RESUME
Introduction: Il n'existe pas de consensus clair sur ce qui constitue un déficit ventilatoire obstructif (DVO) dans des populations pédiatriques. Objectif: Déterminer le pourcentage d'enfants/adolescents présentant un DVO parmi ceux adressés pour spirométrie après avoir pris en compte les définitions avancées par certaines sociétés savantes internationales. [British Columbia (BC), British thoracic-society (BTS), Canadian thoracic society (CTS), European respiratory society et American thoracic society (ERS-ATS), global initiative for asthma (GINA), Irish college of general practitioners (ICGP), national asthma council (NAC), national institute of clinical excellence (NICE), société de pneumologie de langue française et de la société pédiatrique de pneumologie et allergologie (SPLF-SP2A), et South African thoracic society (SATS)]. Méthodes: Cette étude transversale impliquera deux structures médicales à Sousse/Tunisie, et inclura des enfants/adolescents âgés de 6 à 18 ans. Un questionnaire médical sera utilisé, des données anthropométriques et cliniques seront collectées et les données spirométriques seront mesurées par deux spiromètres. Les six définitions suivantes de DVO seront appliquées: i) GINA: volume expiratoire maximal en une seconde (VEMS) < 80 % et VEMS/capacité vitale forcée (CVF) ≤ 0,90; ii) ICGP: VEMS/CVF < 0,70; iii) ERS-ATS ou BTS ou SATS ou SPLF-SP2A ou NAC: z-score du VEMS/CVF < -1,645; iv) NICE: VEMS/CVF < 0,70 ou z- score du VEMS/CVF < -1.645; v) CTS: VEMS/CVF < 0,80 ou z- score du VEMS/CVF < -1,645; et vi) ERS: "z- score du VEMS ou VEMS/CVF" < -1,645 ou "VEMS ou VEMS/CVF" < 0,80. Résultats attendus: Le pourcentage d'enfants/adolescents présentant un DVO variera significativement entre les six définitions. Conclusion: La fréquence du DVO dans cette population dépendra de la définition choisie.
ABSTRACT
Introduction: There is no clear consensus as to what constitutes an obstructive ventilatory impairment (OVI) in pediatric populations. Aim: To determine the percentage of children/adolescents having an OVI among those addressed for spirometry after taking into account the definitions advanced by some international scholarly societies [British Columbia (BC), British thoracic-society (BTS), Canadian thoracic society (CTS), European respiratory society and American thoracic society (ERS-ATS), global initiative for asthma (GINA), Irish college of general practitioners (ICGP), national asthma council (NAC), national institute of clinical excellence (NICE), Société de pneumologie de langue française et de la société pédiatrique de pneumologie et allergologie (SPLF-SP2A), and South African thoracic society (SATS)]. Methods: This bi-centric cross-sectional study will involve two medical structures in Sousse/Tunisia, and will encompass children/adolescents aged 6-18 years. A medical questionnaire will be administered, clinical and anthropometric data will be collected, and the spirometric data will be measured by two spirometers. The following six definitions of OVI will be applied: i) GINA: Forced expiratory volume in 1 second (FEV1 ) < 80% and a FEV1 /forced vital capacity (FVC) ≤ 0.90; ii) ICGP: FEV1 /FVC < 0.70; iii) ERS-ATS or BTS or SATS or SPLF-SP2A or NAC: FEV1 /FVC z-score < -1.645; iv) NICE: FEV1 /FVC < 0.70 or FEV1 /FVC z-score < -1.645; v) CTS: FEV1 /FVC < 0.80 or FEV1 /FVC z-score < -1.645; and vi) ERS: “FEV1 z-score or FEV1 /FVC z-score” < -1.645 or “FEV1 or FEV1 /FVC” < 0.80. Expected results: The percentage of children/adolescents having an OVI will significantly vary between the six definitions. Conclusion: The frequency of OVI in a pediatric population will depend on the definition chosen.
Introduction
Asthma is a very common disease in respiratory affections especially among pediatric population (1).
Until April 2024, asthma is a burden for both the patients by its different symptoms such as dyspnea, cough, and the government by its charges (1).
According to the global initiative for asthma (GINA) (2), confirmation of asthma diagnosis is important, because in 12-50% of people assumed to have severe asthma, it is not found to be the correct diagnosis (3).
