Abstract
In 2018, an outbreak of human rabies caused by the hematophagous bat Desmodus rotundus hit the Brazilian Amazon Basin community of Melgaço, Brazil, resulting in the death of 10 people, 9 of them children. The incidence of rabies has been on the rise among populations in conditions of vulnerability in this ecosystem due to human expansion into sylvatic environments and limited access to public health services. To address this issue, in September 2019, a collaborative effort from national, local, and international institutions promoted and executed a pilot for pre-exposure prophylaxis of a population in high-risk areas for hematophagous bat-mediated rabies. This measure is usually only implemented in response to outbreaks. The pilot was conducted in Portel, in a nearby location to the previous outbreak, with the use of fluvial transportation, and 2987 individuals in 411 dwellings were successfully vaccinated. It established a methodology for pre-exposure prophylaxis for populations in conditions of vulnerability, identifying logistics and costs, as well as characterizing the target riverine population regarding risk factors associated with bites by hematophagous bats. This approach offers a proactive measure to prevent future outbreaks and provides valuable insights into how to address the issue of rabies in remote and difficult-to-reach areas.
Keywords: rabies, public health policy, hematophagous bat, Desmodus rotundus, riverine population, pre-exposure prophylaxis
1. Introduction
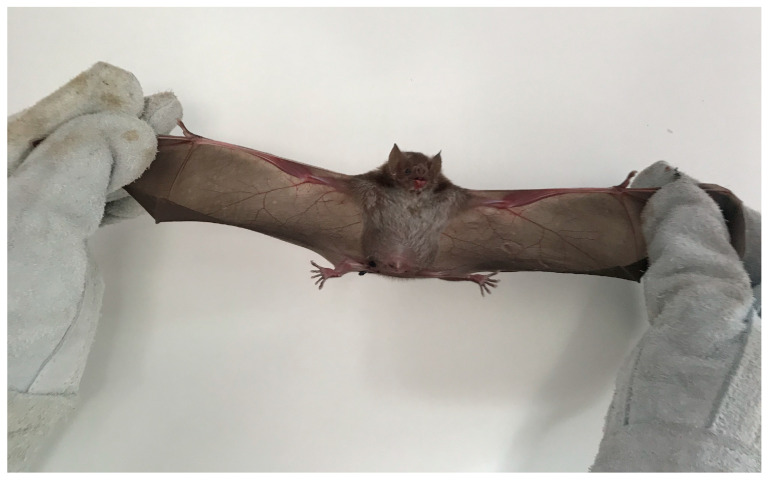
Rabies is an acute zoonotic disease that affects the nervous system and continues to be a major public health concern. While canine rabies is still responsible for 99% of human rabies cases worldwide [1], effective actions and public policies have led to a significant decrease in cases of human rabies mediated by dogs in the Americas [2,3]. As a result of the significant reduction in human rabies mediated by dogs, human rabies through sylvatic cycle has become increasingly apparent, leading to a change in the disease’s epidemiological profile. Rabies mediated by the Desmodus rotundus bat—the common vampire bat (Figure 1)—has emerged as a significant public health issue [4]. D. rotundus is one of the three species of hematophagous bats found only in the Americas [5] and the most abundant and prolific of these species. When feeding, it bites the mammal’s body and licks the blood that flows from the wound on the skin, thereby transmitting rabies since the disease is mainly spread through saliva [6].
Figure 1.
Desmodus rotundus bat captured in the proximity of a dwelling in the Pacajá River in Portel, State of Pará, Brazil. Photo by Felipe Rocha. Portel, 2019.
It is, therefore, crucial to continue investing in public health policies and effective actions to combat the spread of rabies by dogs while also addressing the emerging threat of sylvatic-borne rabies. This requires comprehensive measures to protect at risk populations in conditions of vulnerability, living in remote and difficult-to-reach areas where D. rotundus bats are prevalent, by raising awareness and investing in proactive measures, such as prophylaxis. Sporadically, outbreaks occur in the Amazon region [7,8,9,10,11], and in Brazil, the last occurred in 2018, leading to the death of 10 people—9 of them were children—who were all riverine residents of the Laguna River area in the municipality of Melgaço, State of Pará [8], one of the locations with the lowest Human Development Index (HDI) in Brazil [12].
