Abstract
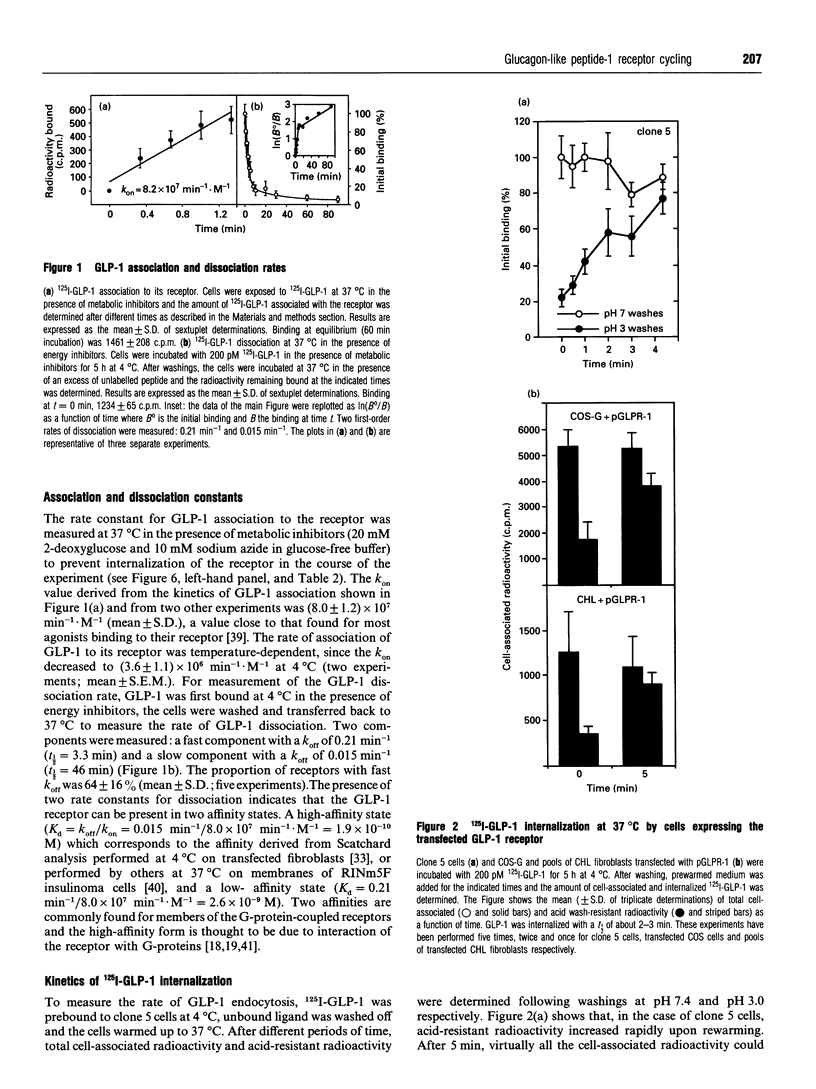
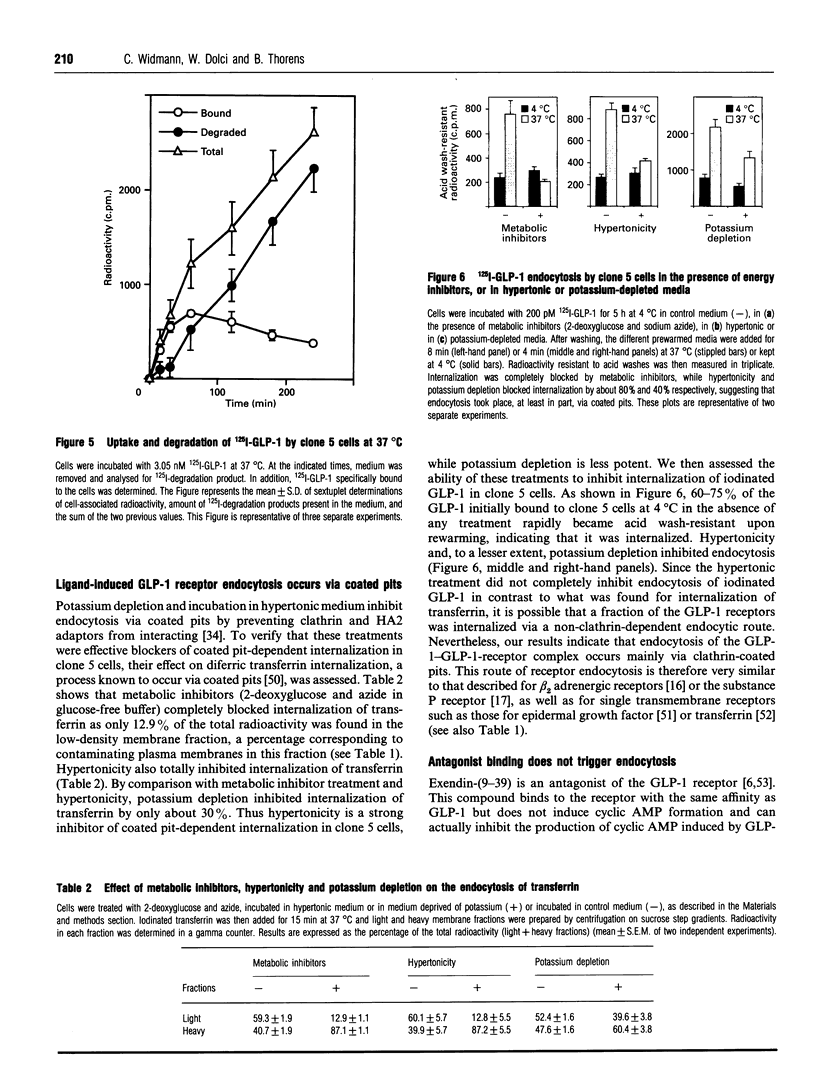
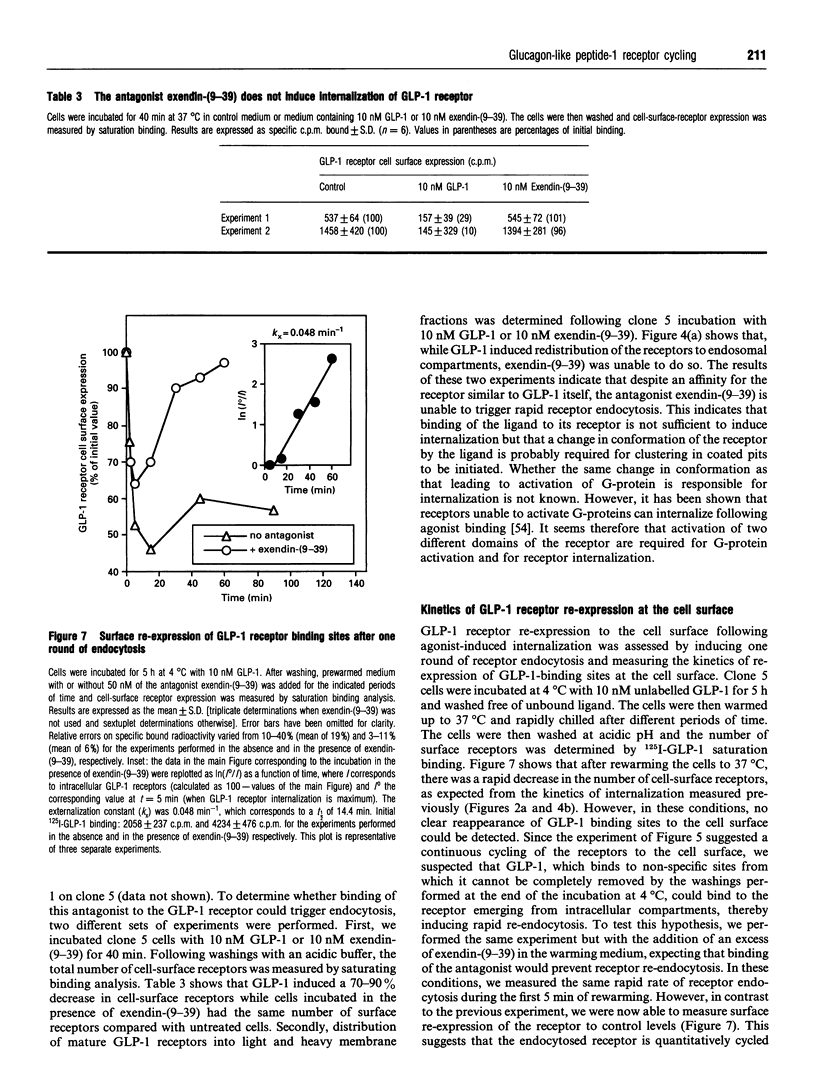
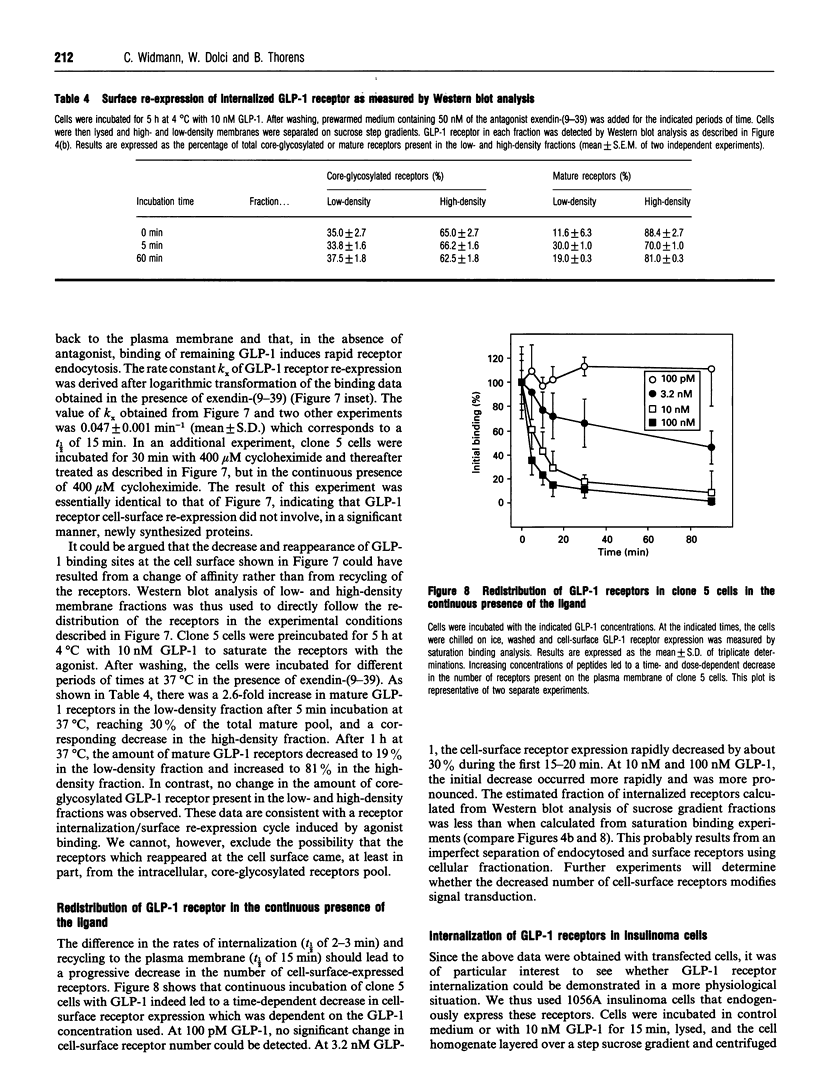
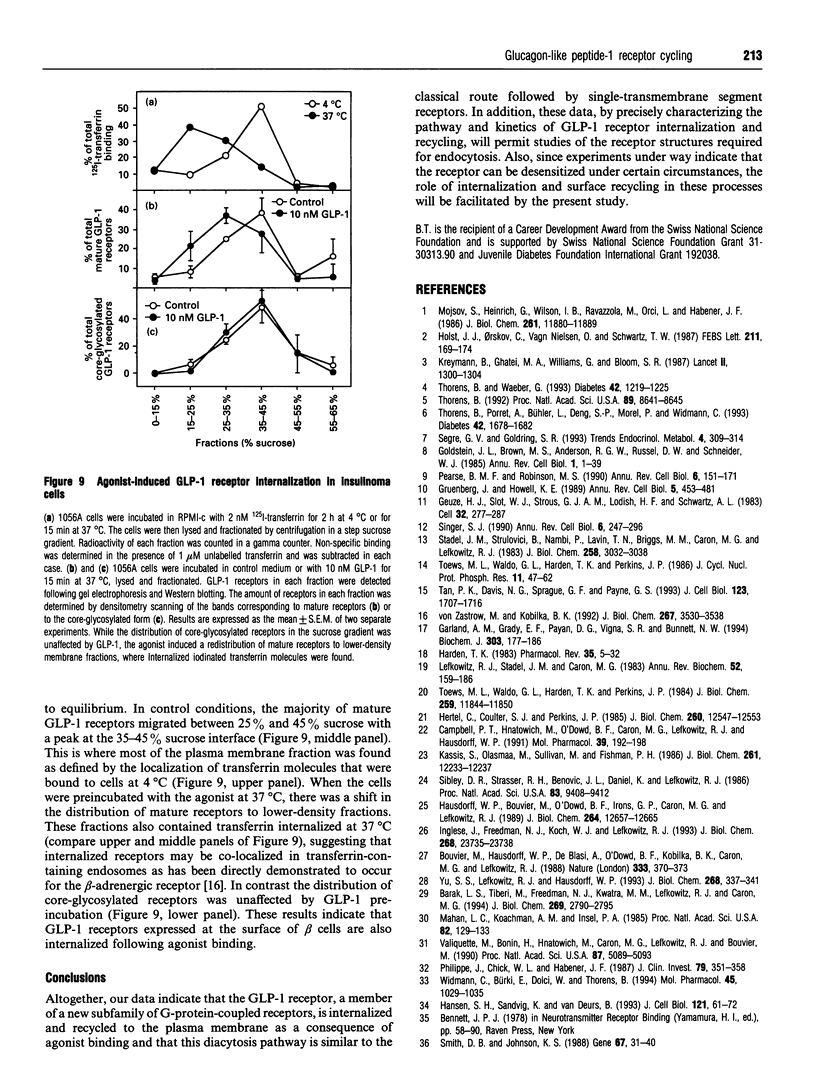
Glucagon-like peptide-1 (GLP-1) is the most potent stimulator of glucose-induced insulin secretion and its pancreatic beta-cell receptor is a member of a new subfamily of G-protein-coupled receptors which includes the receptors for vasoactive intestinal polypeptide, secretin and glucagon. Here we studied agonist-induced GLP-1 receptor internalization in receptor-transfected Chinese hamster lung fibroblasts using three different approaches. First, iodinated GLP-1 bound at 4 degrees C to transfected cells was internalized with a t 1/2 of 2-3 min following warming up of the cells to 37 degrees C. Secondly, exposure to GLP-1 induced a shift in the distribution of the receptors from plasma membrane-enriched to endosomes-enriched membrane fractions, as assessed by Western blot detection of the receptors using specific antibodies. Thirdly, continuous exposure of GLP-1 receptor-expressing cells to iodinated GLP-1 led to a linear accumulation of peptide degradation products in the medium following a lag time of 20-30 min, indicating a continuous cycling of the receptor between the plasma membrane and endosomal compartments. Potassium depletion and hypertonicity inhibited transferrin endocytosis, a process known to occur via coated pit formation, as well as GLP-1 receptor endocytosis. In contrast to GLP-1, the antagonist exendin-(9-39) did not lead to receptor endocytosis. Surface re-expression following one round of GLP-1 receptor