Abstract
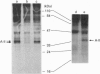
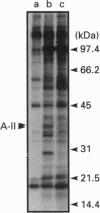
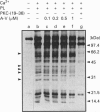
In order to understand how signal transduction occurs during T cell activation, it is necessary to identify the key regulatory molecules whose function is influenced by phosphorylation. Annexins II (A-II) and V (A-V) belong to a large family of Ca(2+)-dependent phospholipid-binding proteins. Among many putative functions, annexins may be involved in signal transduction during cellular proliferation and differentiation. In the present study we show that A-II is phosphorylated in vivo in the Jurkat human T cell line. Indeed, A-II is phosphorylated after stimulation by phorbol myristate acetate and on serine residues after T cell antigen receptor (TcR) stimulation. In cytosol from Jurkat cells, A-II is phosphorylated only by Ca2+/phospholipid-stimulated kinases such as Ca(2+)-dependent protein kinases C (cPKCs). A-V inhibits the phosphorylation of A-II and other substrates of cPKCs and has no effect on kinases activated only by phospholipids. In conclusion, A-II is phosphorylated both in vitro and in vivo in Jurkat cells, and may play a role as a substrate during signal transduction in lymphocytes via the TcR through the PKC pathway. On the other hand, A-V could act as a potent modulator of cPKCs in Jurkat cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acres R. B., Conlon P. J., Mochizuki D. Y., Gallis B. Rapid phosphorylation and modulation of the T4 antigen on cloned helper T cells induced by phorbol myristate acetate or antigen. J Biol Chem. 1986 Dec 5;261(34):16210–16214. [PubMed] [Google Scholar]
- Autero M., Gahmberg C. G. Phorbol diesters increase the phosphorylation of the leukocyte common antigen CD45 in human T cells. Eur J Immunol. 1987 Oct;17(10):1503–1506. doi: 10.1002/eji.1830171018. [DOI] [PubMed] [Google Scholar]
- Baier G., Telford D., Giampa L., Coggeshall K. M., Baier-Bitterlich G., Isakov N., Altman A. Molecular cloning and characterization of PKC theta, a novel member of the protein kinase C (PKC) gene family expressed predominantly in hematopoietic cells. J Biol Chem. 1993 Mar 5;268(7):4997–5004. [PubMed] [Google Scholar]
- Basu A. The potential of protein kinase C as a target for anticancer treatment. Pharmacol Ther. 1993 Sep;59(3):257–280. doi: 10.1016/0163-7258(93)90070-t. [DOI] [PubMed] [Google Scholar]
- Bazzi M. D., Nelsestuen G. L. Protein kinase C and annexins: unusual calcium response elements in the cell. Cell Signal. 1993 Jul;5(4):357–365. doi: 10.1016/0898-6568(93)90075-w. [DOI] [PubMed] [Google Scholar]
- Berry N., Nishizuka Y. Protein kinase C and T cell activation. Eur J Biochem. 1990 Apr 30;189(2):205–214. doi: 10.1111/j.1432-1033.1990.tb15478.x. [DOI] [PubMed] [Google Scholar]
- Blue M. L., Hafler D. A., Craig K. A., Levine H., Schlossman S. F. Phosphorylation of CD4 and CD8 molecules following T cell triggering. J Immunol. 1987 Dec 15;139(12):3949–3954. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Clague M. J. Annexins in the endocytic pathway. Trends Biochem Sci. 1994 Jun;19(6):231–232. doi: 10.1016/0968-0004(94)90143-0. [DOI] [PubMed] [Google Scholar]
- Cai Y. C., Douglass J. In vivo and in vitro phosphorylation of the T lymphocyte type n (Kv1.3) potassium channel. J Biol Chem. 1993 Nov 5;268(31):23720–23727. [PubMed] [Google Scholar]
- Cantrell D. A., Davies A. A., Crumpton M. J. Activators of protein kinase C down-regulate and phosphorylate the T3/T-cell antigen receptor complex of human T lymphocytes. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8158–8162. doi: 10.1073/pnas.82.23.8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Columelli S., Houllier A. M., Pérignon J. L. A protein kinase C inhibitory activity is present in the human T lymphoblast cell line Jurkat. Immunol Lett. 1991 Nov;30(3):297–300. doi: 10.1016/0165-2478(91)90041-8. [DOI] [PubMed] [Google Scholar]