Riverine communities [13] are those who reside on the banks of rivers in remote regions, far from the public health care and basic sanitation services [14,15] offered in urban areas. These communities are surrounded by wild environments and rely on subsistence activities from their surroundings [13]. Due to their proximity and relationship with the environment, riverine communities face various risks and exposures to infectious diseases (Figure 2). As riverine communities are located on the banks of rivers, their dwellings can only be accessed by fluvial transportation, as there are no roads connecting the dwellings across the Amazon jungle. Humans and domestic animals provide a stable feeding source for the common vampire bat [16], leading to an increase in bat–human interactions and the eventual cases of rabies in these environments (Figure 3).
Figure 2.
Typical riverine population dwelling distributed along the banks of the rivers of the Amazon Basin. Photo by Júlio Pompei. Portel, 2019.
Figure 3.
Scar from Desmodus rotundus bite on the thumb of an 11-year-old child. Photo by Felipe Rocha. Portel, 2019.
In infected people, when symptoms are presented, considerable portions of the central nervous system are already compromised, with irreversible clinical progressing to death. As rabies has no cure, the prophylaxis of human cases is the best strategy against the disease through vaccination. When applied timely, the vaccine is effective in preventing the development of rabies [17].
Rabies vaccination prevents the disease in humans and can be administered in two ways: post-exposure prophylaxis (PEP) and pre-exposure prophylaxis (PrEP), with intramuscular (IM) or intradermal (ID) protocols. While Brazil only recommends PrEP for certain high-risk professions, the World Health Organization (WHO) recommends PrEP for individuals residing in areas at risk of rabies and with limited access to PEP [17]. The Ministry of Health of Brazil has promoted specific measures to provide PrEP to at-risk populations, although it has not incorporated PrEP as a public health measure because of a lack of information on the logistics and methodology needed [18].
To address this gap, a pilot was designed and conducted in September 2019 by the Ministry of Health of Brazil (MH), the Pará State Secretary of Health (SESPA), and the Municipal Health Secretaries of Breves, Melgaço, and Portel in collaboration with the Pan American Center for Foot-and-Mouth Disease and Veterinary Public Health of PAHO/WHO (PANAFTOSA/VPH-PAHO/WHO) and the Institute Pasteur of São Paulo State Secretary of Health (IP). The main objective of the pilot was to establish a methodology to deploy PrEP as a public health policy and identify the necessary logistics and costs involved for its implementation focused on the riverine populations in high-risk areas for rabies mediated by hematophagous bats and with limited access to PEP. Additionally, the pilot aimed to identify risk factors associated with bites by hematophagous bats and immunological status before PrEP of the riverine population in the area. This initiative was crucial in paving the way for future public health efforts to prevent the occurrence of rabies outbreaks in at-risk populations.
