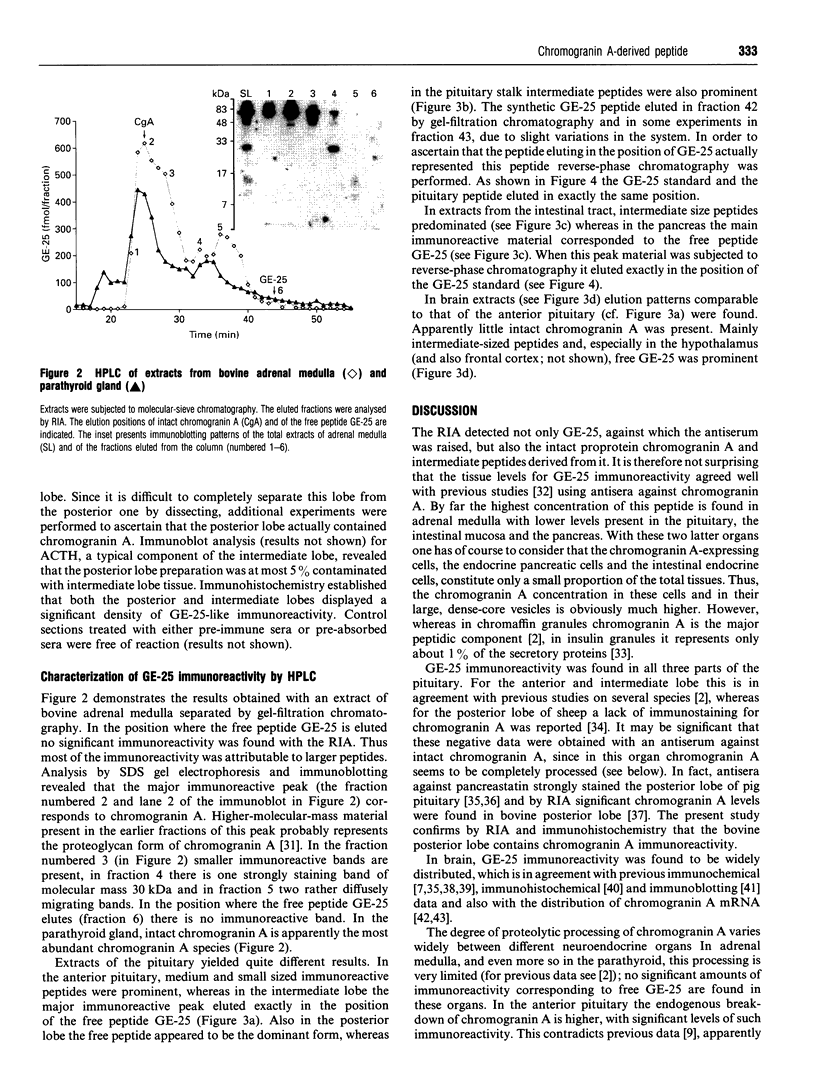
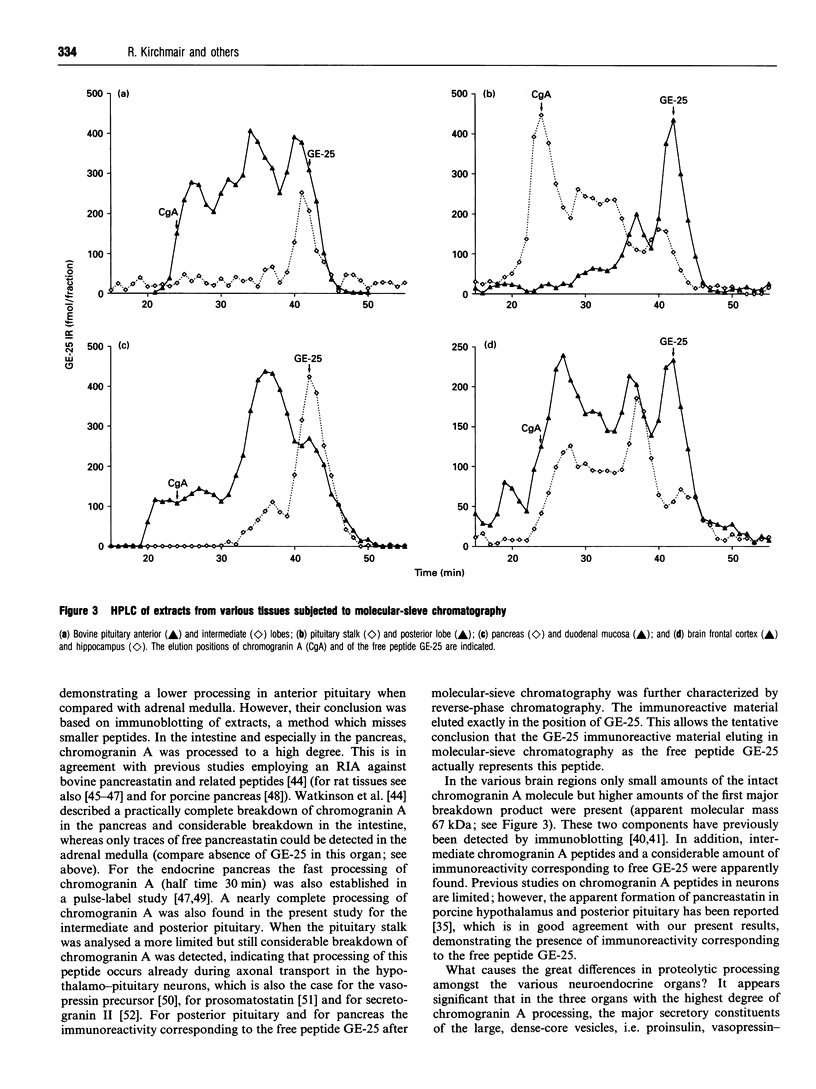
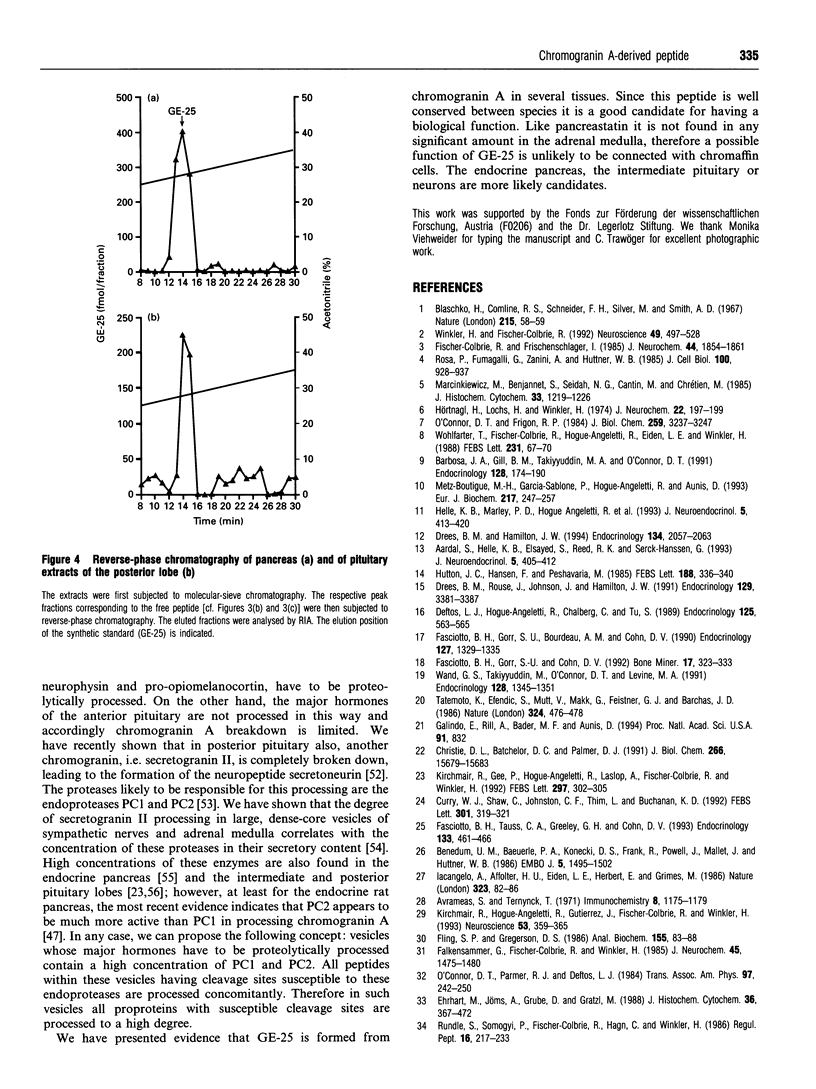
Abstract
We have established a radioimmunoassay for GE-25, a peptide present in the C-terminal end of the primary amino acid sequence of chromogranin A where it is flanked by typical proteolytic cleavage sites. Gel-filtration HPLC was used to characterize the molecular sizes of the immunoreactive molecules. The antiserum recognized not only the free peptide but also larger precursors including the proprotein chromogranin A. The tissues with the highest levels of GE-25 immunoreactivity were in decreasing order: the adrenal medulla, the three lobes of the pituitary gland, intestinal mucosa, pancreas and various brain regions. In adrenal medulla and parathyroid gland most of the immunoreactivity was found to be present as intact chromogranin A and some intermediate-sized peptides, without significant amounts of the free peptide. In anterior pituitary, and even more so in intestine, a shift to smaller peptides was seen. In the posterior and intermediate pituitary and in pancreas the predominant immunoreactive material was apparently represented by the free peptide GE-25. In reverse-phase chromatography this peptide eluted exactly like the synthetic standard, which allows a tentative identification as GE-25. In brain tissue the processing of chromogranin A was intermediate, with significant amounts of immunoreactivity corresponding to GE-25 as well as precursor proteins being present. We suggest that in those organs (endocrine pancreas, intermediate and posterior pituitary) where the major hormones are proteolytically processed there is also a concomitant proteolysis of further susceptible peptides. Since GE-25 is apparently formed in vivo and is well conserved between species it seems a good candidate for having specific physiological functions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aardal S., Helle K. B., Elsayed S., Reed R. K., Serck-Hanssen G. Vasostatins, comprising the N-terminal domain of chromogranin A, suppress tension in isolated human blood vessel segments. J Neuroendocrinol. 1993 Aug;5(4):405–412. doi: 10.1111/j.1365-2826.1993.tb00501.x. [DOI] [PubMed] [Google Scholar]