endocytosis occurred with a half-time of about 15 min. The difference in internalization and surface re-expression rates led to a progressive redistribution of the receptor in intracellular compartments upon continuous exposure to GLP-1. Finally, endogenous GLP-1 receptors expressed by insulinoma cells were also found to be internalized upon agonist binding. Together our data demonstrate that the GLP-1 receptor is internalized upon agonist binding by a route similar to that taken by single transmembrane segment receptors. The characterization of the pathway and kinetics of GLP-1-induced receptor endocytosis will be helpful towards understanding the role of internalization and recycling in the control of signal transduction by this receptor.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barak L. S., Tiberi M., Freedman N. J., Kwatra M. M., Lefkowitz R. J., Caron M. G. A highly conserved tyrosine residue in G protein-coupled receptors is required for agonist-mediated beta 2-adrenergic receptor sequestration. J Biol Chem. 1994 Jan 28;269(4):2790–2795. [PubMed] [Google Scholar]
- Bleil J. D., Bretscher M. S. Transferrin receptor and its recycling in HeLa cells. EMBO J. 1982;1(3):351–355. doi: 10.1002/j.1460-2075.1982.tb01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier M., Hausdorff W. P., De Blasi A., O'Dowd B. F., Kobilka B. K., Caron M. G., Lefkowitz R. J. Removal of phosphorylation sites from the beta 2-adrenergic receptor delays onset of agonist-promoted desensitization. Nature. 1988 May 26;333(6171):370–373. doi: 10.1038/333370a0. [DOI] [PubMed] [Google Scholar]
