.
Recently, the life expectancy of patients with GSD has been extended considerably owing to its early diagnosis and symptomatic treatment (2). Several patients with GSD have been reported to have diabetes (3). Both hyperglycemia and hypoglycemia must be treated for glucose control in GSD patients with diabetes. However, there have been insufficient reports to provide treatment guidelines. Therefore, the effectiveness and safety of these treatments remain unclear.
Intermittently scanned continuous glucose monitoring (isCGM) has been shown to reduce hypoglycemia and facilitate good glucose control in patients with diabetes (4,5). We treated a case of GSD-1a with diabetes using isCGM. Diet and drug therapy improved glucose control without hypoglycemia while monitoring blood glucose levels for 24 h. We report this case in an attempt to determine the most effective and safe treatment for glucose control in patients with GSD-1a and diabetes.
Case Report
A 59-year-old woman with growth retardation since infancy had been diagnosed with hepatomegaly at 4 months old. She had been diagnosed with GSD-1a based on a liver biopsy showing decreased G6PC activity and increased glycogen content at 6 years old. She had been asymptomatic since then, but her life gradually became busy and irregular. At 44 years old, she was hospitalized due to frequent hypoglycemia. She also had liver dysfunction, hyperuricemia, and hyperlipidemia. A 75-g oral glucose tolerance test was performed, and diabetes was suspected [fasting plasma glucose (PG) level, 56 mg/dL; fasting C-peptide level 0.43 ng/mL, PG level, 264 mg/dL at 120 min; C-peptide level 13.96 ng/mL at 120 min; and HbA1c, 4.6%]. A cornstarch diet was started during hospitalization, but the treatment was interrupted. Diabetes was not noted on a medical examination until 58 years old. Struggle avoidance of hypoglycemia and work stress increased her intake of snacks and sweets containing sugar, and diabetes was noted at 59 years old. The patient was admitted to our hospital for glucose control. Before administration, her total calorie intake was 1,576 kcal/day, carbohydrate 253 g/64.2%, protein 62 g/15.7%, fat 35.1 g/20.0%, including 9.6% of fructose, galactose, sucrose, and lactose. Her mother had occasional hypoglycemia but had not been diagnosed with GSD (details are unknown).
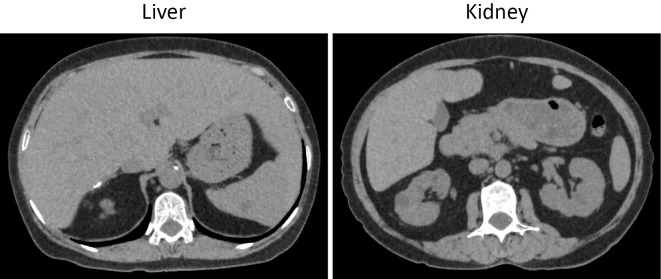
On an examination, she had short stature (139.8 cm) and hepatomegaly but had no abnormal neurological findings. She did not have a doll-like face. Laboratory tests showed liver and renal dysfunction and elevated serum HbA1c, triglyceride, and low-density lipoprotein cholesterol levels (Table). Urine C-peptide 68.8 μg/day indicated non-insulin-dependent diabetes. In the oral meal loading test, the fasting PG level was 103 mg/dL with a C-peptide 1.02 ng/mL, and the PG level was 236 mg/dL with a C-peptide 3.48 ng/mL of 120 min. The lactate level decreased from 48.7 to 39.9 mg/dL after 120 min meal loading. A mutation analysis of G6PC revealed a c.648G>T homozygous mutation. Computed tomography (CT) showed hepatomegaly without a low CT value and an irregular surface in the kidney without atrophy (Fig. 1). Abdominal ultrasonography showed hepatorenal contrast without deep attenuation of the liver, indicating a mild fatty liver.
Table.