2. Materials and Methods
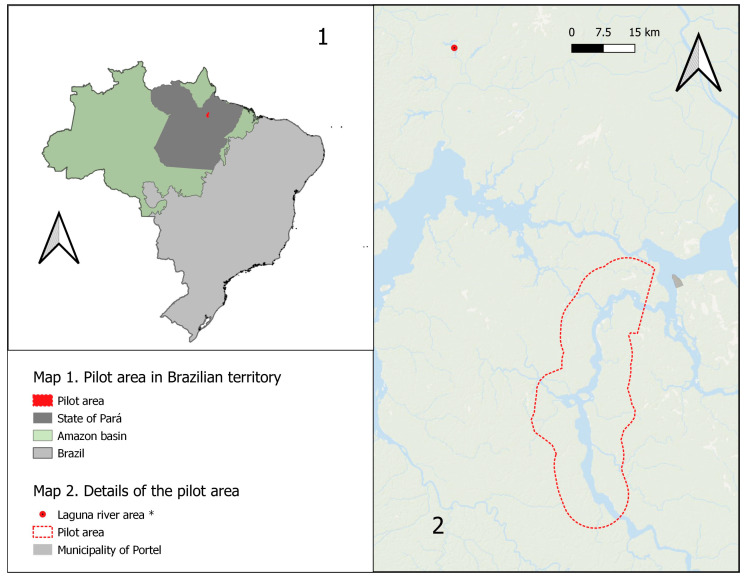
The targeted area of the pilot spanned a 60 km stretch along the Pacajá River in Portel [19], PA, Brazil, and extended up to 8 km along tributary rivers, encompassing a total estimated area of 960 km2, with a total of 59,441.3 km of rivers to navigate (Figure 4). The selection of this area was based on its proximity and ecological and sociocultural resemblance to the region where the last outbreak of human rabies mediated by hematophagous bats occurred in Melgaço, PA, Brazil, in the Laguna River area [8]. It took place between 9th and 25th September, offering the best conditions to navigate the local rivers.
Figure 4.
Pre-exposure prophylaxis pilot area in municipality of Portel, PA, Brazil. * Region where the last outbreak of human rabies mediated by hematophagous bats occurred.
All individuals aged at least 2 years within the designated area received two doses of the VERO rabies vaccine, administered on days zero and seven [17,20,21,22]. Each 0.2 mL ID dose was administered via two applications of 0.1 mL on both forearms. Each vial contained 0.5 mL of vaccine, which was enough to generate two doses of 0.2 mL and half doses of 0.1 mL. The operational base for the pilot was established at a Fluvial Basic Unit of Health (UBSF in Portuguese) (Figure 5) [23]. The infrastructure included six motorboats to facilitate the mobility of teams from the UBSF to dwellings and engaged 41 health professionals.
Figure 5.
Photo of the Fluvial Basic Unit of Health. Photo by Júlio Pompei. Portel, 2019.
Blood samples were collected from random individuals, and an epidemiological survey was conducted by healthcare professionals. The survey aimed to gather information on the possible risk factors associated with bites by hematophagous bats, and blood samples were collected as surveillance measure in public health for the titration of neutralizing antibodies against the rabies virus for future follow-up in the incrementation of titers during the following years.
The epidemiological survey gathered information about both individuals and residence characteristics. Individual characteristics included (i) sex, (ii) age (categorized as child—up to 10 years; adolescent—11 to 17; young adult—18 to 30; adult—31 to 60; and elderly—over 60), and (iii) history of bites by hematophagous bats (variable of interest). Residence characteristics comprised (i) presence of domestic animals in the residence; (ii) history of bites by hematophagous bats in animals; (iii) use of lighting at home during the night; (iv) localization of the residence in relation to the Pacajá River; and (v) history of bites by hematophagous bats in the residence (variable of interest). A Multivariate Generalized Linear Model (MGLN) on software R, version 3.5.1 [24] was applied to explore potential correlations among these variables.
For sample size calculation [24,25], the following parameters were applied: expected proportion of 11% of the population with antibodies against rabies [26]; rapid fluorescent foci inhibition test (RFFIT) with 96% sensitivity and 92% specificity [27]; 7% precision (considering the logistic capacity to collect samples); and 95% confidence interval. The result of the sample size was 150 individuals.
Blood samples were collected prior to administering the first vaccine dose and with the written consent of randomly selected individuals. They were labeled and centrifugated, and the resulting supernatant serum was then transferred to microtubes to facilitate their transport from Pará to the laboratory at the IP, São Paulo, to be subjected to the RFFIT test [27,28,29,30].
