Abstract
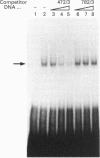
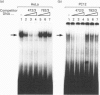
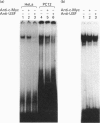
We demonstrate the presence of a functional E box motif in the proximal rat preprotachykinin-A (rPPT) promoter. This element (spanning nucleotides -67 to -47) exhibits the sequence 5'-CACGTG-3' which is recognized and bound by the basic helix-loop-helix family of regulatory proteins. We also show that at least one of the factors bound to this rPPT promoter element in both HeLa and PC12 nuclear extract is the ubiquitously expressed transcription factor, the upstream stimulatory factor (USF). Mutation of this element by insertion of a 10 bp linker into the E box motif, in an rPPT promoter fragment spanning -865 to +92, destroys the ability of this promoter fragment to support reporter gene expression in a PC12 cell model of rPPT promoter activity. The data indicate that this rPPT E box element is likely to function as an important cis-regulatory domain in the rPPT promoter.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball D. W., Compton D., Nelkin B. D., Baylin S. B., de Bustros A. Human calcitonin gene regulation by helix-loop-helix recognition sequences. Nucleic Acids Res. 1992 Jan 11;20(1):117–123. doi: 10.1093/nar/20.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley C. G., Lipkowitz S., Göbel V., Mahon K. A., Bertness V., Green A. R., Gough N. M., Kirsch I. R. Molecular characterization of NSCL, a gene encoding a helix-loop-helix protein expressed in the developing nervous system. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):38–42. doi: 10.1073/pnas.89.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengal E., Ransone L., Scharfmann R., Dwarki V. J., Tapscott S. J., Weintraub H., Verma I. M. Functional antagonism between c-Jun and MyoD proteins: a direct physical association. Cell. 1992 Feb 7;68(3):507–519. doi: 10.1016/0092-8674(92)90187-h. [DOI] [PubMed] [Google Scholar]
- Blackwood E. M., Eisenman R. N. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science. 1991 Mar 8;251(4998):1211–1217. doi: 10.1126/science.2006410. [DOI] [PubMed] [Google Scholar]
- Blackwood E. M., Kretzner L., Eisenman R. N. Myc and Max function as a nucleoprotein complex. Curr Opin Genet Dev. 1992 Apr;2(2):227–235. doi: 10.1016/s0959-437x(05)80278-3. [DOI] [PubMed] [Google Scholar]
- Brown E. R., Roth K. A., Krause J. E. Sexually dimorphic distribution of substance P in specific anterior pituitary cell populations. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1222–1226. doi: 10.1073/pnas.88.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter M. S., Krause J. E. Structure, expression, and some regulatory mechanisms of the rat preprotachykinin gene encoding substance P, neurokinin A, neuropeptide K, and neuropeptide gamma. J Neurosci. 1990 Jul;10(7):2203–2214. doi: 10.1523/JNEUROSCI.10-07-02203.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthew R. W., Chodosh L. A., Sharp P. A. An RNA polymerase II transcription factor binds to an upstream element in the adenovirus major late promoter. Cell. 1985 Dec;43(2 Pt 1):439–448. doi: 10.1016/0092-8674(85)90174-6. [DOI] [PubMed] [Google Scholar]
- Chapman K., Lyons V., Harmar A. J. The sequence of 5' flanking DNA from the rat preprotachykinin gene; analysis of putative transcription factor binding sites. Biochim Biophys Acta. 1993 Mar 20;1172(3):361–363. doi: 10.1016/0167-4781(93)90233-4. [DOI] [PubMed] [Google Scholar]
- Chiwakata C., Brackmann B., Hunt N., Davidoff M., Schulze W., Ivell R. Tachykinin (substance-P) gene expression in Leydig cells of the human and mouse testis. Endocrinology. 1991 May;128(5):2441–2448. doi: 10.1210/endo-128-5-2441. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Martin P. L., Shastry B. S., Roeder R. G. Eukaryotic gene transcription with purified components. Methods Enzymol. 1983;101:582–598. doi: 10.1016/0076-6879(83)01039-3. [DOI] [PubMed] [Google Scholar]
