Abstract
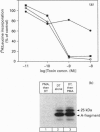
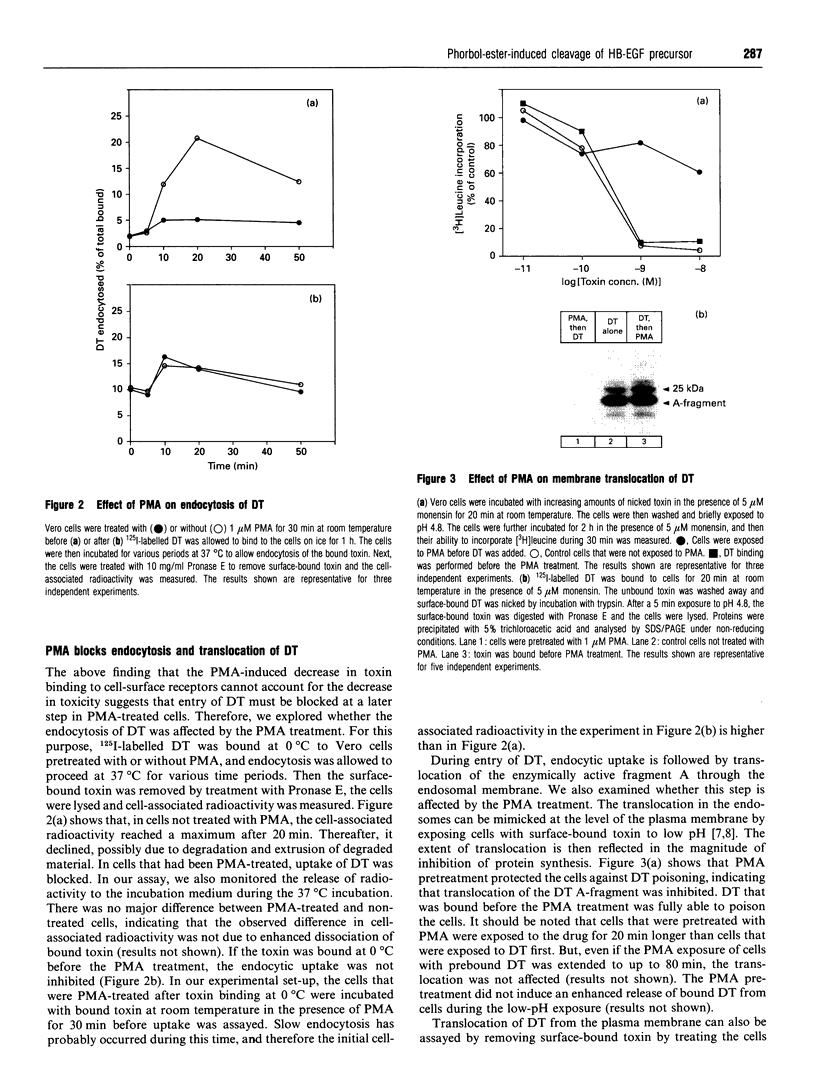
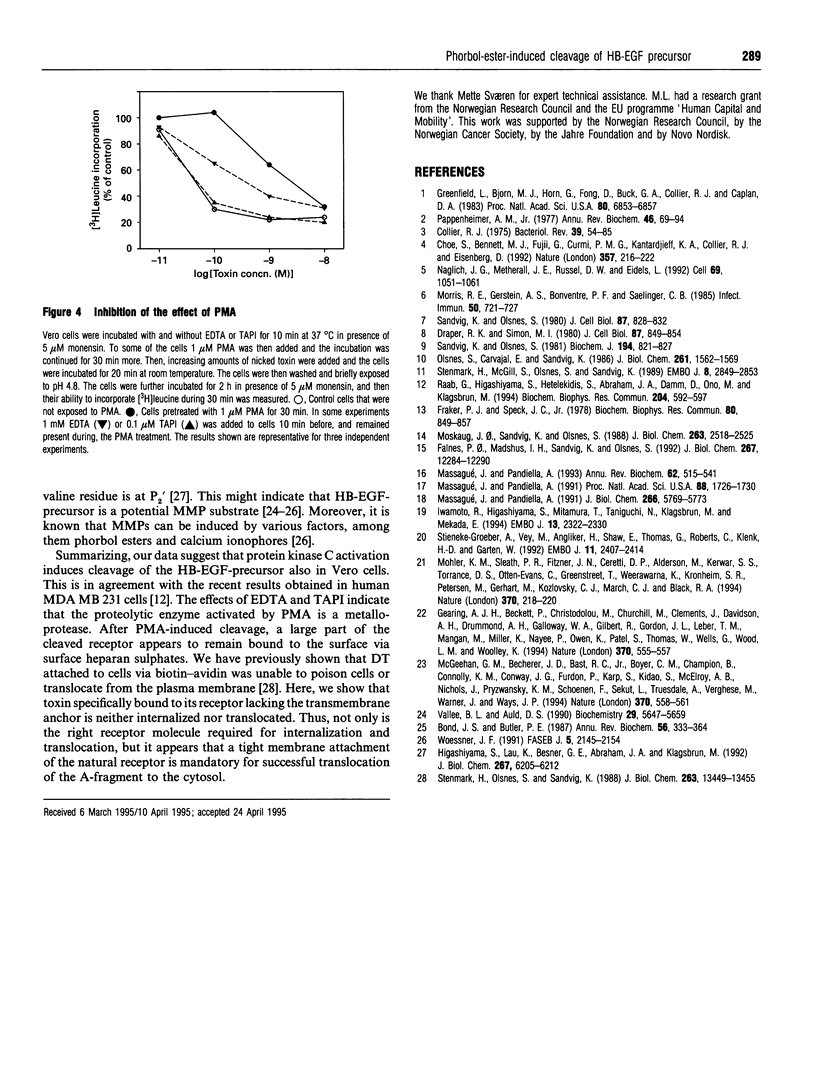
Preincubation of Vero cells with 1 microM phorbol 12-myristate 13-acetate (PMA) decreased the specific binding of diphtheria toxin by about 50%, whereas the toxic effect, endocytic uptake and membrane translocation were completely blocked. Toxin bound to PMA-treated cells was released upon incubation with heparinase. The effect of PMA was abrogated in the presence of EDTA or N-(DL-[2-(hydroxyaminocarbonyl)methyl]-4-methyl-pentanoyl)-L-3-(2' - naphthyl)-alanyl-L-alanine 2-aminoethyl-amide (TAPI), a specific inhibitor of matrix metalloproteases. The results indicate that PMA induces proteolytic cleavage of the diphtheria-toxin receptor [heparin-binding EGF-like growth factor (HB-EGF)-precursor] outside the membrane anchor, and that about 50% of the growth-factor ecto-domain remains associated with the cells, due to binding to surface proteoglycans containing heparan sulphates. Although the cleaved cell-associated HB-EGF binds diphtheria toxin, it does not serve as a functional receptor, since neither toxin internalization nor translocation occurs. Thus the intact HB-EGF precursor is of crucial importance for its function as the diphtheria-toxin receptor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bond J. S., Butler P. E. Intracellular proteases. Annu Rev Biochem. 1987;56:333–364. doi: 10.1146/annurev.bi.56.070187.002001. [DOI] [PubMed] [Google Scholar]
- Choe S., Bennett M. J., Fujii G., Curmi P. M., Kantardjieff K. A., Collier R. J., Eisenberg D. The crystal structure of diphtheria toxin. Nature. 1992 May 21;357(6375):216–222. doi: 10.1038/357216a0. [DOI] [PubMed] [Google Scholar]
- Collier R. J. Diphtheria toxin: mode of action and structure. Bacteriol Rev. 1975 Mar;39(1):54–85. doi: 10.1128/br.39.1.54-85.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper R. K., Simon M. I. The entry of diphtheria toxin into the mammalian cell cytoplasm: evidence for lysosomal involvement. J Cell Biol. 1980 Dec;87(3 Pt 1):849–854. doi: 10.1083/jcb.87.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falnes P. O., Madshus I. H., Sandvig K., Olsnes S. Replacement of negative by positive charges in the presumed membrane-inserted part of diphtheria toxin B fragment. Effect on membrane translocation and on formation of cation channels. J Biol Chem. 1992 Jun 15;267(17):12284–12290. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gearing A. J., Beckett P., Christodoulou M., Churchill M., Clements J., Davidson A. H., Drummond A. H., Galloway W. A., Gilbert R., Gordon J. L. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature. 