3. Results
The pilot vaccinated 2,987 individuals, of which 1842 individuals received two doses of the vaccine (61.7%), and 879 individuals received one dose (38.3%), distributed in 411 registered dwellings. The registration of 266 individuals was lost. The difference between the total vaccine vials used and the total doses applied and recorded results in 12.5% of lost doses. The estimated total cost of the action, adjusted to present values for January 2024 [31,32], was USD 98,863.03 (Table 1).
Table 1.
Costs of pre-exposure prophylaxis pilot against rabies in a riverine population of the Pacajá River, Portel, Pará.
| ITEM | Quantity | Unit Value (BRL) | Present Value * (USD) |
|---|---|---|---|
| 1. UBSF [33]—Depreciation | 15 days | 1,722,060.00 (7300 days of use) |
1109.66 |
| 2. Small motorboats **—Depreciation | 6 units for 15 days | 9000.00 (3650 days of use) |
69.59 |
| 3. Speedboat engines **—Depreciation | 6 units for 15 days | 13,000.00 (3650 days of use) |
100.52 |
| 4. Fuel a. Displacement UBSF [33,34] b. Operating UBSF [33,34] c. Motorboats [34] |
2880 L 6120 L 1080 L |
4.516/L | 14,275.36 |
| 5. Oil [34] | 48 bottles | 28.90 | 435.02 |
| 6. Per diem for the professionals ** | 340 per diem | 135.00 | 14,394.13 |
| 7. Inputs (syringes, gloves, cotton, alcohol, artboards, pens, printing, etc.) ** | 3135.98 | ||
| 8. VERO cells human rabies vaccines [35] | 2760 vials | 73.45 | 63,573.13 |
| 9. GPS ** | 6 pieces of equipment | 940.50 | 1769.63 |
| TOTAL (adjusted for January 2024) | 98,863.03 | ||
| Cost per individual (considering 2987 individuals) | 33.10 | ||
| Cost per dose applied (considering 4563 doses) | 21.67 | ||
| Cost by km2 covered (considering 960 km2) | 102.98 | ||
| Cost by km of rivers navigated (considering 59,441.3 km) | 1.66 | ||
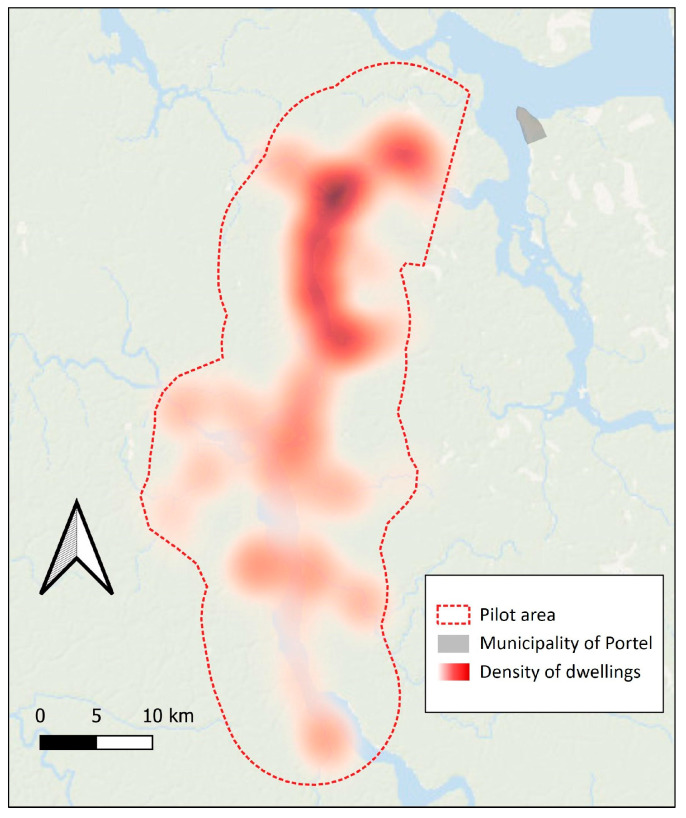
Table 2 presents demographic characteristics found in the pilot area considering the vaccinated population and the total number of dwellings attended. The coordinates of the dwellings in the pilot area indicate higher density on the banks of the Pacajá River and higher density in the region closer to the urban center of Portel (Figure 6).
