Abstract
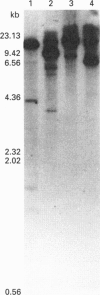
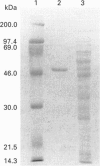
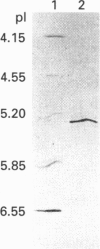
A human brain cDNA clone encoding glutathione synthetase (EC 6.3.2.3) has been sequenced and expressed in Escherichia coli. The protein is 474 amino acids in length with a subunit molecular mass of 52,352 Da. The recombinant protein exhibits glutathione synthetase activity and occurs as a homodimer. The recombinant glutathione synthetase was purified to homogeneity and had a specific activity of 1.73 mumol/min per mg of protein, an isoelectric point of 5.35 and a pH optimum between 7.0 and 7.5. Southern blots of human genomic DNA hybridized with the glutathione synthetase cDNA revealed a relatively simple pattern of strongly hybridizing fragments, indicating the absence of a large gene family and suggesting that there may be only one glutathione synthetase gene in the human genome.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. D., Kerlavage A. R., Fields C., Venter J. C. 3,400 new expressed sequence tags identify diversity of transcripts in human brain. Nat Genet. 1993 Jul;4(3):256–267. doi: 10.1038/ng0793-256. [DOI] [PubMed] [Google Scholar]
- Baker R. T., Smith S. A., Marano R., McKee J., Board P. G. Protein expression using cotranslational fusion and cleavage of ubiquitin. Mutagenesis of the glutathione-binding site of human Pi class glutathione S-transferase. J Biol Chem. 1994 Oct 14;269(41):25381–25386. [PubMed] [Google Scholar]
- Beutler E., Gelbart T., Pegelow C. Erythrocyte glutathione synthetase deficiency leads not only to glutathione but also to glutathione-S-transferase deficiency. J Clin Invest. 1986 Jan;77(1):38–41. doi: 10.1172/JCI112298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E., Mathai C. K., Smith J. E. Biochemical variants of glucose-6-phosphate dehydrogenase giving rise to congenital nonspherocytic hemolytic disease. Blood. 1968 Feb;31(2):131–150. [PubMed] [Google Scholar]
- Board P. G., Pierce K. Expression of human glutathione S-transferase 2 in Escherichia coli. Immunological comparison with the basic glutathione S-transferases isoenzymes from human liver. Biochem J. 1987 Dec 15;248(3):937–941. doi: 10.1042/bj2480937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Gushima H., Miya T., Murata K., Kimura A. Purification and characterization of glutathione synthetase from Escherichia coli B. J Appl Biochem. 1983 Jun;5(3):210–218. [PubMed] [Google Scholar]
- Gushima H., Yasuda S., Soeda E., Yokota M., Kondo M., Kimura A. Complete nucleotide sequence of the E. coli glutathione synthetase gsh-II. Nucleic Acids Res. 1984 Dec 21;12(24):9299–9307. doi: 10.1093/nar/12.24.9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habenicht A., Hille S., Knöchel W. Molecular cloning of the large subunit of glutathione synthetase from Xenopus laevis embryos. Biochim Biophys Acta. 1993 Sep 23;1174(3):295–298. doi: 10.1016/0167-4781(93)90202-o. [DOI] [PubMed] [Google Scholar]
- Hibi T., Kato H., Nishioka T., Oda J., Yamaguchi H., Katsube Y., Tanizawa K., Fukui T. Use of adenosine (5')polyphospho(5')pyridoxals to study the substrate-binding region of glutathione synthetase from Escherichia coli B. Biochemistry. 1993 Feb 16;32(6):1548–1554. doi: 10.1021/bi00057a020. [DOI] [PubMed] [Google Scholar]