Laboratory Data on the Admission of the Patient.
| <General> | <Biochemistry> | <Coagulation> | <Diabetes-related> | |||||||||||||||
| Height | 139.8 | cm | TP | 7.4 | g/dL | PT | 11.3 | sec | HbA1c | 8.0 | % | |||||||
| Weight | 40.2 | kg | Alb | 4.1 | g/dL | PT-INR | 0.89 | Fasting plasma glucose | 132 | mg/dL | ||||||||
| BMI | 20.8 | kg/m2 | T-Bil | 0.6 | mg/dL | APTT | 26.5 | sec | Fasting insulin | 1.3 | μU/mL | |||||||
| AST | 54 | IU/L | D-Dimer | <0.3 | μg/mL | Fasting C-peptide | 1.02 | ng/mL | ||||||||||
| <CBC> | ALT | 40 | IU/L | GAD-Ab | <0.5 | U/mL | ||||||||||||
| WBC | 5,420 | /μL | ALP | 96 | U/L | <VBG> | ||||||||||||
| Neu | 38.7 | % | LDH | 149 | IU/L | pH | 7.26 | <Meal loading test> | ||||||||||
| Eo | 3.1 | % | γ-GTP | 158 | IU/L | pCO2 | 41.3 | mmHg | 0 min | 120 min | ||||||||
| Ba | 0.4 | % | CPK | 60 | U/L | pO2 | 68.6 | mmHg | Plasma glucose (mg/dL) | 103 | 236 | |||||||
| Ly | 50.4 | % | Amylase | 85 | U/L | HCO3- | 17.8 | mmol/L | C-peptide (ng/mL) | 1.02 | 3.48 | |||||||
| Mono | 7.4 | % | BUN | 20 | mg/dL | BE | -8.6 | mEq/L | Lactate (mg/dL) | 48.7 | 39.9 | |||||||
| RBC | 426 | /μL | Cre | 0.88 | mg/dL | Lactate | 63.1 | mg/dL | ||||||||||
| Hb | 13.4 | g/dL | eGFR | 51.2 | mL/min/1.73m2 | <24h Urine test> | ||||||||||||
| Ht | 39.8 | % | Cystatin C | 1.16 | mg/L | <Tumor marker> | C-peptide | 68.8 | μg/day | |||||||||
| Plt | 244 | /μL | UA | 6.3 | mg/dL | AFP | 1.7 | ng/mL | Albmin | 36.2 | mg/day | |||||||
| Na | 139 | mEq/L | PIVKA-2 | 17 | mAU/mL | |||||||||||||
| K | 3.7 | mEq/L | CEA | 1.8 | ng/mL | |||||||||||||
| <Urine> | Cl | 104 | mEq/L | CA19-9 | 34.0 | U/mL | <Mutation analysis of G6PC gene> | |||||||||||
| Protein | (1+) | Ca | 9.2 | mg/dL | c.648G>T homozygous mutation | |||||||||||||
| Glucose | (1+) | T-Cho | 346 | mg/dL | <Ketone body level> | |||||||||||||
| Blood | (-) | TG | 456 | mg/dL | Total ketone body | 133 | mmol/L | |||||||||||
| Ketone | (-) | HDL-Cho | 59 | mg/dL | Acetoacetic acid | 43.0 | mmol/L | |||||||||||
| LDL-Cho | 218 | mg/dL | 3-hydroxybutric acid | 89.5 | mmol/L | |||||||||||||
| Protein | 0.1 | g/day | CRP | 0.8 | mg/dL | |||||||||||||
| Albumin | 26.2 | mg/day | ||||||||||||||||
Figure 1.
CT findings of the (A) liver and (B) kidney.
After hospitalization, we started diet therapy with a total energy limit of 1,300 kcal/day, including carbohydrates at 175 g/53.8%, protein at 65 g/20.0%, and fat at 45 g/31.2%, excluding dairy products and fruits and with restricted carbohydrates, such as fructose, galactose, sucrose, and lactose, accounting for ≤5%. We also replaced all instances of sugar with a low-calorie sweetener. Supplements were used between meals (once per 50 kcal) and exercise.