The spirometry is very useful for the diagnosis, the control and the treatment of asthma (4).
In practice, performing spirometry, before and after bronchodilator, is needed to assess baseline lung function and seek objective evidence of obstructive ventilatory impairment (OVI) (2).
The OVI in pediatric patients is a global concern affecting both low-middle and high-income countries (5).
A major challenge in this context is the lack of consensus regarding its diagnostic criteria, which makes the interpretation of spirometry results a complex task (6).
What constitutes an OVI in pediatric populations? At least six different international definitions exist (2, 7-16).
First, according to the GINA (2), it is an association between a low forced expiratory volume in 1 second (FEV1) and a FEV1/forced vital capacity (FVC) ratio ≤ 0.90.
Second, according to the Irish college of general practitioners (ICGP) (7), it is a FEV1/FVC < 0.70.
Third, according to the European respiratory society and American thoracic society (ERS-ATS) (8), the South African thoracic society (SATS) (9), the French language pulmonology society and the pediatric society of pulmonology and allergology (SPLF-SP2A) (10), the British thoracic society (BTS) (11), and the national asthma council (NAC) (12), it is a FEV1 / FVC z-score < -1.645.
Fourth, according to the national institute of clinical excellence (NICE) (13), it is a FEV1 / FVC < 0.70 or a FEV1 / FVC z-score < -1.645.
Fifth, according to the British Columbia (BC) (14), and the Canadian thoracic society (CTS) (15), it is a FEV1/FVC < 0.80 or a FEV1/FVC z-score < -1.645.
Finally, according to the ERS (16), it is a “" FEV1 z-score or FEV 1/FVC z-score” < -1.645 or “" FEV 1 or FEV 1 /FVC” < 0.80.
When examining the aforementioned definitions, it appears that three approaches were used for diagnosing an OVI in pediatric patients.
The first approach (ie; operational approach) relies on specific fixed cutoff values as proposed by the GINA (2), and ICGP (7).
The second approach (ie; physiological approach) is based on z-scores, as advocated by the ERS-ATS (8), SATS (9), SPLF-SP2A (10), BTS (11), and NAC (12 ).
The third approach (ie; physiological and/or operational approaches) is based on both aforementioned methods as advocated by the NICE(13), CTS(15), BC( 14), and ERS (16).
Therefore, a clear consensus on what constitutes an OVI is notably absent, and there is no definitive answer to the following two questions: i) Which spirometric parameter(s) should be used to validate the diagnosis of an OVI? Is it “" FEV1/FVC” (7,8-12,14,15) or “" FEV1 and FEV1/FVC” (2), or “" FEV1 or FEV1/FVC”?’ (16); and ii) Which lower limit of normal (LLN) should be applied? Is it a LLN presented as fixed thresholds of 0.70 (7, 13), 0.80 (14-16) or 0.90 (2) (ie; operational approach), or derived from z-scores (ie; physiological approach) (8 -16)?
The lack of a clear consensus on what constitutes an OVI leads to varying interpretations of spirometry results in pediatric populations.
A consequential issue emanating from this disparity is the potential for patients to be diagnosed with OVI by one set of criteria while being considered free from OVI by another, introducing ambiguity into clinical practice.
The main goal of this study design is to ascertain the frequency of OVI in pediatric population, analyzing the diagnostic criteria utilized by different scholarly societies.
A secondary objective involves describing the clinical and functional characteristics specific to each pediatric population, highlighting the consequences of employing distinct diagnostic criteria.
This approach aims to provide insights into the varied aspects of OVI in pediatric patients and the impact of different diagnostic standards on clinical and functional attributes.
Population and methods
Study design
This real life bicentric cross-sectional investigation is set to be conducted at two distinct public and private medical structures in Sousse (Tunisia) (ie; laboratory of physiology and functional explorations at the Farhat HACHED Hospital, and international center for functional explorations).
The study is scheduled to start in February 2024.
Approval for this research has been secured from the hospital's medical and research ethics committee at Farhat Hached's hospital (Approval No: 0212/2023).
A bilingual consent and information document, presented in Arabic and French, will be provided to participants and their parents or legal guardians.