- Coméra C., Rothhut B., Cavadore J. C., Vilgrain I., Cochet C., Chambaz E., Russo-Marie F. Further characterization of four lipocortins from human peripheral blood mononuclear cells. J Cell Biochem. 1989 Jul;40(3):361–370. doi: 10.1002/jcb.240400312. [DOI] [PubMed] [Google Scholar]
- Coméra C., Rothhut B., Russo-Marie F. Identification and characterization of phospholipase A2 inhibitory proteins in human mononuclear cells. Eur J Biochem. 1990 Feb 22;188(1):139–146. doi: 10.1111/j.1432-1033.1990.tb15381.x. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Creutz C. E. The annexins and exocytosis. Science. 1992 Nov 6;258(5084):924–931. doi: 10.1126/science.1439804. [DOI] [PubMed] [Google Scholar]
- Davies A. A., Crumpton M. J. Identification of calcium-binding proteins associated with the lymphocyte plasma membrane. Biochem Biophys Res Commun. 1985 Apr 30;128(2):571–577. doi: 10.1016/0006-291x(85)90084-1. [DOI] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Identification of a cellular protein substrate phosphorylated by the avian sarcoma virus-transforming gene product. Cell. 1980 Oct;21(3):829–836. doi: 10.1016/0092-8674(80)90446-8. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Ruscetti F. W. Association of protein kinase C activation with IL 2 receptor expression. J Immunol. 1986 Feb 15;136(4):1266–1273. [PubMed] [Google Scholar]
- Gerke V., Weber K. Identity of p36K phosphorylated upon Rous sarcoma virus transformation with a protein purified from brush borders; calcium-dependent binding to non-erythroid spectrin and F-actin. EMBO J. 1984 Jan;3(1):227–233. doi: 10.1002/j.1460-2075.1984.tb01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudie R. B., Horne C. H., Wilkinson P. C. A simple method for producing antibody specific to a single selected diffusible antigen. Lancet. 1966 Dec 3;2(7475):1224–1226. doi: 10.1016/s0140-6736(66)92305-1. [DOI] [PubMed] [Google Scholar]
- Gould K. L., Woodgett J. R., Isacke C. M., Hunter T. The protein-tyrosine kinase substrate p36 is also a substrate for protein kinase C in vitro and in vivo. Mol Cell Biol. 1986 Jul;6(7):2738–2744. doi: 10.1128/mcb.6.7.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug H., Sarre T. F. Protein kinase C isoenzymes: divergence in signal transduction? Biochem J. 1993 Apr 15;291(Pt 2):329–343. doi: 10.1042/bj2910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isacke C. M., Trowbridge I. S., Hunter T. Modulation of p36 phosphorylation in human cells: studies using anti-p36 monoclonal antibodies. Mol Cell Biol. 1986 Jul;6(7):2745–2751. doi: 10.1128/mcb.6.7.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson N., Nguyen Van P., Söling H. D., Weber K. Functionally distinct serine phosphorylation sites of p36, the cellular substrate of retroviral protein kinase; differential inhibition of reassociation with p11. EMBO J. 1986 Dec 20;5(13):3455–3460. doi: 10.1002/j.1460-2075.1986.tb04669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone S. A., Hubaishy I., Waisman D. M. Phosphorylation of annexin II tetramer by protein kinase C inhibits aggregation of lipid vesicles by the protein. J Biol Chem. 1992 Dec 25;267(36):25976–25981. [PubMed] [Google Scholar]
- Johnstone S. A., Hubaishy I., Waisman D. M. Regulation of annexin I-dependent aggregation of phospholipid vesicles by protein kinase C. Biochem J. 1993 Sep 15;294(Pt 3):801–807. doi: 10.1042/bj2940801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Acid and base hydrolysis of phosphoproteins bound to immobilon facilitates analysis of phosphoamino acids in gel-fractionated proteins. Anal Biochem. 1989 Jan;176(1):22–27. doi: 10.1016/0003-2697(89)90266-2. [DOI] [PubMed] [Google Scholar]