- Campbell P. T., Hnatowich M., O'Dowd B. F., Caron M. G., Lefkowitz R. J., Hausdorff W. P. Mutations of the human beta 2-adrenergic receptor that impair coupling to Gs interfere with receptor down-regulation but not sequestration. Mol Pharmacol. 1991 Feb;39(2):192–198. [PubMed] [Google Scholar]
- Cheung A. H., Dixon R. A., Hill W. S., Sigal I. S., Strader C. D. Separation of the structural requirements for agonist-promoted activation and sequestration of the beta-adrenergic receptor. Mol Pharmacol. 1990 Jun;37(6):775–779. [PubMed] [Google Scholar]
- Dautry-Varsat A., Ciechanover A., Lodish H. F. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder S., Miller K., Moehren G., Ullrich A., Schlessinger J., Hopkins C. R. Kinase activity controls the sorting of the epidermal growth factor receptor within the multivesicular body. Cell. 1990 May 18;61(4):623–634. doi: 10.1016/0092-8674(90)90474-s. [DOI] [PubMed] [Google Scholar]
- Garland A. M., Grady E. F., Payan D. G., Vigna S. R., Bunnett N. W. Agonist-induced internalization of the substance P (NK1) receptor expressed in epithelial cells. Biochem J. 1994 Oct 1;303(Pt 1):177–186. doi: 10.1042/bj3030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Lodish H. F., Schwartz A. L. Intracellular site of asialoglycoprotein receptor-ligand uncoupling: double-label immunoelectron microscopy during receptor-mediated endocytosis. Cell. 1983 Jan;32(1):277–287. doi: 10.1016/0092-8674(83)90518-4. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S., Anderson R. G., Russell D. W., Schneider W. J. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- Gruenberg J., Howell K. E. Membrane traffic in endocytosis: insights from cell-free assays. Annu Rev Cell Biol. 1989;5:453–481. doi: 10.1146/annurev.cb.05.110189.002321. [DOI] [PubMed] [Google Scholar]