- Arden S. D., Rutherford N. G., Guest P. C., Curry W. J., Bailyes E. M., Johnston C. F., Hutton J. C. The post-translational processing of chromogranin A in the pancreatic islet: involvement of the eukaryote subtilisin PC2. Biochem J. 1994 Mar 15;298(Pt 3):521–528. doi: 10.1042/bj2980521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. Peroxidase labelled antibody and Fab conjugates with enhanced intracellular penetration. Immunochemistry. 1971 Dec;8(12):1175–1179. doi: 10.1016/0019-2791(71)90395-8. [DOI] [PubMed] [Google Scholar]
- Barbosa J. A., Gill B. M., Takiyyuddin M. A., O'Connor D. T. Chromogranin A: posttranslational modifications in secretory granules. Endocrinology. 1991 Jan;128(1):174–190. doi: 10.1210/endo-128-1-174. [DOI] [PubMed] [Google Scholar]
- Benedum U. M., Baeuerle P. A., Konecki D. S., Frank R., Powell J., Mallet J., Huttner W. B. The primary structure of bovine chromogranin A: a representative of a class of acidic secretory proteins common to a variety of peptidergic cells. EMBO J. 1986 Jul;5(7):1495–1502. doi: 10.1002/j.1460-2075.1986.tb04388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschko H., Comline R. S., Schneider F. H., Silver M., Smith A. D. Secretion of a chromaffin granule protein, chromogranin, from the adrenal gland after splanchnic stimulation. Nature. 1967 Jul 1;215(5096):58–59. doi: 10.1038/215058a0. [DOI] [PubMed] [Google Scholar]
- Curry W. J., Johnston C. F., Shaw C., Buchanan K. D. Distribution and partial characterisation of immunoreactivity to the putative C-terminus of rat pancreastatin. Regul Pept. 1990 Oct 8;30(3):207–219. doi: 10.1016/0167-0115(90)90096-f. [DOI] [PubMed] [Google Scholar]
- Curry W. J., Shaw C., Johnston C. F., Thim L., Buchanan K. D. Isolation and primary structure of a novel chromogranin A-derived peptide, WE-14, from a human midgut carcinoid tumour. FEBS Lett. 1992 Apr 27;301(3):319–321. doi: 10.1016/0014-5793(92)80266-j. [DOI] [PubMed] [Google Scholar]
- Deftos L. J., Hogue-Angeletti R., Chalberg C., Tu S. PTHrP secretion is stimulated by CT and inhibited by CgA peptides. Endocrinology. 1989 Jul;125(1):563–565. doi: 10.1210/endo-125-1-563. [DOI] [PubMed] [Google Scholar]
- Drees B. M., Hamilton J. W. Processing of chromogranin A by bovine parathyroid secretory granules: production and secretion of N-terminal fragments. Endocrinology. 1994 May;134(5):2057–2063. doi: 10.1210/endo.134.5.8156905. [DOI] [PubMed] [Google Scholar]
- Drees B. M., Rouse J., Johnson J., Hamilton J. W. Bovine parathyroid glands secrete a 26-kDa N-terminal fragment of chromogranin-A which inhibits parathyroid cell secretion. Endocrinology. 1991 Dec;129(6):3381–3387. doi: 10.1210/endo-129-6-3381. [DOI] [PubMed] [Google Scholar]