Participants will be asked to give their oral consent and parents or legal guardian will be asked to sign the written consent.
Sample size
The sample size was calculated according to this predictive equation (17, 18): N = (Zα/2 2x p (1-p))/Δ2, where
• “" N” was the number of needed children/adolescents;
• “"Zα/2” was the normal deviate for type 1 error (Zα/2 =3.29 for 0.1% level of significance);
• “"p” was the frequency of OVI in children/adolescentsaddressed for spirometry in a given medical structure;and
• “"Δ” was the accuracy (= 6%).
According to Ndukwu et al. (19), 27.6% (p=0.276) of 145 Nigerian children aged 6 to 12 years who performed a spirometry had an OVI diagnosed in front of a z-score FEV1/FVC < -1.645, with z-score FVC > -1.645.
The sample size is therefore 601 consecutive children/adolescents.
The assumption of 50% for inclusion and exclusion criteria (20) gave a revised sample of 1202 children/adolescents [=601/(1.0–0.50)].
Population
The study will involve a single visit, scheduled in the morning between 8 a.m. and 15 p.m.
The population source for this research comprises children/adolescents aged 6 to 18 years exhibiting respiratory clinical manifestations.
The target population consists of children/adolescents referred by physicians (eg; paediatricians, pulmonologists, general practitioners) to undergo a spirometry test during the study period.
Inclusion criteria will incorporate paediatric patients who will be referred to the specified above cited two medical structures due to clinical symptoms indicative of respiratory diseases including dyspnoea, cough or for routine monitoring of their respiratory conditions such as asthma and/or allergy.
The following non-inclusion criteria will be applied: respiratory acute exacerbation, short acting β2-agonist or anticholinergic agents use before four hours of testing, long β2-agonists use before 24 hours of testing (21), other nationalities besides Tunisian or Algerian, and active smokers who smoked daily at the time of the survey (22).
Unacceptable and/or non-reproducible spirometric tests (23), and a history of the upper respiratory tract infection during the three weeks prior to investigation will be also applied as non-inclusion criteria.
Applied protocol
From the presentation of the parents or legal guardians of the child/adolescent to the aforementioned two medicals structures, the following explorations will be conducted in this order: medical questionnaire, anthropometric data, spirometry, and possibly other tests such as bronchodilation test, skin tests taking into account the exams reported in the referral drafted by the attending physician
Applied questionnaire
A standardized medical questionnaire, previously applied in local studies (24-26), will be administered for clinical assessment and detailed medical history.
The questions will be presented in Arabic, the native language, to facilitate effective communication and understanding.
The questionnaire encompasses various items:
i) Socioeconomic and demographic data: schooling level (eg; primary, secondary, high school, unschooled), nationality (eg; Tunisian, Algerian, other), exposure to humidity, pollution, plants, or animals (yes, no); ii) Indications of respiratory functional explorations (if reported in the referral letter) such as asthma diagnosis or control, allergy, dyspnea, chronic cough, rhinitis, repeated bronchitis, and chest tightness; iii) Family history of atopy (yes, no); iv) Neonatal history: Birth weight (in kg), term of birth (eg; premature, full term), delivery mode (eg; natural childbirth, caesarian section);
v) Personal data: History of passive smoking (defined as regular exposure to tobacco smoke from external sources, such as parents or caregivers who smoke in close proximity to the patient (27)), rhinitis or conjunctivitis, asthma, other respiratory diseases, or non-respiratory diseases such as ear, nose, and throat conditions;
vi) Asthma data (if applicable): duration and medical treatments;
vii) Respiratory symptoms like cough, phlegm, dyspnea, signs of allergic rhinitis such as runny nose, pruritus, and sternutation;
viii) Dyspnea level (if applicable) using the five levels of the modified medical research council dyspnea scale [0(breathless with strenuous exercise); 1 (short of breath when hurrying on the level or walking up a slight hill); 2(walking slower than people of the same age on the level because of breathlessness or stopping for breath when walking at own pace on the level); 3 (stopping for breath after walking about 100 yards or after a few minutes on the level); and 4 (too breathless to leave the house or breathless when dressing)] (28);
ix) Practice of skin tests (yes, no) and resulting outcomes(if applicable) expressed as negative or positive reactions( 29); and x) Current respiratory treatments other than asthma treatments.
