Abstract
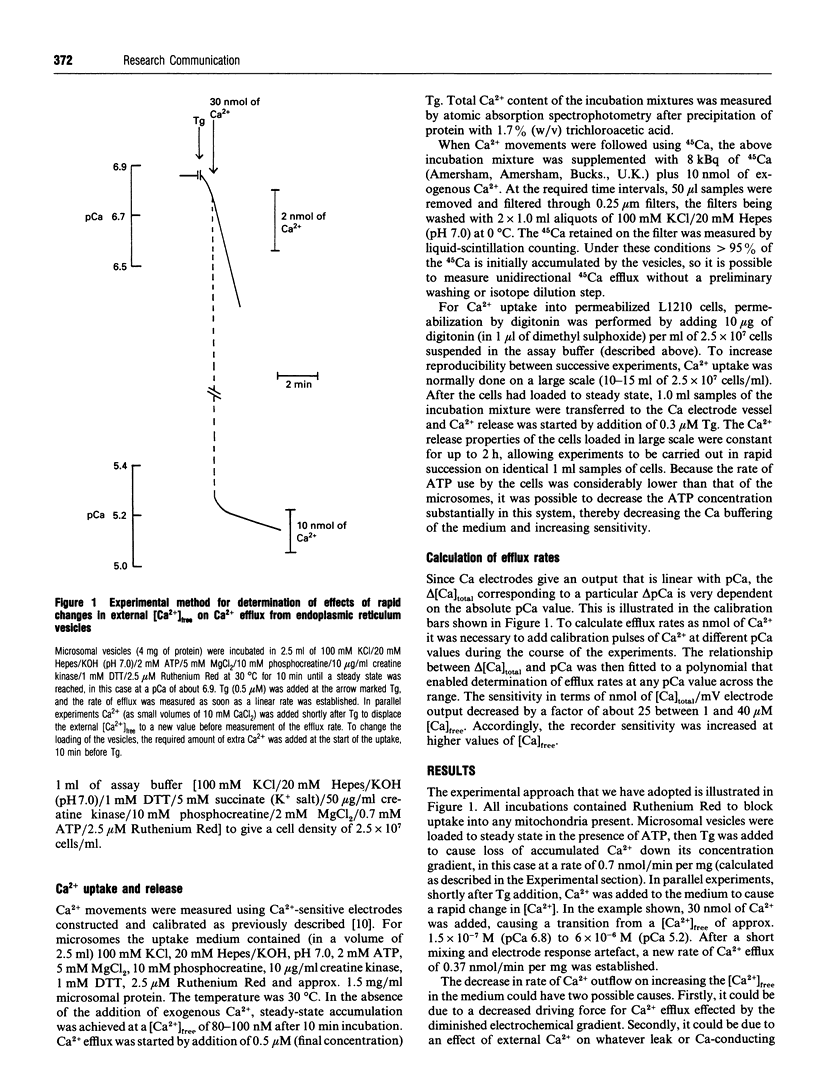
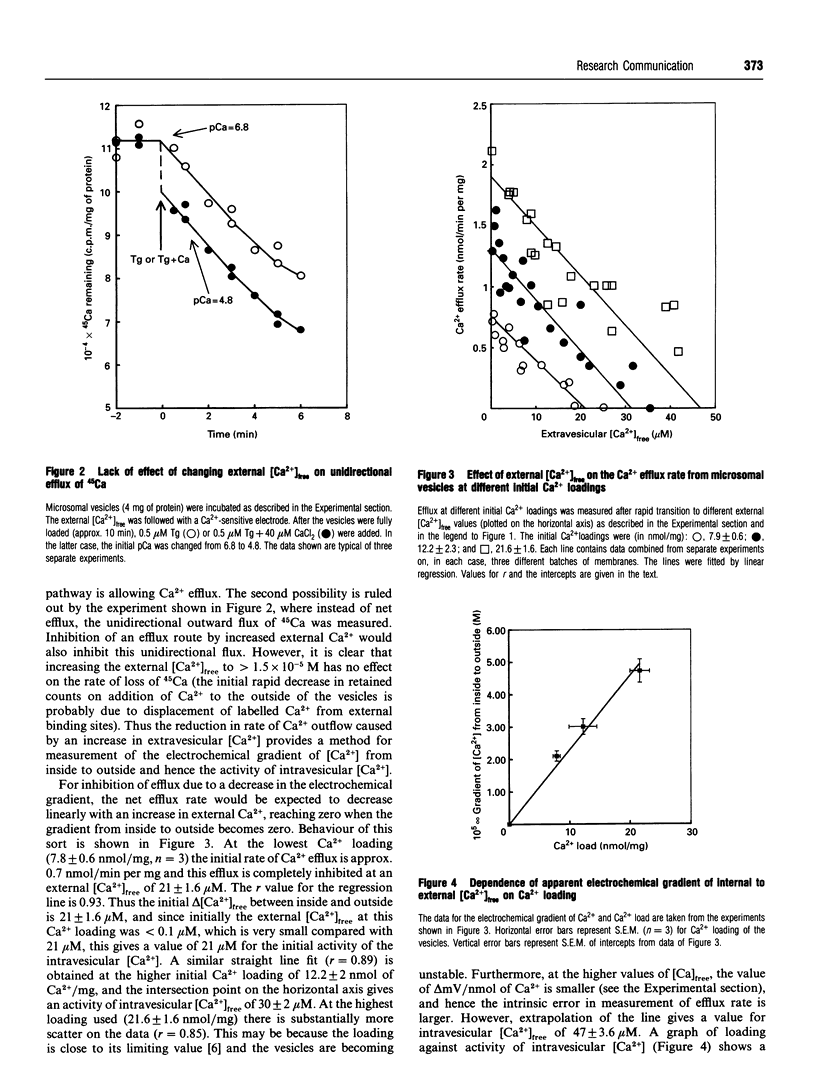
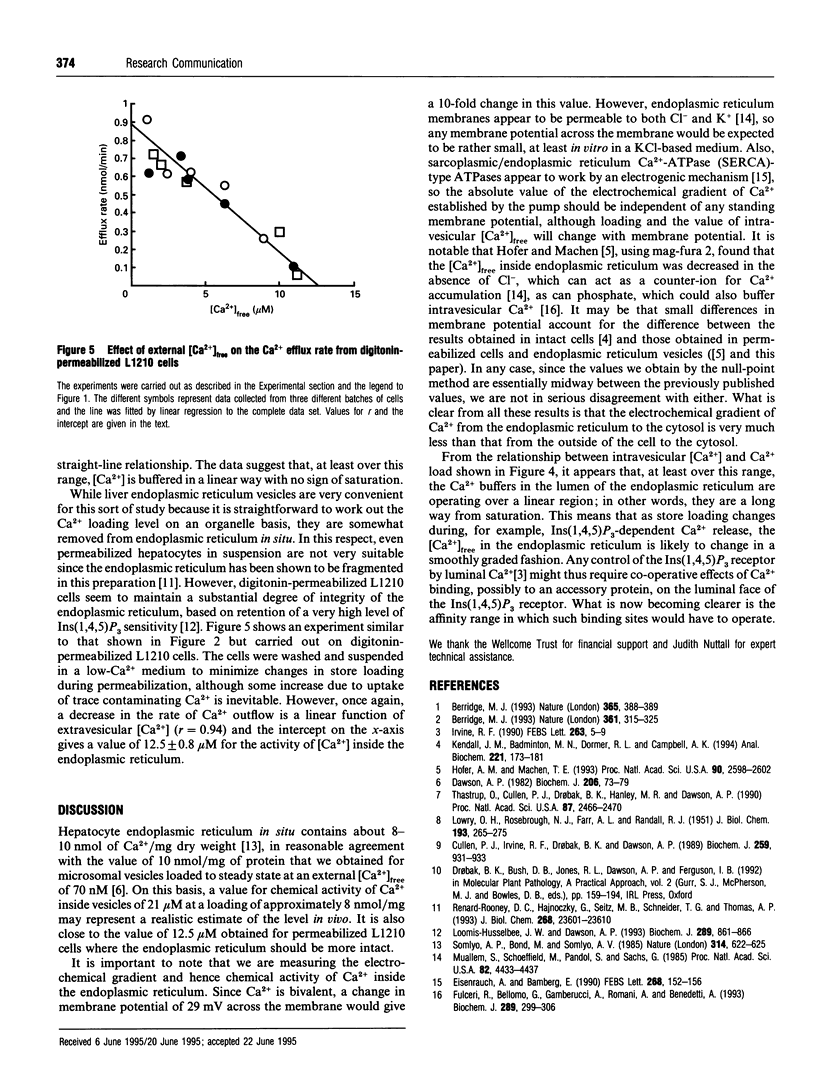
The rate of unidirectional efflux of 45Ca from rat liver microsomal vesicles loaded with 45Ca and then treated with thapsigargin is not inhibited by increased [Ca2+] in the external medium, although the net efflux rate is substantially inhibited. We have used this property to measure the electrochemical gradient of Ca2+ from the inside to the outside of the vesicles at a series of Ca2+ loadings, by measuring the external [Ca2+]free at which there is zero net efflux. At a loading of 7.9 +/- 0.6 nmol/mg of microsomal protein, the apparent internal [Ca2+]free is 21 +/- 1.6 microM. As the loading is increased, the internal [Ca2+]free increases linearly up to a value of 47 +/- 3.6 microM at a loading of 21.6 +/- 1.6 nmol/mg. Using a similar technique, the value for [Ca2+]free in the endoplasmic reticulum of permeabilized L1210 cells was found to be 12.5 microM.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Cell signalling. A tale of two messengers. Nature. 1993 Sep 30;365(6445):388–389. doi: 10.1038/365388a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Cullen P. J., Irvine R. F., Drøbak B. K., Dawson A. P. Inositol 1,3,4,5-tetrakisphosphate causes release of Ca2+ from permeabilized mouse lymphoma L1210 cells by its conversion into inositol 1,4,5-trisphosphate. Biochem