- Ericsson A., Geenen V., Robert F., Legros J. J., Vrindts-Gevaert Y., Franchimont P., Brene S., Persson H. Expression of preprotachykinin-A and neuropeptide-Y messenger RNA in the thymus. Mol Endocrinol. 1990 Aug;4(8):1211–1218. doi: 10.1210/mend-4-8-1211. [DOI] [PubMed] [Google Scholar]
- Fisher F., Crouch D. H., Jayaraman P. S., Clark W., Gillespie D. A., Goding C. R. Transcription activation by Myc and Max: flanking sequences target activation to a subset of CACGTG motifs in vivo. EMBO J. 1993 Dec 15;12(13):5075–5082. doi: 10.1002/j.1460-2075.1993.tb06201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor P. D., Sawadogo M., Roeder R. G. The adenovirus major late transcription factor USF is a member of the helix-loop-helix group of regulatory proteins and binds to DNA as a dimer. Genes Dev. 1990 Oct;4(10):1730–1740. doi: 10.1101/gad.4.10.1730. [DOI] [PubMed] [Google Scholar]
- He X., Rosenfeld M. G. Mechanisms of complex transcriptional regulation: implications for brain development. Neuron. 1991 Aug;7(2):183–196. doi: 10.1016/0896-6273(91)90257-z. [DOI] [PubMed] [Google Scholar]
- Helke C. J., Krause J. E., Mantyh P. W., Couture R., Bannon M. J. Diversity in mammalian tachykinin peptidergic neurons: multiple peptides, receptors, and regulatory mechanisms. FASEB J. 1990 Apr 1;4(6):1606–1615. [PubMed] [Google Scholar]
- Hurd Y. L., Brown E. E., Finlay J. M., Fibiger H. C., Gerfen C. R. Cocaine self-administration differentially alters mRNA expression of striatal peptides. Brain Res Mol Brain Res. 1992 Mar;13(1-2):165–170. doi: 10.1016/0169-328x(92)90058-j. [DOI] [PubMed] [Google Scholar]
- Johnson J. E., Birren S. J., Anderson D. J. Two rat homologues of Drosophila achaete-scute specifically expressed in neuronal precursors. Nature. 1990 Aug 30;346(6287):858–861. doi: 10.1038/346858a0. [DOI] [PubMed] [Google Scholar]
- Johnson J. E., Birren S. J., Saito T., Anderson D. J. DNA binding and transcriptional regulatory activity of mammalian achaete-scute homologous (MASH) proteins revealed by interaction with a muscle-specific enhancer. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3596–3600. doi: 10.1073/pnas.89.8.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler J. A., Black I. B. Nerve growth factor stimulates development of substance P in the embryonic spinal cord. Brain Res. 1981 Mar 9;208(1):135–145. doi: 10.1016/0006-8993(81)90626-0. [DOI] [PubMed] [Google Scholar]
- Kuramoto H., Kondo H., Fujita T. Substance P-like immunoreactivity in adrenal chromaffin cells and intra-adrenal nerve fibers of rats. Histochemistry. 1985;82(6):507–512. doi: 10.1007/BF00489970. [DOI] [PubMed] [Google Scholar]
- Lenardo M., Pierce J. W., Baltimore D. Protein-binding sites in Ig gene enhancers determine transcriptional activity and inducibility. Science. 1987 Jun 19;236(4808):1573–1577. doi: 10.1126/science.3109035. [DOI] [PubMed] [Google Scholar]
- Lindsay R. M., Harmar A. J. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature. 1989 Jan 26;337(6205):362–364. doi: 10.1038/337362a0. [DOI] [PubMed] [Google Scholar]
- Lipkowitz S., Göbel V., Varterasian M. L., Nakahara K., Tchorz K., Kirsch I. R. A comparative structural characterization of the human NSCL-1 and NSCL-2 genes. Two basic helix-loop-helix genes expressed in the developing nervous system. J Biol Chem. 1992 Oct 15;267(29):21065–21071. [PubMed] [Google Scholar]
- Lüscher B., Eisenman R. N. New light on Myc and Myb. Part I. Myc. Genes Dev. 1990 Dec;4(12A):2025–2035. doi: 10.1101/gad.4.12a.2025. [DOI] [PubMed] [Google Scholar]