Table 2.
Demographic characteristics of the pilot population.
| Average number of individuals per dwelling (size of family) | 7.26 individuals/dwelling |
| Density (number of individuals per km2) | 2.83 inhabitants/km2 |
| Proportion by sex | Men—50.8% Women—49.2% |
| Proportion by age | Child—30.50% Adolescent—22.01% Young adult—20.32% Adult—22.31% Elderly—4.85% |
Figure 6.
Distribution and heat map of the density of registered dwellings in the pilot area for vaccination.
A total of 192 individuals were sampled for serological characterization of the population of antibody titers against rabies before vaccination and future serological follow-up; however, only 170 questionnaires were considered due to errors and lack of information in the questionnaires. These 170 individuals were distributed in 105 dwellings and were sampled to characterize the risk of bites by hematophagous bats and for the epidemiological survey. The results of the MGLM for the risk characterization of dwellings and individuals in relation to the occurrence of bites by hematophagous bats can be observed in Table 3. Of the 170 blood samples collected for the titration of neutralizing antibodies, 4 were discarded, and 166 were submitted to the RFFIT test, with an average titer of 0.0291 IU/mL. Of these 166, 2.41% (4 samples, I.C. 0.94–6.03%) presented values equal to or greater than 0.1 IU/mL [26], which are values considered high for individuals who were never vaccinated. Out of the individuals with high antibody titers, only two reported being bitten by hematophagous bats.
Table 3.
Results of the Multivariate General Linear Model to characterize risk for dwellings and individuals in relation to the occurrence of bites by the Desmodus rotundus bat in humans.
| Multivariate GLM to Characterize Risk | Correlated Feature | Ratio | Odds Ratio | p-Value |
|---|---|---|---|---|
| Dwellings | Occurrence of bite by D. rotundus in domestic animals | 66/90 | 2.85 | 0.0604 |
| Dwellings | Maintenance of light source at night |
70/105 | 0.79 | 0.6485 |
| Dwellings | Residence located in affluent rivers |
41/105 | 5.39 | 0.0008 |
| Individuals | Sex: Male | 94/170 | 1.36 | 0.372 |
| Individuals | Age category: Adolescent | 32/170 | 0.50 | 0.282 |
| Individuals | Age category: Young adult | 40/170 | 0.55 | 0.327 |
| Individuals | Age category: Adult | 58/170 | 1.42 | 0.518 |
| Individuals | Age category: Elderly | 20/170 | 2.49 | 0.168 |
4. Discussion
The PrEP pilot was a successful collaboration between various public service sectors, reference institutes, and international organizations aiming to promote public health and protect populations in conditions of vulnerability. The pilot provided parameters on how PrEP can be replicated as a public health measure in ecologically and epidemiologically similar areas. To determine which populations were eligible to receive the vaccine, certain criteria should be established, such as assessing the risk of hematophagous bat attacks and limitations on access to public health services. Furthermore, the PrEP pilot is a model of effective cooperation and strategic planning to improve the health outcomes of at-risk populations.
The UBSF that served as the operational base for the pilot was essential in the ecological scenario in which it was carried out. Centralizing the necessary supplies and vaccines supported the vaccinator teams in executing the measures. This structure would not only be valuable for vaccination but would also support the promotion of other health actions in marginalized populations.
Vaccinators with experience in the application of the tuberculosis vaccine [36] were employed to ensure the correct application of ID rabies vaccines. Each dwelling was visited twice, on days 0 and 7, and on some occasions, the number of individuals present in each dwelling varied between the two visits because of labor reasons, for example. Almost 40% of the population received just one dose of the vaccine.