- Göke R., Fehmann H. C., Linn T., Schmidt H., Krause M., Eng J., Göke B. Exendin-4 is a high potency agonist and truncated exendin-(9-39)-amide an antagonist at the glucagon-like peptide 1-(7-36)-amide receptor of insulin-secreting beta-cells. J Biol Chem. 1993 Sep 15;268(26):19650–19655. [PubMed] [Google Scholar]
- Göke R., Trautmann M. E., Haus E., Richter G., Fehmann H. C., Arnold R., Göke B. Signal transmission after GLP-1(7-36)amide binding in RINm5F cells. Am J Physiol. 1989 Sep;257(3 Pt 1):G397–G401. doi: 10.1152/ajpgi.1989.257.3.G397. [DOI] [PubMed] [Google Scholar]
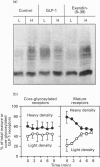
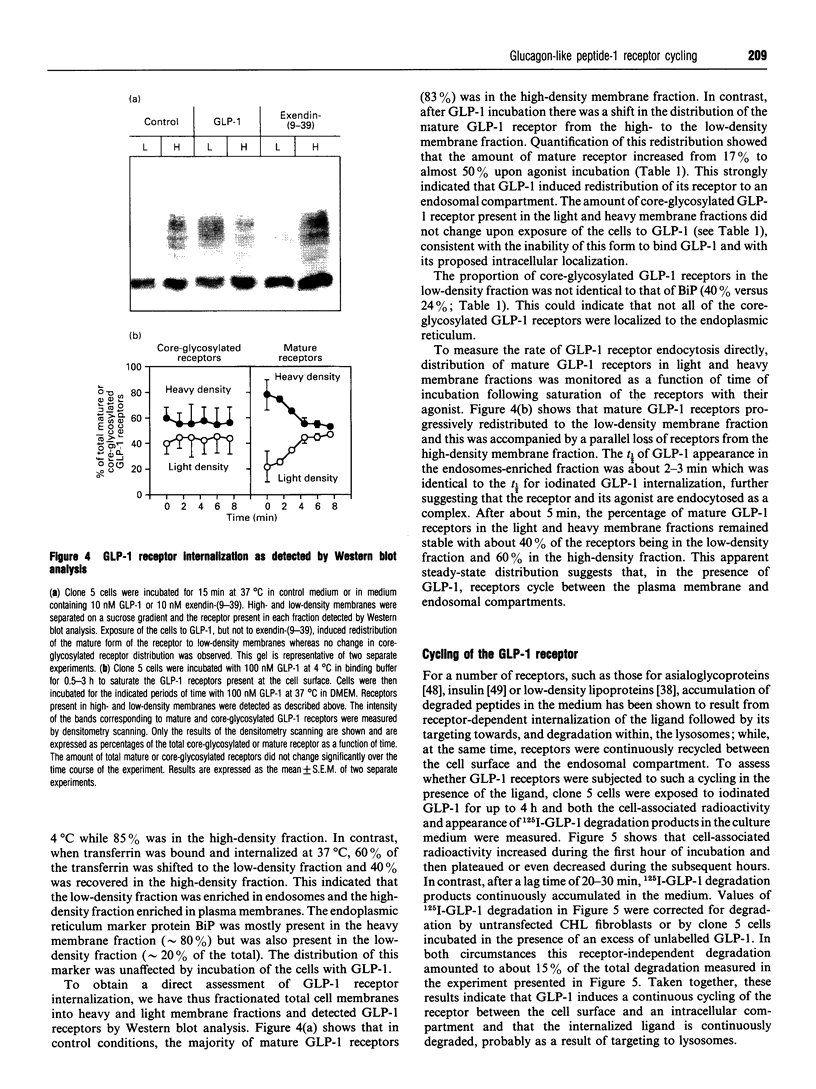
- Haas I. G. BiP (GRP78), an essential hsp70 resident protein in the endoplasmic reticulum. Experientia. 1994 Nov 30;50(11-12):1012–1020. doi: 10.1007/BF01923455. [DOI] [PubMed] [Google Scholar]
- Hansen S. H., Sandvig K., van Deurs B. Clathrin and HA2 adaptors: effects of potassium depletion, hypertonic medium, and cytosol acidification. J Cell Biol. 1993 Apr;121(1):61–72. doi: 10.1083/jcb.121.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden T. K. Agonist-induced desensitization of the beta-adrenergic receptor-linked adenylate cyclase. Pharmacol Rev. 1983 Mar;35(1):5–32. [PubMed] [Google Scholar]
- Hausdorff W. P., Bouvier M., O'Dowd B. F., Irons G. P., Caron M. G., Lefkowitz R. J. Phosphorylation sites on two domains of the beta 2-adrenergic receptor are involved in distinct pathways of receptor desensitization. J Biol Chem. 1989 Jul 25;264(21):12657–12665. [PubMed] [Google Scholar]