- Maggio J. E. Tachykinins. Annu Rev Neurosci. 1988;11:13–28. doi: 10.1146/annurev.ne.11.030188.000305. [DOI] [PubMed] [Google Scholar]
- Mendelson S. C., Morrison C. F., McAllister J., Paterson J. M., Dobson S. P., Mulderry P. K., Quinn J. P. Repression of preprotachykinin-A promoter activity is mediated by a proximal promoter element. Neuroscience. 1995 Apr;65(3):837–847. doi: 10.1016/0306-4522(94)00554-i. [DOI] [PubMed] [Google Scholar]
- Mendelson S. C., Quinn J. P. Characterisation of potential regulatory elements within the rat preprotachykinin-A promoter. Neurosci Lett. 1995 Jan 23;184(2):125–128. doi: 10.1016/0304-3940(94)11186-m. [DOI] [PubMed] [Google Scholar]
- Miyamoto N. G., Moncollin V., Egly J. M., Chambon P. Specific interaction between a transcription factor and the upstream element of the adenovirus-2 major late promoter. EMBO J. 1985 Dec 16;4(13A):3563–3570. doi: 10.1002/j.1460-2075.1985.tb04118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori N., Schoenherr C., Vandenbergh D. J., Anderson D. J. A common silencer element in the SCG10 and type II Na+ channel genes binds a factor present in nonneuronal cells but not in neuronal cells. Neuron. 1992 Jul;9(1):45–54. doi: 10.1016/0896-6273(92)90219-4. [DOI] [PubMed] [Google Scholar]
- Morrison C. F., McAllister J., Dobson S. P., Mulderry P. K., Quinn J. P. An activator element within the preprotachykinin-A promoter. Mol Cell Neurosci. 1994 Apr;5(2):165–175. doi: 10.1006/mcne.1994.1018. [DOI] [PubMed] [Google Scholar]
- Morrison C. F., McAllister J., Lyons V., Chapman K., Quinn J. P. The rat preprotachykinin-A promoter is regulated in PC12 cells by the synergistic action of multiple stimuli. Neurosci Lett. 1994 Nov 7;181(1-2):117–120. doi: 10.1016/0304-3940(94)90573-8. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Vaessin H., Caudy M., Jan L. Y., Jan Y. N., Cabrera C. V., Buskin J. N., Hauschka S. D., Lassar A. B. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989 Aug 11;58(3):537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- Navankasattusas S., Sawadogo M., van Bilsen M., Dang C. V., Chien K. R. The basic helix-loop-helix protein upstream stimulating factor regulates the cardiac ventricular myosin light-chain 2 gene via independent cis regulatory elements. Mol Cell Biol. 1994 Nov;14(11):7331–7339. doi: 10.1128/mcb.14.11.7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawa H., Patterson P. H. Separation and partial characterization of neuropeptide-inducing factors in heart cell conditioned medium. Neuron. 1990 Feb;4(2):269–277. doi: 10.1016/0896-6273(90)90101-k. [DOI] [PubMed] [Google Scholar]
- Nawa H., Sah D. W. Different biological activities in conditioned media control the expression of a variety of neuropeptides in cultured sympathetic neurons. Neuron. 1990 Feb;4(2):279–287. doi: 10.1016/0896-6273(90)90102-l. [DOI] [PubMed] [Google Scholar]
- Noguchi K., Ruda M. A. Gene regulation in an ascending nociceptive pathway: inflammation-induced increase in preprotachykinin mRNA in rat lamina I spinal projection neurons. J Neurosci. 1992 Jul;12(7):2563–2572. doi: 10.1523/JNEUROSCI.12-07-02563.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson J. M., Mendelson S. C., McAllister J., Morrison C. F., Dobson S., Grace C., Quinn J. P. Three immediate early gene response elements in the proximal preprotachykinin-A promoter in two functionally distinct domains. Neuroscience. 1995 Jun;66(4):921–932. doi: 10.1016/0306-4522(95)00041-g. [DOI] [PubMed] [Google Scholar]
- Peleg S., Abruzzese R. V., Cooper C. W., Gagel R. F. Down-regulation of calcitonin gene transcription by vitamin D requires two widely separated enhancer sequences. Mol Endocrinol. 1993 Aug;7(8):999–1008. doi: 10.1210/mend.7.8.8232320. [DOI] [PubMed] [Google Scholar]