- Egger C., Kirchmair R., Hogue-Angeletti R., Fischer-Colbrie R., Winkler H. Different degrees of processing of secretogranin II in large dense core vesicles of bovine adrenal medulla and sympathetic axons correlate with their content of soluble PC1 and PC2. Neurosci Lett. 1993 Sep 3;159(1-2):199–201. doi: 10.1016/0304-3940(93)90833-7. [DOI] [PubMed] [Google Scholar]
- Egger C., Kirchmair R., Kapelari S., Fischer-Colbrie R., Hogue-Angeletti R., Winkler H. Bovine posterior pituitary: presence of p65 (synaptotagmin), PC1, PC2 and secretoneurin in large dense core vesicles. Neuroendocrinology. 1994 Feb;59(2):169–175. doi: 10.1159/000126655. [DOI] [PubMed] [Google Scholar]
- Ehrhart M., Jörns A., Grube D., Gratzl M. Cellular distribution and amount of chromogranin A in bovine endocrine pancreas. J Histochem Cytochem. 1988 May;36(5):467–472. doi: 10.1177/36.5.3282005. [DOI] [PubMed] [Google Scholar]
- Erickson J. D., Lloyd R., Trojanowski J. Q., Iacangelo A., Eiden E. Sites of synthesis of chromogranins A and B in the human brain. Neuropeptides. 1992 Apr;21(4):239–244. doi: 10.1016/0143-4179(92)90028-u. [DOI] [PubMed] [Google Scholar]
- Fasciotto B. H., Gorr S. U., Bourdeau A. M., Cohn D. V. Autocrine regulation of parathyroid secretion: inhibition of secretion by chromogranin-A (secretory protein-I) and potentiation of secretion by chromogranin-A and pancreastatin antibodies. Endocrinology. 1990 Sep;127(3):1329–1335. doi: 10.1210/endo-127-3-1329. [DOI] [PubMed] [Google Scholar]
- Fasciotto B. H., Gorr S. U., Cohn D. V. Autocrine inhibition of parathyroid cell secretion requires proteolytic processing of chromogranin A. Bone Miner. 1992 Jun;17(3):323–333. doi: 10.1016/0169-6009(92)90783-a. [DOI] [PubMed] [Google Scholar]
- Fasciotto B. H., Trauss C. A., Greeley G. H., Cohn D. V. Parastatin (porcine chromogranin A347-419), a novel chromogranin A-derived peptide, inhibits parathyroid cell secretion. Endocrinology. 1993 Aug;133(2):461–466. doi: 10.1210/endo.133.2.8344192. [DOI] [PubMed] [Google Scholar]
- Fischer-Colbrie R., Frischenschlager I. Immunological characterization of secretory proteins of chromaffin granules: chromogranins A, chromogranins B, and enkephalin-containing peptides. J Neurochem. 1985 Jun;44(6):1854–1861. doi: 10.1111/j.1471-4159.1985.tb07179.x. [DOI] [PubMed] [Google Scholar]