- Hertel C., Coulter S. J., Perkins J. P. A comparison of catecholamine-induced internalization of beta-adrenergic receptors and receptor-mediated endocytosis of epidermal growth factor in human astrocytoma cells. Inhibition by phenylarsine oxide. J Biol Chem. 1985 Oct 15;260(23):12547–12553. [PubMed] [Google Scholar]
- Holst J. J., Orskov C., Nielsen O. V., Schwartz T. W. Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett. 1987 Jan 26;211(2):169–174. doi: 10.1016/0014-5793(87)81430-8. [DOI] [PubMed] [Google Scholar]
- Hopkins C. R., Trowbridge I. S. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A431 cells. J Cell Biol. 1983 Aug;97(2):508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglese J., Freedman N. J., Koch W. J., Lefkowitz R. J. Structure and mechanism of the G protein-coupled receptor kinases. J Biol Chem. 1993 Nov 15;268(32):23735–23738. [PubMed] [Google Scholar]
- Jacobson K., Ishihara A., Inman R. Lateral diffusion of proteins in membranes. Annu Rev Physiol. 1987;49:163–175. doi: 10.1146/annurev.ph.49.030187.001115. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kreymann B., Williams G., Ghatei M. A., Bloom S. R. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987 Dec 5;2(8571):1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- Kurz J. B., Perkins J. P. Isoproterenol-initiated beta-adrenergic receptor diacytosis in cultured cells. Mol Pharmacol. 1992 Feb;41(2):375–381. [PubMed] [Google Scholar]
- Lefkowitz R. J., Stadel J. M., Caron M. G. Adenylate cyclase-coupled beta-adrenergic receptors: structure and mechanisms of activation and desensitization. Annu Rev Biochem. 1983;52:159–186. doi: 10.1146/annurev.bi.52.070183.001111. [DOI] [PubMed] [Google Scholar]