- Read M. L., Clark A. R., Docherty K. The helix-loop-helix transcription factor USF (upstream stimulating factor) binds to a regulatory sequence of the human insulin gene enhancer. Biochem J. 1993 Oct 1;295(Pt 1):233–237. doi: 10.1042/bj2950233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985 Nov;43(1):165–175. doi: 10.1016/0092-8674(85)90021-2. [DOI] [PubMed] [Google Scholar]
- Singh H., Sen R., Baltimore D., Sharp P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Sirito M., Lin Q., Maity T., Sawadogo M. Ubiquitous expression of the 43- and 44-kDa forms of transcription factor USF in mammalian cells. Nucleic Acids Res. 1994 Feb 11;22(3):427–433. doi: 10.1093/nar/22.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon D. L., Amati B., Land H. Distinct DNA binding preferences for the c-Myc/Max and Max/Max dimers. Nucleic Acids Res. 1993 Nov 25;21(23):5372–5376. doi: 10.1093/nar/21.23.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein B., Baldwin A. S., Jr, Ballard D. W., Greene W. C., Angel P., Herrlich P. Cross-coupling of the NF-kappa B p65 and Fos/Jun transcription factors produces potentiated biological function. EMBO J. 1993 Oct;12(10):3879–3891. doi: 10.1002/j.1460-2075.1993.tb06066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K. Mechanisms for diversity in gene expression patterns. Neuron. 1991 Aug;7(2):177–181. doi: 10.1016/0896-6273(91)90256-y. [DOI] [PubMed] [Google Scholar]
- Symes A. J., Craig R. K., Brickell P. M. Loss of transcriptional repression contributes to the ectopic expression of the calcitonin/alpha-CGRP gene in a human lung carcinoma cell line. FEBS Lett. 1992 Jul 20;306(2-3):229–233. doi: 10.1016/0014-5793(92)81006-8. [DOI] [PubMed] [Google Scholar]
- Tverberg L. A., Russo A. F. Regulation of the calcitonin/calcitonin gene-related peptide gene by cell-specific synergy between helix-loop-helix and octamer-binding transcription factors. J Biol Chem. 1993 Jul 25;268(21):15965–15973. [PubMed] [Google Scholar]
- Vallejo M., Ron D., Miller C. P., Habener J. F. C/ATF, a member of the activating transcription factor family of DNA-binding proteins, dimerizes with CAAT/enhancer-binding proteins and directs their binding to cAMP response elements. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4679–4683. doi: 10.1073/pnas.90.10.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villares R., Cabrera C. V. The achaete-scute gene complex of D. melanogaster: conserved domains in a subset of genes required for neurogenesis and their homology to myc. Cell. 1987 Jul 31;50(3):415–424. doi: 10.1016/0092-8674(87)90495-8. [DOI] [PubMed] [Google Scholar]
- Weinstock J. V., Blum A., Walder J., Walder R. Eosinophils from granulomas in murine schistosomiasis mansoni produce substance P. J Immunol. 1988 Aug 1;141(3):961–966. [PubMed] [Google Scholar]
- Weintraub H., Davis R., Tapscott S., Thayer M., Krause M., Benezra R., Blackwell T. K., Turner D., Rupp R., Hollenberg S. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991 Feb 15;251(4995):761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- Wuenschell C. W., Mori N., Anderson D. J. Analysis of SCG10 gene expression in transgenic mice reveals that neural specificity is achieved through selective derepression. Neuron. 1990 Apr;4(4):595–602. doi: 10.1016/0896-6273(90)90117-x. [DOI] [PubMed] [Google Scholar]
- Yoon S. O., Chikaraishi D. M. Tissue-specific transcription of the rat tyrosine hydroxylase gene requires synergy between an AP-1 motif and an overlapping E box-containing dyad. Neuron. 1992 Jul;9(1):55–67. doi: 10.1016/0896-6273(92)90220-8. [DOI] [PubMed] [Google Scholar]