Respiratory exacerbation will be defined as emergency room visits, hospitalizations or unscheduled doctor visits for events requiring the use of systemic corticosteroids or an increase from a stable maintenance dose for at least three days prior to the spirometry (24 ).
Sex, age and anthropometric data
The patient's sex (boy, girl), will be recorded.
Decimal age will be taken as the number of complete years from birth to the date of the study, and patients will be divided into five 2-year age groups.
Standing height (cm) and weight (kg) will be determined.
Height will be measured without shoes, with the patient standing as tall as possible against a stadiometer (Detecto scale Webb city, Missouri, USA) or an ultrasonic stadiometer in the private medical structure.
Body mass index (BMI, kg/m2) will be calculated.
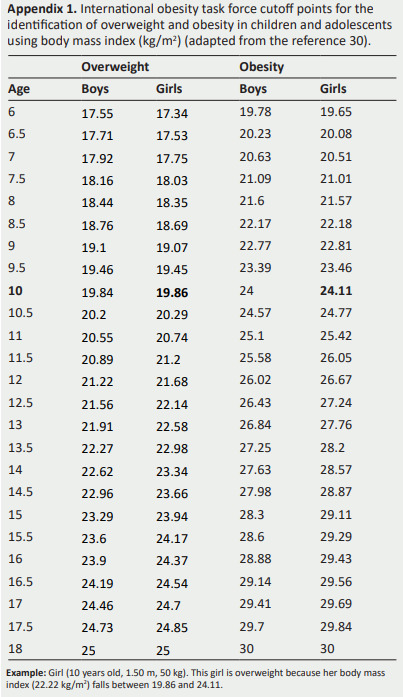
Normal weight, overweight and obesity will be defined using growth curves’ references reaching values of 25 kg/m2 for overweight and 30 kg/m2 for obesity (30).
The international obesity task force cutoff points for theidentification of overweight and obesity will be applied(Appendix 1) (30)
Appendix 1. International obesity task force cutoff points for the identification of overweight and obesity in children and adolescents using body mass index (kg/m2 ) (adapted from the reference 30).
Spirometry measurements
In each of the aforementioned medical structures, all spirometric tests will be performed by qualified persons, according to the most update international recommendations (23).
In public and private medical structures, the used types of spirometers will be Medisoft (Sorinnes, Belgium), and MicroLab (CareFusion Basingstoke, UK), respectively.
In the above-cited medical structures, the turbine calibration will be daily performed using a 3-l syringe.
Testing will take place in a calm and comfortable environment, separate from the waiting area and other patients undergoing testing.
The patient will sit upright, with shoulders slightly back and chin slightly elevated.
During the respiratory maneuvers, a nose clip will be used.
The spirometric technique was previously described (24, 25).
The FVC maneuver had three distinct phases: Maximal inspiration, a blast of exhalation, and continued complete exhalation to the end of testing.
Patients will be verbally encouraged to continue exhaling air at the end of the maneuver to obtain optimal effort.
Precise spirometric evaluations will rely on rigorous acceptability and repeatability criteria (24, 25).
For acceptability, patients must demonstrate no cough during the first second of expiration and no glottic closure during this critical period (23).
Post-1 second, glottic closure should be entirely absent (23).
End-of-test criteria (EOFE) [eg; expiratory plateau (< 25 mL in the last second of expiration), expiratory time surpassing 15 seconds, FVC within repeatability tolerance] will be verified (23 ).
Only acceptable respiratory maneuvers free from faulty zero-flow settings, obstructed mouthpieces or spirometers, or leaks post-maximal inspiration following EOFE will be accepted (23).
If maximal inspiration postEOFE exceeds FVC, then the difference between forced inspiratory vital capacity and FVC must be < 100 mL or 5% of FVC, whichever is greater (23).
A minimum of three reproducible FVC measurements will be obtained.
FVC and FEV1 of the best two of the three selected curves must varied less than 15 mL (23).
The best FVC and the best FEV1 will be computed, even if the two values did not come from the same curve (23).
The following two spirometric data will be determined: FVC (L), FEV1 (L).
FEV1/FVC ratio (absolute value) will be calculated.