- Mahan L. C., Koachman A. M., Insel P. A. Genetic analysis of beta-adrenergic receptor internalization and down-regulation. Proc Natl Acad Sci U S A. 1985 Jan;82(1):129–133. doi: 10.1073/pnas.82.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall S., Green A., Olefsky J. M. Evidence for recycling of insulin receptors in isolated rat adipocytes. J Biol Chem. 1981 Nov 25;256(22):11464–11470. [PubMed] [Google Scholar]
- Mojsov S., Heinrich G., Wilson I. B., Ravazzola M., Orci L., Habener J. F. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem. 1986 Sep 5;261(25):11880–11889. [PubMed] [Google Scholar]
- Pearse B. M., Robinson M. S. Clathrin, adaptors, and sorting. Annu Rev Cell Biol. 1990;6:151–171. doi: 10.1146/annurev.cb.06.110190.001055. [DOI] [PubMed] [Google Scholar]
- Philippe J., Chick W. L., Habener J. F. Multipotential phenotypic expression of genes encoding peptide hormones in rat insulinoma cell lines. J Clin Invest. 1987 Feb;79(2):351–358. doi: 10.1172/JCI112819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A. L., Fridovich S. E., Lodish H. F. Kinetics of internalization and recycling of the asialoglycoprotein receptor in a hepatoma cell line. J Biol Chem. 1982 Apr 25;257(8):4230–4237. [PubMed] [Google Scholar]
- Sibley D. R., Strasser R. H., Benovic J. L., Daniel K., Lefkowitz R. J. Phosphorylation/dephosphorylation of the beta-adrenergic receptor regulates its functional coupling to adenylate cyclase and subcellular distribution. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9408–9412. doi: 10.1073/pnas.83.24.9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J. The structure and insertion of integral proteins in membranes. Annu Rev Cell Biol. 1990;6:247–296. doi: 10.1146/annurev.cb.06.110190.001335. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Stadel J. M., Strulovici B., Nambi P., Lavin T. N., Briggs M. M., Caron M. G., Lefkowitz R. J. Desensitization of the beta-adrenergic receptor of frog erythrocytes. Recovery and characterization of the down-regulated receptors in sequestered vesicles. J Biol Chem. 1983 Mar 10;258(5):3032–3038. [PubMed] [Google Scholar]
- Tan P. K., Davis N. G., Sprague G. F., Payne G. S. Clathrin facilitates the internalization of seven transmembrane segment receptors for mating pheromones in yeast. J Cell Biol. 1993 Dec;123(6 Pt 2):1707–1716. doi: 10.1083/jcb.123.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8641–8645. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B., Porret A., Bühler L., Deng S. P., Morel P., Widmann C. Cloning and functional expression of the human islet GLP-1 receptor. Demonstration that exendin-4 is an agonist and exendin-(9-39) an antagonist of the receptor. Diabetes. 1993 Nov;42(11):1678–1682. doi: 10.2337/diab.42.11.1678. [DOI] [PubMed] [Google Scholar]
- Thorens B., Waeber G. Glucagon-like peptide-I and the control of insulin secretion in the normal state and in NIDDM. Diabetes. 1993 Sep;42(9):1219–1225. doi: 10.2337/diab.42.9.1219. [DOI] [PubMed] [Google Scholar]
- Toews M. L., Waldo G. L., Harden T. K., Perkins J. P. Comparison of binding of 125I-iodopindolol to control and desensitized cells at 37 degrees and on ice. J Cyclic Nucleotide Protein Phosphor Res. 1986;11(1):47–62. [PubMed] [Google Scholar]