One of the challenges faced was retrieving the entire volume of vaccine from the vials. This was primarily due to factors such as the constant agitation of the small motorboats used to transport the vaccination teams and the design of the vial cap, which resulted in the formation of bubbles. The formation of bubbles and the design of the vial resulted in the loss of doses, meaning that for future PrEP actions under similar conditions, an additional 20% of vials must be considered to ensure that there are enough doses for the entire population.
The environmental conditions also affected the pilot’s records. Although Brazil’s National Immunization Program records show that at least 4829 doses were administered, there were a significant number of individuals who were vaccinated but not registered; records were lost due to adverse working conditions. As a result, the proportion of lost doses may be lower than the 12.5% presented in the results. The wastage seen in the pilot was considerably lower than the 15% of vaccine wastage that is acceptable according to the GAVI Alliance for the COVID-19 vaccine [37], resulting in a vaccine waste cost of USD 7946.00 (the wastage in reality is lower considering the lost records counted as vaccine waste).
The cost of human rabies vaccines accounted for 65.6% of the PrEP pilot’s total cost. An alternative to the ID method would be to adopt the IM protocol, which involves administering two doses of vaccine on days zero and seven [17,19,20,21]; therefore, the IM protocol would require twice as many vaccines, which would represent 78.3% of the total cost of the action and increase the overall cost by 64%. From a financial perspective, the ID protocol has a significant advantage over the IM protocol, as studies have shown that both protocols provide equal immunological protection to vaccinated individuals [17].
When considering operational costs, efforts could be combined to carry out multiple public health measures simultaneously, increasing the efficiency of resource use and promoting health among marginalized populations. The pilot area is also affected by other neglected tropical diseases, such as leishmaniasis, malaria, and yellow fever [38]. The effort to carry out rabies PrEP could be combined with control measures for other diseases, vaccination and surveillance actions, disease treatment distribution, data collection, epidemiological surveys, etc.
There was a higher concentration of dwellings in the region closest to the urban center. The reason for this could be better access to services and infrastructure offered by the city. As one moves away from the urban center toward the rivers, the density of isolated dwellings decreases, and communities and clusters of dwellings become more prevalent.
All mammals, including bats, are susceptible to rabies. Bats contract and die from rabies, and as the symptoms progress, it makes it difficult for a bat to maintain flight and its feeding behavior. To transmit rabies to other mammals, the virus would have to be present in the bat saliva before symptoms appear or at the beginning of the disease. The mechanisms responsible for the maintenance and transmission of rabies between D. rotundus bats are grooming, physical disputes, and other social interactions [6]. It is not possible to determine if a bat is positive for rabies unless symptoms are present (suggesting clinical diagnosis) or samples of brain material are taken for rabies diagnosis. This means any bite from D. rotundus bats represents a risk for rabies transmission. It is important to note that any wildlife mammal bite event is classified as high risk for rabies by the WHO [16].
The pilot identified that 45.7% of dwellings and 34.4% of individuals in the population had a history of bites by hematophagous bats at least once in their lives. This highlights the risk of rabies that the riverine population in the Amazon Basin faces. It is worth noting that this information relies on the memory of the interviewed individuals.
The presence or absence of domestic animals in the dwellings was not significant to the occurrence of hematophagous bat bites in humans. However, a correlation was found between the occurrence of bites in domestic animals and the location of dwellings with the occurrence of bites in humans. This suggests that the location of dwellings may be a factor that influences the occurrence of hematophagous bat bites in humans. It is worth noting that D. rotundus prefers domestic animals over wild animals [39], and this preference may explain the correlation found between the occurrence of bites in domestic animals and the occurrence of bites in humans in nearby dwellings.
The D. rotundus is a bat species that relies on a stable feeding source to maintain its territory, as it does not store energy reserves and can die of starvation within two days without feeding [40]. It travels up to 10 km in search of food [41], highlighting the importance of having stable feeding sources within its territory, even if those sources are different mammal or bird species [39]. Dwellings located in areas farther from the main river, such as the Pacajá River, may have easier access as feeding sources for the bats. The affluent rivers of Pacajá enter the interior of the forest, a region of common vampire bat habitats, and as a result, dwellings located in affluent rivers are more exposed to contact with these bats.