1994 Aug 18;370(6490):555–557. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- Greenfield L., Bjorn M. J., Horn G., Fong D., Buck G. A., Collier R. J., Kaplan D. A. Nucleotide sequence of the structural gene for diphtheria toxin carried by corynebacteriophage beta. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6853–6857. doi: 10.1073/pnas.80.22.6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama S., Lau K., Besner G. E., Abraham J. A., Klagsbrun M. Structure of heparin-binding EGF-like growth factor. Multiple forms, primary structure, and glycosylation of the mature protein. J Biol Chem. 1992 Mar 25;267(9):6205–6212. [PubMed] [Google Scholar]
- Iwamoto R., Higashiyama S., Mitamura T., Taniguchi N., Klagsbrun M., Mekada E. Heparin-binding EGF-like growth factor, which acts as the diphtheria toxin receptor, forms a complex with membrane protein DRAP27/CD9, which up-regulates functional receptors and diphtheria toxin sensitivity. EMBO J. 1994 May 15;13(10):2322–2330. doi: 10.1002/j.1460-2075.1994.tb06516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J., Pandiella A. Membrane-anchored growth factors. Annu Rev Biochem. 1993;62:515–541. doi: 10.1146/annurev.bi.62.070193.002503. [DOI] [PubMed] [Google Scholar]
- McGeehan G. M., Becherer J. D., Bast R. C., Jr, Boyer C. M., Champion B., Connolly K. M., Conway J. G., Furdon P., Karp S., Kidao S. Regulation of tumour necrosis factor-alpha processing by a metalloproteinase inhibitor. Nature. 1994 Aug 18;370(6490):558–561. doi: 10.1038/370558a0. [DOI] [PubMed] [Google Scholar]
- Mohler K. M., Sleath P. R., Fitzner J. N., Cerretti D. P., Alderson M., Kerwar S. S., Torrance D. S., Otten-Evans C., Greenstreet T., Weerawarna K. Protection against a lethal dose of endotoxin by an inhibitor of tumour necrosis factor processing. Nature. 1994 Jul 21;370(6486):218–220. doi: 10.1038/370218a0. [DOI] [PubMed] [Google Scholar]
- Morris R. E., Gerstein A. S., Bonventre P. F., Saelinger C. B. Receptor-mediated entry of diphtheria toxin into monkey kidney (Vero) cells: electron microscopic evaluation. Infect Immun. 1985 Dec;50(3):721–727. doi: 10.1128/iai.50.3.721-727.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskaug J. O., Sandvig K., Olsnes S. Low pH-induced release of diphtheria toxin A-fragment in Vero cells. Biochemical evidence for transfer to the cytosol. J Biol Chem. 1988 Feb 15;263(5):2518–2525. [PubMed] [Google Scholar]