- Huang C. S., He W., Meister A., Anderson M. E. Amino acid sequence of rat kidney glutathione synthetase. Proc Natl Acad Sci U S A. 1995 Feb 14;92(4):1232–1236. doi: 10.1073/pnas.92.4.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Majerus P. W., Brauner M. J., Smith M. B., Minnich V. Glutathione synthesis in human erythrocytes. II. Purification and properties of the enzymes of glutathione biosynthesis. J Clin Invest. 1971 Aug;50(8):1637–1643. doi: 10.1172/JCI106652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Meister A. Glutathione synthetase from rat kidney. Methods Enzymol. 1985;113:393–399. doi: 10.1016/s0076-6879(85)13052-1. [DOI] [PubMed] [Google Scholar]
- Mooz E. D., Meister A. Tripeptide (glutathione) synthetase. Purification, properties, and mechanism of action. Biochemistry. 1967 Jun;6(6):1722–1734. doi: 10.1021/bi00858a022. [DOI] [PubMed] [Google Scholar]
- Mutoh N., Nakagawa C. W., Ando S., Tanabe K., Hayashi Y. Cloning and sequencing of the gene encoding the large subunit of glutathione synthetase of Schizosaccharomyces pombe. Biochem Biophys Res Commun. 1991 Nov 27;181(1):430–436. doi: 10.1016/s0006-291x(05)81437-8. [DOI] [PubMed] [Google Scholar]
- Nakagawa C. W., Mutoh N., Hayashi Y. Glutathione synthetase from the fission yeast. Purification and its unique heteromeric subunit structure. Biochem Cell Biol. 1993 Sep-Oct;71(9-10):447–453. doi: 10.1139/o93-066. [DOI] [PubMed] [Google Scholar]
- Nasmyth K. A. The regulation of yeast mating-type chromatin structure by SIR: an action at a distance affecting both transcription and transposition. Cell. 1982 Sep;30(2):567–578. doi: 10.1016/0092-8674(82)90253-7. [DOI] [PubMed] [Google Scholar]
- Oppenheimer L., Wellner V. P., Griffith O. W., Meister A. Glutathione synthetase. Purification from rat kidney and mapping of the substrate binding sites. J Biol Chem. 1979 Jun 25;254(12):5184–5190. [PubMed] [Google Scholar]
- Peters J. M., Dalrymple B. P., Jorgensen W. K. Sequence of a putative glutathione synthetase II gene and flanking regions from Anaplasma centrale. Biochem Biophys Res Commun. 1992 Feb 14;182(3):1040–1046. doi: 10.1016/0006-291x(92)91836-f. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Prchal J. T., Crist W. M., Roper M., Wellner V. P. Hemolytic anemia, recurrent metabolic acidosis, and incomplete albinism associated with glutathione synthetase deficiency. Blood. 1983 Oct;62(4):754–757. [PubMed] [Google Scholar]
- Rathbun W. B., Sethna S. S., Van Buskirk G. Purification and properties of glutathione synthetase from bovine lens. Exp Eye Res. 1977 Feb;24(2):145–158. doi: 10.1016/0014-4835(77)90255-x. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Saraste M., Sibbald P. R., Wittinghofer A. The P-loop--a common motif in ATP- and GTP-binding proteins. Trends Biochem Sci. 1990 Nov;15(11):430–434. doi: 10.1016/0968-0004(90)90281-f. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Kato H., Nishioka T., Oda J. Mutational and proteolytic studies on a flexible loop in glutathione synthetase from Escherichia coli B: the loop and arginine 233 are critical for the catalytic reaction. Biochemistry. 1992 Mar 3;31(8):2259–2265. doi: 10.1021/bi00123a007. [DOI] [PubMed] [Google Scholar]
- Wellner V. P., Sekura R., Meister A., Larsson A. Glutathione synthetase deficiency, an inborn error of metabolism involving the gamma-glutamyl cycle in patients with 5-oxoprolinuria (pyroglutamic aciduria). Proc Natl Acad Sci U S A. 1974 Jun;71(6):2505–2509. doi: 10.1073/pnas.71.6.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]