Those data will be expressed as percentages of predicted normal values derived from the 2012 spirometric global lung initiative (GLI) norms (Caucasian group) which are applicable to Tunisian and Algerian pediatric populations (31, 32 ).
Z-scores for FVC, FEV1, and FEV1/FVC will be calculated, and any z-score falling below the LLN (ie; < -1.645) will be regarded as abnormal (33-35 ).
An Excel file processing functionality will be used.
The Excel file will include the following input values in this order: age (year), height (cm), sex (F for females, M for males), ethnicity (ie; 1 for Caucasian), FEV1(L), and FVC (L).
The Excel file will be upload on the calculator page (http://gli-calculator.ersnet.org/index.html).
The software will calculate the FEV1 /FVC ratio, and for each spirometric data several outcomes (ie; predicted value,LLN, and z-score) will be determined.2.4.4 OVI definitions
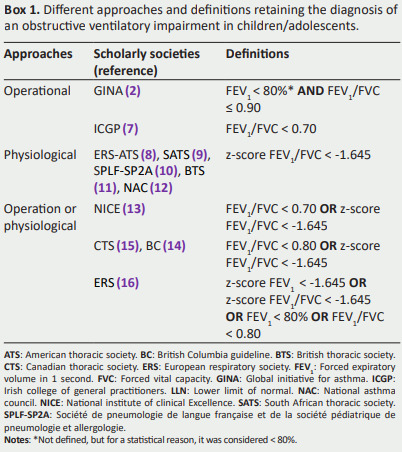
Box 1 provides a comprehensive overview of distinct diagnostic approaches and definitions endorsed by various international scholarly societies ( 2, 7- 16) for identifying OVI in children/adolescents.
It is capital to highlight that the GINA (2), designates FEV1 as ""low" without a specified threshold.
For a statistical reason, in the GINA definition, a “"low” FEV1 will be considered lower than 80%.
Box 1. Different approaches and definitions retaining the diagnosis of an obstructive ventilatory impairment in children/adolescents.
Statistical analysis
The Kolmogorov-Smirnov test will be used to analyze the variables distribution.
If the variables’ distribution will be normal and variances will be equal, quantitative data will be expressed by their means ± standard deviation and 95% confidence interval.
Alternatively, when normality assumptions will be violated or variances will differ, medians (interquartile) will be used for result expression.
Categorical data will be expressed as number (%).
The Cochrane test will be used to compare the percentages of children/adolescents with an OVI according to the six definitions.
When applicable, significant differences between the percentages will be tested using the McNemar test.
The two-tailed Chi-2 test will be used to compare the percentages of patients having an OVI [reference (ie, ERS- ATS (8)) vs. the remaining five definitions].
In order to compare the accuracy of the six definitions, we will perform two actions.
First, we will arbitrarily consider the ERS- ATS 2022 definition as a « standard»for the OVI diagnosis (8), and we will calculate the sensitivity, specificity, positive and negative predictive values (36) for the other five definitions by comparison to the results given by the “"standard” method.
Second, we will compare some important clinical data [eg; exposure to humidity, pollution, plants, or animals, some personal data like body mass (corpulence status), history of passive smoking, rhinitis or conjunctivitis, asthma, other respiratory or ear, nose, and throat diseases, presence of some respiratory symptoms like cough, phlegm, dyspnea, and skin tests outcomes] between the six definitions.
All mathematical computations and statistical procedures will be performed using Statistica statistical software(StatSoft, Inc. (2011). STATISTICA, version 10).
Significance was set at the 0.05 level.
Expected results
The diverse definitions and diagnostic criteria used by different scholarly societies contribute to the potential for patients to be labelled with an OVI by one set of standards and deemed normal by another.
This inherent variability in interpretation introduces complexity into clinical practice and underscores the urgent need for a standardized approach in diagnosing OVI in paediatric populations.