- Fling S. P., Gregerson D. S. Peptide and protein molecular weight determination by electrophoresis using a high-molarity tris buffer system without urea. Anal Biochem. 1986 May 15;155(1):83–88. doi: 10.1016/0003-2697(86)90228-9. [DOI] [PubMed] [Google Scholar]
- Helle K. B., Marley P. D., Angeletti R. H., Aunis D., Galindo E., Small D. H., Livett B. G. Chromogranin A: secretion of processed products from the stimulated retrogradely perfused bovine adrenal gland. J Neuroendocrinol. 1993 Aug;5(4):413–420. doi: 10.1111/j.1365-2826.1993.tb00502.x. [DOI] [PubMed] [Google Scholar]
- Hutton J. C., Davidson H. W., Grimaldi K. A., Peshavaria M. Biosynthesis of betagranin in pancreatic beta-cells. Identification of a chromogranin A-like precursor and its parallel processing with proinsulin. Biochem J. 1987 Jun 1;244(2):449–456. doi: 10.1042/bj2440449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton J. C., Hansen F., Peshavaria M. beta-Granins: 21 kDa co-secreted peptides of the insulin granule closely related to adrenal medullary chromogranin A. FEBS Lett. 1985 Sep 2;188(2):336–340. doi: 10.1016/0014-5793(85)80398-7. [DOI] [PubMed] [Google Scholar]
- Hutton J. C., Peshavaria M., Johnston C. F., Ravazzola M., Orci L. Immunolocalization of betagranin: a chromogranin A-related protein of the pancreatic B-cell. Endocrinology. 1988 Mar;122(3):1014–1020. doi: 10.1210/endo-122-3-1014. [DOI] [PubMed] [Google Scholar]
- Hörtnagl H., Lochs H., Winkler H. Immunological studies on the acidic chromogranins and on dopamine beta-hydroxylase (EC 1.14.2.1) of bovine chromaffin granules. J Neurochem. 1974 Jan;22(1):197–199. doi: 10.1111/j.1471-4159.1974.tb12201.x. [DOI] [PubMed] [Google Scholar]
- Iacangelo A., Affolter H. U., Eiden L. E., Herbert E., Grimes M. Bovine chromogranin A sequence and distribution of its messenger RNA in endocrine tissues. Nature. 1986 Sep 4;323(6083):82–86. doi: 10.1038/323082a0. [DOI] [PubMed] [Google Scholar]
- Jensen T. B., Fahrenkrug J., Sundler F. Immunocytochemical localisation of pancreastatin and chromogranin A in porcine neuroendocrine tissues. Regul Pept. 1991 Oct 29;36(2):283–297. doi: 10.1016/0167-0115(91)90063-m. [DOI] [PubMed] [Google Scholar]
- Kar S., Bretherton-Watt D., Gibson S. J., Steel J. H., Gentleman S. M., Roberts G. W., Valentino K., Tatemoto K., Ghatei M. A., Bloom S. R. Novel peptide pancreastatin: its occurrence and codistribution with chromogranin A in the central nervous system of the pig. J Comp Neurol. 1989 Oct 22;288(4):627–639. doi: 10.1002/cne.902880409. [DOI] [PubMed] [Google Scholar]