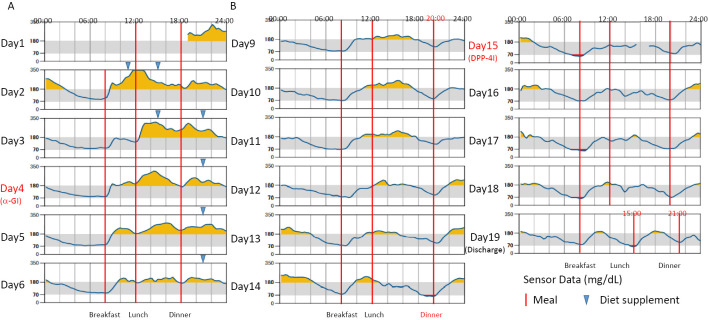
isCGM was used for glucose monitoring. However, because hyperglycemia during the daytime did not improve, we concomitantly decreased food supplementation between meals. On Day 4, we started α-GI voglibose at 0.6 mg daily (Fig. 2A). With this treatment, hyperglycemia during the day tended to improve. Hypoglycemia was not observed after discontinuing food supplementation between meals and changing the time from 6:00 pm to 8:00 pm to suit the patient's daily life, but hyperglycemia after breakfast and at night remained (Fig. 2B).
Figure 2.
Glucose monitoring using isCGM in the present patient. The target glucose level was 70-180 mg/dL, and we showed the effect of treatment based on the time below range (TBR), time in range (TIR), and time above range (TAR) daily. A: Administration of the α-GI voglibose (0.6 mg/day) was initiated on day 4. B: Food supplementation between meals was discontinued, and the timing of dinner was moved from 18:00 to 20:00 on day 9. DPP-4 inhibitor (DPP-4I) sitagliptin (50 mg/day) was added on day 15.
For more effective glucose control, the DPP-4 inhibitor sitagliptin 50 mg daily was started on day 15, and hyperglycemia improved (Fig. 2C). Blood glucose levels were maintained at >65 mg/dL in the isCGM group, and there was no symptomatic hypoglycemia. Sitagliptin was decreased to 25 mg daily at discharge because of concerns about hypoglycemia with an increase in her physical activity. She did not have any gastrointestinal symptoms, such as diarrhea or constipation, after treatment with an α-GI or DPP-4 inhibitor. In this case, a sodium-glucose co-transporter 2 (SGLT2) inhibitor and metformin were not used because of the risk of elevated ketone bodies and lactic acid levels. Febuxostat 10 mg/day, rosuvastatin 2.5 mg/day, and pemafibrate 0.1 mg/day, were started. She underwent several examinations for microangiopathy and macroangiopathy during hospitalization; however, such severe complications were not observed.
Discussion
This case of GSD-1a associated with diabetes is extremely rare. As the lifespan of patients with GSD is extended, the management of GSD with diabetes is becoming an even more important issue than before. The presence of both hyperglycemia and hypoglycemia in the same patient make glucose control especially problematic. Diet therapy is important for the treatment of GSD-1a. In addition to proper calorie setting, it is necessary to limit restricted carbohydrates to ≤5% of the diet in order to reduce the occurrence of hyperlactacidemia, dyslipidemia, and obesity.
In the present case, liver dysfunction, hyperuricemia, and dyslipidemia were observed at 44 years old, and diabetes was suspected. The patient was considered to have metabolic disorders associated with GSD-1a (6). Previously, GSD-1a was reported to cause insulin resistance owing to the accumulation of G6P in the liver, which causes de novo lipid production, leading to fatty liver (7). In previous reports of GSD-1a combined with impaired glucose tolerance, obesity and hyperinsulinemia have been associated (3,8). However, obesity was not observed in the present case. It has also been reported that insulin secretion is delayed in the postprandial state, causing hyperglycemia (9). There is another report of GSD-1a in diabetes without obesity treated with insulin therapy (10). In this case, another 14 years had passed before this hospitalization, and diet therapy alone did not improve hyperglycemia, which is why we thought that there was insulin deficiency in this case. Therefore, α-GI and DPP-4 inhibitors may be suitable for patient GSD-1a with diabetes without obesity.
Incretin-related drugs, including DPP-4 inhibitors, enhance insulin secretion and suppress glucagon secretion in a glucose-dependent manner (11). Under hypoglycemic conditions, the action of these drugs in lowering BG levels is absent. In the present case, additional therapy with a DPP-4 inhibitor improved glucose control. Among patients with a low glycemic status, disorders of gluconeogenesis might become obvious in GSD-1a patients with diabetes throughout the treatment. In the present GSD-1a patient, the isCGM data did not show severe hypoglycemia during treatment with α-GI and DPP-4i. Therefore, DPP-4i may be a treatment options for GSD-1a patients with diabetes.