References
- Baker JA. 2022 year in review: Pediatric asthma. Respir Care. 2023;68(10):1430–1437. doi: 10.4187/respcare.10913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global strategy for asthma management and prevention, 2023
- Hashimoto S, Bel EH. Current treatment of severe asthma. Clin Exp Allergy. 2012;42(5):693–705. doi: 10.1111/j.1365-2222.2011.03936.x. [DOI] [PubMed] [Google Scholar]
- Tian C, Xiong S, Li S, Song X, Zhang Y, Jiang X. Spirometry in the diagnosis of cough variant asthma in children. Pediatr Pulmonol. 2024;59(2):291–299. doi: 10.1002/ppul.26745. [DOI] [PubMed] [Google Scholar]
- Mortimer K, Reddel HK, Pitrez PM, Bateman ED. Asthma management in low and middle income countries: case for change. Eur Respir J. 2022;60(3):2103179. doi: 10.1183/13993003.03179-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdesslem M, Ghannouchi I, Ben Saad H. The pressing need for standardised diagnostic criteria for obstructive ventilatory impairment in adults and children. Int J Tuberc Lung Dis. 2024;28(3):166–167. doi: 10.5588/ijtld.23.0510. [DOI] [PubMed] [Google Scholar]
- Asthma - diagnosis, assessment and management in general practice quick reference guide
- Stanojevic S, Kaminsky DA, Miller MR, Thompson B, Aliverti A, Barjaktarevic I. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J. 2022;60(1):2101499. doi: 10.1183/13993003.01499-2021. [DOI] [PubMed] [Google Scholar]
- Maree DM, Swanepoel RA, Swart F, Gray DM, Masekela R, Allwood BW. Position statement for adult and paediatric spirometry in South Africa: 2022 update. Afr J Thorac Crit Care Med. 2022;28(4):10.7196/AJTCCM.2022.v28i4.287. doi: 10.7196/AJTCCM.2022.v28i4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raherison-Semjen C, Guilleminault L, Billiart I, Chenivesse C, De Oliveira A, Izadifar A. Update of the 2021 recommendations for the management and follow-up of adult asthmatic patients under the guidance of the French society of pulmonology and the paediatric society of pulmonology and allergology. Long version. Rev Mal Respir. 2021;38(10):1048–1083. doi: 10.1016/j.rmr.2021.08.002. [DOI] [PubMed] [Google Scholar]
- British thoracic society. BTS/SIGN British guideline on the management of asthma. Link. 2024.
- National asthma council Australia Australian asthma handbook, version 2.0. Link. 2024.
- National institute for health and care excellence Asthma: diagnosis, monitoring and chronic asthma management. NICE guideline [NG80] Link. 2024.
- British Columbia. Asthma diagnosis, education and management.
- Yang CL, Hicks EA, Mitchell P, Reisman J, Podgers D, Hayward KM. Canadian thoracic society 2021 guideline update: Diagnosis and management of asthma in preschoolers, children and adults. Can J Respir Cri Care Sleep Med. 2021;5(6):348–361. [Google Scholar]
- Gaillard EA, Moeller A. Evidence-based European guidelines for the diagnosis of asthma in children aged 5-16 years. Lancet Respir Med. 2021;9(6):558–560. doi: 10.1016/S2213-2600(21)00183-1. [DOI] [PubMed] [Google Scholar]
- Serhier Z, Bendahhou K, Ben Abdelaziz A, Bennani MO. Methodological sheet n degrees 1: How to calculate the size of a sample for an observational study? Tunis Med. 2020;98(1):1–7. [PubMed] [Google Scholar]
- Methnani J, Latiri I, Dergaa I, Chamari K, Ben Saad H. ChatGPT for sample-size calculation in sports medicine and exercise sciences: A cautionary note. Int J Sports Physiol Perform. 2023;18(10):1219–123. doi: 10.1123/ijspp.2023-0109. [DOI] [PubMed] [Google Scholar]
- Ndukwu CI, Ozoh OB, Ale BM, Ayuk AC, Elo-Ilo JC, Awokola BI. Spirometry abnormalities and its associated factors among primary school children in a Nigerian city. Clin Med Insights Pediatr. 2021;15 doi: 10.1177/11795565211001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constant C, Sampaio I, Negreiro F, Aguiar P, Silva AM, Salgueiro M. Respiratory disease screening in school-aged children using portable spirometry. J Pediatr (Rio J) 2011;87(2):123–130. doi: 10.2223/JPED.2069. [DOI] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Khalil S, Ben Abdelaziz A, Zanina Y, Ben Yahia F, Khelil M, Zoghlami C. Epidemiology of smoking in the male population in Tunisia. HSHS Study 6. Tunis Med. 2022;100(10):683–695. [PMC free article] [PubMed] [Google Scholar]
- Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL. Standardization of spirometry 2019 update. An official American thoracic society and European respiratory society technical statement. Am J Respir Crit Care Med. 2019;200(8):e70–e88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guezguez F, Knaz H, Anane I, Bougrida M, Ben Saad H. The 'clinically significant' bronchodilator responsiveness (BDR) in children: a comparative study between six definitions of scholarly societies and a mini-review. Expert Rev Respir Med. 2021;15(6):823–832. doi: 10.1080/17476348.2021.1906653. [DOI] [PubMed] [Google Scholar]
- Bougrida M, Bourahli MK, Aissaoui A, Rouatbi S, Mehdioui H, Ben Saad H. Spirometric reference values for children living in Constantine (Eastern region of Algeria) Tunis Med. 2012;90(1):51–61. [PubMed] [Google Scholar]
- Ben Fraj S, Miladi A, Guezguez F, Ben Rejeb M, Bouguila J, Gargouri I. Does Ramadan fasting affect spirometric data of healthy adolescents? Clin Med Insights Pediatr. 2019;13:1179556519862280. doi: 10.1177/1179556519862280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolton K, Dietrich K, Auinger P, Lanphear BP, Hornung R. Exposure to environmental tobacco smoke and cognitive abilities among U.S. children and adolescents. Environ Health Perspect. 2005;113(1):98–103. doi: 10.1289/ehp.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2(5147):257–266. doi: 10.1136/bmj.2.5147.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes J, Borrego L, Romeira A, Pinto P. Skin prick tests and allergy diagnosis. Allergol Immunopathol (Madr) 2009;37(3):155–164. doi: 10.1016/S0301-0546(09)71728-8. [DOI] [PubMed] [Google Scholar]
- Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes. 2012;7(4):284–294. doi: 10.1111/j.2047-6310.2012.00064.x. [DOI] [PubMed] [Google Scholar]
- Ben Saad H. Review of the current use of global lung function initiative norms for spirometry (GLI-2012) and static lung volumes (GLI-2021) in Great Arab Maghreb (GAM) countries and steps required to improve their utilization. Libyan J Med. 2022;17(1):2031596. doi: 10.1080/19932820.2022.2031596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masekela R, Hall GL, Stanojevic S, Sartorius B, MacGinty R, Saad HB, et al An urgent need for African spirometry reference equations: the Paediatric and Adult African Spirometry study. Int J Tuberc Lung Dis. 2019;23(8):952–958. doi: 10.5588/ijtld.18.0442. [DOI] [PubMed] [Google Scholar]
- Ben Saad H. Interpretation of respiratory functional explorations of deficiency and incapacity in adult. Tunis Med. 2020;98(11):797–815. [PubMed] [Google Scholar]
- Ben Saad H. In 2023, it is vital to standardize the interpretation of spirometry in children. Pediatr Pulmonol. 2023;58(8):2187–2188. doi: 10.1002/ppul.26489. [DOI] [PubMed] [Google Scholar]
- Guezguez F, Ghannouchi I, Sayhi A, Charfedi E, Yahyaoui A, Rouatbi S, et al How to interpret parameters of routine lung function tests in 2023? Tunis Med. 2023;101(3):323–333. [PMC free article] [PubMed] [Google Scholar]
- Monaghan TF, Rahman SN, Agudelo CW, Wein AJ, Lazar JM, Everaert K, et al Foundational statistical principles in medical research: Sensitivity, specificity, positive predictive value, and negative predictive value. Medicina (Kaunas) 2021;57(5):503. doi: 10.3390/medicina57050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergaa I, Chamari K, Zmijewski P, Ben Saad H. From human writing to artificial intelligence generated text: examining the prospects and potential threats of ChatGPT in academic writing. Biol Sport. 2023;40(2):615–622. doi: 10.5114/biolsport.2023.125623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergaa I, Saad HB. Artificial intelligence and promoting open access in academic publishing. Tunis Med. 2023;101(6):533–536. [PMC free article] [PubMed] [Google Scholar]