- Kawakubo A., Takatsuki K., Yoneda M., Kurokawa M., Suzuki A., Semba R., Kato K. Highly sensitive enzyme immunoassay for bovine chromogranin A: application for studies of regional distribution in bovine central nervous system. J Mol Neurosci. 1989;1(4):215–223. [PubMed] [Google Scholar]
- Kirchmair R., Gee P., Hogue-Angeletti R., Laslop A., Fischer-Colbrie R., Winkler H. Immunological characterization of the endoproteases PC1 and PC2 in adrenal chromaffin granules and in the pituitary gland. FEBS Lett. 1992 Feb 10;297(3):302–305. doi: 10.1016/0014-5793(92)80560-4. [DOI] [PubMed] [Google Scholar]
- Kirchmair R., Hogue-Angeletti R., Gutierrez J., Fischer-Colbrie R., Winkler H. Secretoneurin--a neuropeptide generated in brain, adrenal medulla and other endocrine tissues by proteolytic processing of secretogranin II (chromogranin C). Neuroscience. 1993 Mar;53(2):359–365. doi: 10.1016/0306-4522(93)90200-y. [DOI] [PubMed] [Google Scholar]
- Larsen P. J., Bersani M., Holst J. J., Møller M., Mikkelsen J. D. Distribution and characterization of different molecular products of pro-somatostatin in the hypothalamus and posterior pituitary lobe of the Mongolian gerbil (Meriones unguiculatus). J Neurosci. 1992 Mar;12(3):946–961. doi: 10.1523/JNEUROSCI.12-03-00946.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahata S. K., Mahata M., Marksteiner J., Sperk G., Fischer-Colbrie R., Winkler H. Distribution of mRNAs for Chromogranins A and B and Secretogranin II in Rat Brain. Eur J Neurosci. 1991;3(9):895–904. doi: 10.1111/j.1460-9568.1991.tb00101.x. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz M., Benjannet S., Seidah N. G., Cantin M., Chrétien M. Immunocytochemical localization of a novel pituitary protein (7B2) within the rat brain and hypophysis. J Histochem Cytochem. 1985 Dec;33(12):1219–1226. doi: 10.1177/33.12.4067275. [DOI] [PubMed] [Google Scholar]
- Metz-Boutigue M. H., Garcia-Sablone P., Hogue-Angeletti R., Aunis D. Intracellular and extracellular processing of chromogranin A. Determination of cleavage sites. Eur J Biochem. 1993 Oct 1;217(1):247–257. doi: 10.1111/j.1432-1033.1993.tb18240.x. [DOI] [PubMed] [Google Scholar]
- Nolan J. A., Trojanowski J. Q., Hogue-Angeletti R. Neurons and neuroendocrine cells contain chromogranin: detection of the molecule in normal bovine tissues by immunochemical and immunohistochemical methods. J Histochem Cytochem. 1985 Aug;33(8):791–798. doi: 10.1177/33.8.3894497. [DOI] [PubMed] [Google Scholar]