- Kaplan R., Jaye M., Burgess W. H., Schlaepfer D. D., Haigler H. T. Cloning and expression of cDNA for human endonexin II, a Ca2+ and phospholipid binding protein. J Biol Chem. 1988 Jun 15;263(17):8037–8043. [PubMed] [Google Scholar]
- Keutzer J. C., Hirschhorn R. R. The growth-regulated gene 1B6 is identified as the heavy chain of calpactin I. Exp Cell Res. 1990 May;188(1):153–159. doi: 10.1016/0014-4827(90)90291-h. [DOI] [PubMed] [Google Scholar]
- Khanna N. C., Tokuda M., Waisman D. M. Phosphorylation of lipocortins in vitro by protein kinase C. Biochem Biophys Res Commun. 1986 Dec 15;141(2):547–554. doi: 10.1016/s0006-291x(86)80208-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lisanti M. P., Scherer P. E., Tang Z., Sargiacomo M. Caveolae, caveolin and caveolin-rich membrane domains: a signalling hypothesis. Trends Cell Biol. 1994 Jul;4(7):231–235. doi: 10.1016/0962-8924(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Martin F., Derancourt J., Capony J. P., Watrin A., Cavadore J. C. A 36 kDa monomeric protein and its complex with a 10 kDa protein both isolated from bovine aorta are calpactin-like proteins that differ in their Ca2+-dependent calmodulin-binding and actin-severing properties. Biochem J. 1988 May 1;251(3):777–785. doi: 10.1042/bj2510777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel A. E., Bouic P., Lattanze G. R., Stevenson H. C., Miller P., Dirienzo W., Stefanini G. F., Galbraith R. M. Reaction of T lymphocytes with anti-T3 induces translocation of C-kinase activity to the membrane and specific substrate phosphorylation. J Immunol. 1987 May 15;138(10):3519–3524. [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Oudinet J. P., Russo-Marie F., Cavadore J. C., Rothhut B. Protein kinase C-dependent phosphorylation of annexins I and II in mesangial cells. Biochem J. 1993 May 15;292(Pt 1):63–68. doi: 10.1042/bj2920063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D. J., Min H. K., Rhee S. G. Inhibition of CD3-linked phospholipase C by phorbol ester and by cAMP is associated with decreased phosphotyrosine and increased phosphoserine contents of PLC-gamma 1. J Biol Chem. 1992 Jan 25;267(3):1496–1501. [PubMed] [Google Scholar]
- Park D. J., Rho H. W., Rhee S. G. CD3 stimulation causes phosphorylation of phospholipase C-gamma 1 on serine and tyrosine residues in a human T-cell line. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5453–5456. doi: 10.1073/pnas.88.12.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premack B. A., Gardner P. Signal transduction by T-cell receptors: mobilization of Ca and regulation of Ca-dependent effector molecules. Am J Physiol. 1992 Dec;263(6 Pt 1):C1119–C1140. doi: 10.1152/ajpcell.1992.263.6.C1119. [DOI] [PubMed] [Google Scholar]
- Radke K., Gilmore T., Martin G. S. Transformation by Rous sarcoma virus: a cellular substrate for transformation-specific protein phosphorylation contains phosphotyrosine. Cell. 1980 Oct;21(3):821–828. doi: 10.1016/0092-8674(80)90445-6. [DOI] [PubMed] [Google Scholar]
- Raynal P., Hullin F., Ragab-Thomas J. M., Fauvel J., Chap H. Annexin 5 as a potential regulator of annexin 1 phosphorylation by protein kinase C. In vitro inhibition compared with quantitative data on annexin distribution in human endothelial cells. Biochem J. 1993 Jun 15;292(Pt 3):759–765. doi: 10.1042/bj2920759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynal P., Pollard H. B. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim Biophys Acta. 1994 Apr 5;1197(1):63–93. doi: 10.1016/0304-4157(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Rothhut B., Comera C., Prieur B., Errasfa M., Minassian G., Russo-Marie F. Purification and characterization of a 32-kDa phospholipase A2 inhibitory protein (lipocortin) from human peripheral blood mononuclear cells. FEBS Lett. 1987 Jul 13;219(1):169–175. doi: 10.1016/0014-5793(87)81211-5. [DOI] [PubMed] [Google Scholar]