- Toews M. L., Waldo G. L., Harden T. K., Perkins J. P. Relationship between an altered membrane form and a low affinity form of the beta-adrenergic receptor occurring during catecholamine-induced desensitization. Evidence for receptor internalization. J Biol Chem. 1984 Oct 10;259(19):11844–11850. [PubMed] [Google Scholar]
- Valiquette M., Bonin H., Hnatowich M., Caron M. G., Lefkowitz R. J., Bouvier M. Involvement of tyrosine residues located in the carboxyl tail of the human beta 2-adrenergic receptor in agonist-induced down-regulation of the receptor. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5089–5093. doi: 10.1073/pnas.87.13.5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo G. L., Northup J. K., Perkins J. P., Harden T. K. Characterization of an altered membrane form of the beta-adrenergic receptor produced during agonist-induced desensitization. J Biol Chem. 1983 Nov 25;258(22):13900–13908. [PubMed] [Google Scholar]
- Widmann C., Bürki E., Dolci W., Thorens B. Signal transduction by the cloned glucagon-like peptide-1 receptor: comparison with signaling by the endogenous receptors of beta cell lines. Mol Pharmacol. 1994 May;45(5):1029–1035. [PubMed] [Google Scholar]
- Yu S. S., Lefkowitz R. J., Hausdorff W. P. Beta-adrenergic receptor sequestration. A potential mechanism of receptor resensitization. J Biol Chem. 1993 Jan 5;268(1):337–341. [PubMed] [Google Scholar]
- von Zastrow M., Kobilka B. K. Ligand-regulated internalization and recycling of human beta 2-adrenergic receptors between the plasma membrane and endosomes containing transferrin receptors. J Biol Chem. 1992 Feb 15;267(5):3530–3538. [PubMed] [Google Scholar]