- O'Connor D. T. Chromogranin: widespread immunoreactivity in polypeptide hormone producing tissues and in serum. Regul Pept. 1983 Jul;6(3):263–280. doi: 10.1016/0167-0115(83)90145-3. [DOI] [PubMed] [Google Scholar]
- O'Connor D. T., Frigon R. P. Chromogranin A, the major catecholamine storage vesicle soluble protein. Multiple size forms, subcellular storage, and regional distribution in chromaffin and nervous tissue elucidated by radioimmunoassay. J Biol Chem. 1984 Mar 10;259(5):3237–3247. [PubMed] [Google Scholar]
- O'Connor D. T., Parmer R. J., Deftos L. J. Chromogranin A: studies in the endocrine system. Trans Assoc Am Physicians. 1984;97:242–250. [PubMed] [Google Scholar]
- Rosa P., Fumagalli G., Zanini A., Huttner W. B. The major tyrosine-sulfated protein of the bovine anterior pituitary is a secretory protein present in gonadotrophs, thyrotrophs, mammotrophs, and corticotrophs. J Cell Biol. 1985 Mar;100(3):928–937. doi: 10.1083/jcb.100.3.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundle S., Somogyi P., Fischer-Colbrie R., Hagn C., Winkler H., Chubb I. W. Chromogranin A, B and C: immunohistochemical localization in ovine pituitary and the relationship with hormone-containing cells. Regul Pept. 1986 Dec 30;16(3-4):217–233. doi: 10.1016/0167-0115(86)90021-2. [DOI] [PubMed] [Google Scholar]
- Russell J. T., Brownstein M. J., Gainer H. Time course of appearance and release of [35S]cysteine labelled neurophysins and peptides in the neurohypophysis. Brain Res. 1981 Feb 2;205(2):299–311. doi: 10.1016/0006-8993(81)90341-3. [DOI] [PubMed] [Google Scholar]
- Schmidt W. E., Siegel E. G., Lamberts R., Gallwitz B., Creutzfeldt W. Pancreastatin: molecular and immunocytochemical characterization of a novel peptide in porcine and human tissues. Endocrinology. 1988 Sep;123(3):1395–1404. doi: 10.1210/endo-123-3-1395. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Day R., Chrétien M. The family of pro-hormone and pro-protein convertases. Biochem Soc Trans. 1993 Aug;21(3):685–691. doi: 10.1042/bst0210685. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Marcinkiewicz M., Benjannet S., Gaspar L., Beaubien G., Mattei M. G., Lazure C., Mbikay M., Chrétien M. Cloning and primary sequence of a mouse candidate prohormone convertase PC1 homologous to PC2, Furin, and Kex2: distinct chromosomal localization and messenger RNA distribution in brain and pituitary compared to PC2. Mol Endocrinol. 1991 Jan;5(1):111–122. doi: 10.1210/mend-5-1-111. [DOI] [PubMed] [Google Scholar]