- Naglich J. G., Metherall J. E., Russell D. W., Eidels L. Expression cloning of a diphtheria toxin receptor: identity with a heparin-binding EGF-like growth factor precursor. Cell. 1992 Jun 12;69(6):1051–1061. doi: 10.1016/0092-8674(92)90623-k. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Carvajal E., Sandvig K. Interactions between diphtheria toxin entry and anion transport in vero cells. III. Effect on toxin binding and anion transport of tumor-promoting phorbol esters, vanadate, fluoride, and salicylate. J Biol Chem. 1986 Feb 5;261(4):1562–1569. [PubMed] [Google Scholar]
- Pandiella A., Massagué J. Cleavage of the membrane precursor for transforming growth factor alpha is a regulated process. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1726–1730. doi: 10.1073/pnas.88.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandiella A., Massagué J. Multiple signals activate cleavage of the membrane transforming growth factor-alpha precursor. J Biol Chem. 1991 Mar 25;266(9):5769–5773. [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr Diphtheria toxin. Annu Rev Biochem. 1977;46:69–94. doi: 10.1146/annurev.bi.46.070177.000441. [DOI] [PubMed] [Google Scholar]
- Raab G., Higashiyama S., Hetelekidis S., Abraham J. A., Damm D., Ono M., Klagsbrun M. Biosynthesis and processing by phorbol ester of the cells surface-associated precursor form of heparin-binding EGF-like growth factor. Biochem Biophys Res Commun. 1994 Oct 28;204(2):592–597. doi: 10.1006/bbrc.1994.2500. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Diphtheria toxin entry into cells is facilitated by low pH. J Cell Biol. 1980 Dec;87(3 Pt 1):828–832. doi: 10.1083/jcb.87.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Effects of retinoids and phorbol esters on the sensitivity of different cell lines to the polypeptide toxins modeccin, abrin, ricin and diphtheria toxin. Biochem J. 1981 Mar 15;194(3):821–827. doi: 10.1042/bj1940821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H., McGill S., Olsnes S., Sandvig K. Permeabilization of the plasma membrane by deletion mutants of diphtheria toxin. EMBO J. 1989 Oct;8(10):2849–2853. doi: 10.1002/j.1460-2075.1989.tb08432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H., Olsnes S., Sandvig K. Requirement of specific receptors for efficient translocation of diphtheria toxin A fragment across the plasma membrane. J Biol Chem. 1988 Sep 15;263(26):13449–13455. [PubMed] [Google Scholar]
- Stieneke-Gröber A., Vey M., Angliker H., Shaw E., Thomas G., Roberts C., Klenk H. D., Garten W. Influenza virus hemagglutinin with multibasic cleavage site is activated by furin, a subtilisin-like endoprotease. EMBO J. 1992 Jul;11(7):2407–2414. doi: 10.1002/j.1460-2075.1992.tb05305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Auld D. S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 1990 Jun 19;29(24):5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]