J. 1989 May 1;259(3):931–933. doi: 10.1042/bj2590931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. P. Kinetic properties of the Ca2+-accumulation system of a rat liver microsomal fraction. Biochem J. 1982 Jul 15;206(1):73–79. doi: 10.1042/bj2060073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenrauch A., Bamberg E. Voltage-dependent pump currents of the sarcoplasmic reticulum Ca2(+)-ATPase in planar lipid membranes. FEBS Lett. 1990 Jul 30;268(1):152–156. doi: 10.1016/0014-5793(90)80996-v. [DOI] [PubMed] [Google Scholar]
- Fulceri R., Bellomo G., Gamberucci A., Romani A., Benedetti A. Physiological concentrations of inorganic phosphate affect MgATP-dependent Ca2+ storage and inositol trisphosphate-induced Ca2+ efflux in microsomal vesicles from non-hepatic cells. Biochem J. 1993 Jan 1;289(Pt 1):299–306. doi: 10.1042/bj2890299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer A. M., Machen T. E. Technique for in situ measurement of calcium in intracellular inositol 1,4,5-trisphosphate-sensitive stores using the fluorescent indicator mag-fura-2. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2598–2602. doi: 10.1073/pnas.90.7.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F. 'Quantal' Ca2+ release and the control of Ca2+ entry by inositol phosphates--a possible mechanism. FEBS Lett. 1990 Apr 9;263(1):5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- Kendall J. M., Badminton M. N., Dormer R. L., Campbell A. K. Changes in free calcium in the endoplasmic reticulum of living cells detected using targeted aequorin. Anal Biochem. 1994 Aug 15;221(1):173–181. doi: 10.1006/abio.1994.1394. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loomis-Husselbee J. W., Dawson A. P. A steady-state mechanism can account for the properties of inositol 2,4,5-trisphosphate-stimulated Ca2+ release from permeabilized L1210 cells. Biochem J. 1993 Feb 1;289(Pt 3):861–866. doi: 10.1042/bj2890861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muallem S., Schoeffield M., Pandol S., Sachs G. Inositol trisphosphate modification of ion transport in rough endoplasmic reticulum. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4433–4437. doi: 10.1073/pnas.82.13.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard-Rooney D. C., Hajnóczky G., Seitz M. B., Schneider T. G., Thomas A. P. Imaging of inositol 1,4,5-trisphosphate-induced Ca2+ fluxes in single permeabilized hepatocytes. Demonstration of both quantal and nonquantal patterns of Ca2+ release. J Biol Chem. 1993 Nov 5;268(31):23601–23610. [PubMed] [Google Scholar]
- Somlyo A. P., Bond M., Somlyo A. V. Calcium content of mitochondria and endoplasmic reticulum in liver frozen rapidly in vivo. Nature. 1985 Apr 18;314(6012):622–625. doi: 10.1038/314622a0. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]