- Smeekens S. P., Avruch A. S., LaMendola J., Chan S. J., Steiner D. F. Identification of a cDNA encoding a second putative prohormone convertase related to PC2 in AtT20 cells and islets of Langerhans. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):340–344. doi: 10.1073/pnas.88.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P., Hodgson A. J., DePotter R. W., Fischer-Colbrie R., Schober M., Winkler H., Chubb I. W. Chromogranin immunoreactivity in the central nervous system. Immunochemical characterisation, distribution and relationship to catecholamine and enkephalin pathways. Brain Res. 1984 Dec;320(2-3):193–230. doi: 10.1016/0165-0173(84)90007-9. [DOI] [PubMed] [Google Scholar]
- Tatemoto K., Efendić S., Mutt V., Makk G., Feistner G. J., Barchas J. D. Pancreastatin, a novel pancreatic peptide that inhibits insulin secretion. Nature. 1986 Dec 4;324(6096):476–478. doi: 10.1038/324476a0. [DOI] [PubMed] [Google Scholar]
- Wand G. S., Takiyyuddin M., O'Connor D. T., Levine M. A. A proposed role for chromogranin A as a glucocorticoid-responsive autocrine inhibitor of proopiomelanocortin secretion. Endocrinology. 1991 Mar;128(3):1345–1351. doi: 10.1210/endo-128-3-1345. [DOI] [PubMed] [Google Scholar]
- Watkinson A., Jönsson A. C., Davison M., Young J., Lee C. M., Moore S., Dockray G. J. Heterogeneity of chromogranin A-derived peptides in bovine gut, pancreas and adrenal medulla. Biochem J. 1991 Jun 1;276(Pt 2):471–479. doi: 10.1042/bj2760471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler R., Marksteiner J., Bellmann R., Wohlfarter T., Schober M., Fischer-Colbrie R., Sperk G., Winkler H. Chromogranins in rat brain: characterization, topographical distribution and regulation of synthesis. Brain Res. 1990 Nov 5;532(1-2):87–94. doi: 10.1016/0006-8993(90)91746-4. [DOI] [PubMed] [Google Scholar]
- Winkler H., Fischer-Colbrie R. The chromogranins A and B: the first 25 years and future perspectives. Neuroscience. 1992 Aug;49(3):497–528. doi: 10.1016/0306-4522(92)90222-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarter T., Fischer-Colbrie R., Hogue-Angeletti R., Eiden L. E., Winkler H. Processing of chromogranin A within chromaffin granules starts at C- and N-terminal cleavage sites. FEBS Lett. 1988 Apr 11;231(1):67–70. doi: 10.1016/0014-5793(88)80704-x. [DOI] [PubMed] [Google Scholar]