- Rothhut B., Coméra C., Cortial S., Haumont P. Y., Diep Le K. H., Cavadore J. C., Conard J., Russo-Marie F., Lederer F. A 32 kDa lipocortin from human mononuclear cells appears to be identical with the placental inhibitor of blood coagulation. Biochem J. 1989 Nov 1;263(3):929–935. doi: 10.1042/bj2630929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo-Marie F. Lipocortins: an update. Prostaglandins Leukot Essent Fatty Acids. 1991 Feb;42(2):83–89. doi: 10.1016/0952-3278(91)90072-d. [DOI] [PubMed] [Google Scholar]
- Schlaepfer D. D., Haigler H. T. Characterization of Ca2+-dependent phospholipid binding and phosphorylation of lipocortin I. J Biol Chem. 1987 May 15;262(14):6931–6937. [PubMed] [Google Scholar]
- Schlaepfer D. D., Jones J., Haigler H. T. Inhibition of protein kinase C by annexin V. Biochemistry. 1992 Feb 18;31(6):1886–1891. doi: 10.1021/bi00121a043. [DOI] [PubMed] [Google Scholar]
- Shackelford D. A., Trowbridge I. S. Identification of lymphocyte integral membrane proteins as substrates for protein kinase C. Phosphorylation of the interleukin-2 receptor, class I HLA antigens, and T200 glycoprotein. J Biol Chem. 1986 Jun 25;261(18):8334–8341. [PubMed] [Google Scholar]
- Shackelford D. A., Trowbridge I. S. Induction of expression and phosphorylation of the human interleukin 2 receptor by a phorbol diester. J Biol Chem. 1984 Oct 10;259(19):11706–11712. [PubMed] [Google Scholar]
- Shibata S., Sato H., Maki M. Calphobindins (placental annexins) inhibit protein kinase C. J Biochem. 1992 Oct;112(4):552–556. doi: 10.1093/oxfordjournals.jbchem.a123937. [DOI] [PubMed] [Google Scholar]
- Siegel J. N., Klausner R. D., Rapp U. R., Samelson L. E. T cell antigen receptor engagement stimulates c-raf phosphorylation and induces c-raf-associated kinase activity via a protein kinase C-dependent pathway. J Biol Chem. 1990 Oct 25;265(30):18472–18480. [PubMed] [Google Scholar]
- Soula M., Fagard R., Fischer S. Interaction of human immunodeficiency virus glycoprotein 160 with CD4 in Jurkat cells increases p56lck autophosphorylation and kinase activity. Int Immunol. 1992 Feb;4(2):295–299. doi: 10.1093/intimm/4.2.295. [DOI] [PubMed] [Google Scholar]
- Soula M., Rothhut B., Camoin L., Guillaume J. L., Strosberg D., Vorherr T., Burn P., Meggio F., Fischer S., Fagard R. Anti-CD3 and phorbol ester induce distinct phosphorylated sites in the SH2 domain of p56lck. J Biol Chem. 1993 Dec 25;268(36):27420–27427. [PubMed] [Google Scholar]
- Szamel M., Resch K. T-cell antigen receptor-induced signal-transduction pathways--activation and function of protein kinases C in T lymphocytes. Eur J Biochem. 1995 Feb 15;228(1):1–15. doi: 10.1111/j.1432-1033.1995.tb20221.x. [DOI] [PubMed] [Google Scholar]
- Touqui L., Rothhut B., Shaw A. M., Fradin A., Vargaftig B. B., Russo-Marie F. Platelet activation--a role for a 40K anti-phospholipase A2 protein indistinguishable from lipocortin. Nature. 1986 May 8;321(6066):177–180. doi: 10.1038/321177a0. [DOI] [PubMed] [Google Scholar]
- Tsutsumi A., Kubo M., Fujii H., Freire-Moar J., Turck C. W., Ransom J. T. Regulation of protein kinase C isoform proteins in phorbol ester-stimulated Jurkat T lymphoma cells. J Immunol. 1993 Mar 1;150(5):1746–1754. [PubMed] [Google Scholar]
- Weiss A., Koretzky G., Schatzman R. C., Kadlecek T. Functional activation of the T-cell antigen receptor induces tyrosine phosphorylation of phospholipase C-gamma 1. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5484–5488. doi: 10.1073/pnas.88.13.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]