Management of hypoglycemia and associated metabolic disorders is very important in GSD. In particular, GSD-1a is considered to have strong hypoglycemic symptoms in early childhood due to the impairment of gluconeogenesis in the liver. The c.648G>T mutation of G6PC found in this case is a dominant mutation in Japan, and hypoglycemic symptoms are reported to be relatively mild in patients with this mutation (12). Therefore, there is no contradiction in that there was no severe hypoglycemia in early childhood in this case. Patients with the G6PC gene homozygous mutation c.648G>T tend to have higher serum uric acid, lactic acid, and triglyceride levels than those with heterozygous mutations (12). Similarly, compared to GSD type 1b (GSD-1b), GSD-1a has higher prevalence of metabolic syndrome, which is attributed to excess G6P accumulation in endoplasmic reticulum of liver (13). As the lifespan of patients with GSD-1a improves, the risk of complication with not only hypoglycemia but also hepatic disorders, renal disorders, hyperlipidemia, and hyperuricemia due to glycogen deposition in organs increases. Therefore, management of these complications is considered an important clinical issue.
Although GSD-1a with diabetes is still a rare disease, the present findings suggest a potential treatment option, which will lead to further discussion.
The patient provided her informed consent for the publication of her clinical details and images.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Rajas F, Labrune P, Mithieux G. Glycogen storage disease type 1 and diabetes: learning by comparing and contrasting the two disorders. Diabetes Metab 39: 377-387, 2013. [DOI] [PubMed] [Google Scholar]
- 2. Rake JP, Visser G, Labrune P, Leonard JV, Ullrich K, Smit PG, European Study on Glycogen Storage Disease Type 1 (ESGSD 1). Guidelines for management of glycogen storage disease type 1 - European Study on Glycogen Storage Disease Type 1 (ESGSD1). Eur J Pediatr 161: S112-S119, 2002. [DOI] [PubMed] [Google Scholar]
- 3. Cohn A, Ohri A. Diabetes mellitus in a patient with glycol storage disease type 1a: a case report. J Med Case Rep 11: 319, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bolinder J, Antuna R, Geelhoed-duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomized controlled trial. Lancet 388: 2254-2263, 2016. [DOI] [PubMed] [Google Scholar]
- 5. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Ratman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes 8: 55-73, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moses SW. Historical highlights and unsolved problems in glycogen storage disease type 1. Eur J Pediatr 161: S2-S9, 2002. [DOI] [PubMed] [Google Scholar]
- 7. Rajas F, Labrune P, Mithieux G. Glycogen storage disease type 1 and diabetes: learning by comparing and contrasting the two disorders. Diabetes Metab 39: 377-387, 2013. [DOI] [PubMed] [Google Scholar]
- 8. Katayama D, Baba H, Kuwabara T, et al. SGLT2 inhibition alleviated hyperglycemia, glucose intolerance, and dumping syndrome-like symptoms in a patient with glycogen storage disease type 1a: a case report. J Med Case Rep 16: 75, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lockwood DH, Merimee TJ, Edgar PJ, et al. Insulin secretion in type 1 glycogen storage disease. Diabetes 18: 755-758, 1969. [DOI] [PubMed] [Google Scholar]
- 10. Yuan X, Ma W, Wu X, et al. Successful treatment of diabetes associated with glycogen storage disease type 1a. Diabet Med 38: e14373, 2021. [DOI] [PubMed] [Google Scholar]
- 11. Degn KB, Brock B, Juhl CB, et al. Effect of intravenous infusion of exenatide (synthetic exendin-4) on glucose-dependent insulin secretion and counter regulation during hypoglycemia. Diabetes 53: 2397-2403, 2004. [DOI] [PubMed] [Google Scholar]
- 12. Kim YM, Choi JH, Lee BH, Kim GH, Kim KM, Yoo HW. Predominance of the c.648G > T G6PC gene mutation and late complications in Korean patients with glycogen storage disease type 1a. Orphanet J Rare Dis 15: 45, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Melis D, Rossi A, Pivonello R, et al. Glycogen storage disease type 1a (GSD1a) but not Glycogen storage disease type 1b (GSD1b) is associated to an increased risk of metabolic syndrome: possible role of microsomal glucose 6-phosphate accumulation. Orphanet J Rare Dis 10: 91, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]