No correlation was found between age group or sex and a history of bites. However, it was observed that elderly and adult individuals were more likely to have experienced a bite by a hematophagous bat because of a longer lifetime, raising the chances of being bitten by D. rotundus. Eleven individuals claimed to have been bitten by hematophagous bats in recent months, of which four were children, two were adolescents, one was a young adult, and four were adults. Children and adolescents, who were targeted in six of the eleven recent events, had a combined age range of 0–17 years, while young adults and adults had a combined age range of 18–60 years. More than half of the recent attacks had occurred in younger individuals, with a smaller range of ages.
Blood samples revealed the presence of four individuals with rabies antibody titers considered high for those who have never received the vaccine, considering that some individuals who had received the vaccine may not remember doing so. The difficulty in recalling exposure events also could lead to underestimation of bite rates by D. rotundus bats, as well as exposure through other sources, such as wild and domestic animals, which also represent a risk for rabies. The highest titer value found was 0.13 IU/mL, which is lower than titers reported in other studies (reaching 2.8 IU/mL) [26].
Overall, there are three recommendations for future PrEP actions to reduce vaccine wastage, decrease the number of individuals receiving only one dose, and prevent the loss of registration: (1) improve communication prior to the action to ensure a higher number of individuals are present at their residences during the vaccinators’ first visits; (2) enhance the quality of training provided to vaccinators, emphasizing the necessity and importance of proper registration; and (3) establish the ID protocol for administering PrEP to populations in conditions of vulnerability in high-risk areas instead of the IM protocol, thereby reducing vaccine costs.
5. Conclusions
This pilot project showcases the viability of implementing public policies for PrEP against rabies by administering vaccinations to populations residing in regions susceptible to bites from hematophagous bats. This effort distinguishes itself through its large-scale execution, benefiting thousands of individuals residing in vulnerable conditions, and with that, countries such as Brazil and Perú have been implementing PrEP as a public health policy [42]. It is essential to note that PrEP does not obviate the need for PEP following an exposure incident. However, given the remote nature of the target population, the implementation of PrEP as a public health intervention offers a critical layer of protection. Furthermore, PrEP should be complemented by additional strategies, such as health education and vaccination against other infectious diseases [43]. The targeted population inhabits areas of vulnerability and faces persistent risks of exposure to bites from Desmodus rotundus bats, thereby heightening the likelihood of contracting bat-borne rabies. Throughout the pilot project, a commitment to health equity served as a guiding principle embraced by all stakeholders involved.
Acknowledgments
The entirety of the pilot’s costs, spanning conceptualization, planning, and execution, were generously funded by the MH of Brazil, the SESPA, the PANAFTOSA/VPH-PAHO/WHO, and the IP. All the vaccines administered throughout the pilot were acquired and provided by the MH of Brazil. The utilization of the UBSF was granted by the local government of Melgaço Municipality through its Health Secretary. Furthermore, the IP conducted all serological analyses of the collected samples. We extend our heartfelt gratitude to all the local health professionals who dedicated their efforts and actively participated in this pilot, providing crucial assistance to the riverine families. Special appreciation goes to the riverine communities along the Pacajá River, who welcomed us into their homes with open hearts. It is important to note that the findings and conclusions presented in this report solely reflect the perspectives of the authors and do not necessarily represent the views of the MH of Brazil, the SESPA, the PAHO/WHO, or the IP. The authors (Felipe Rocha, Julio Cesar Augusto Pompei, Marco Antonio Natal Vigilato, Daniel Magalhães Lima, Raphael Schneider Vianna, Ottorino Cosivi) are staff member of the Pan American Health Organization. The authors alone are responsible for the views expressed in this publication, and they do not necessarily represent the decisions or policies of the Pan American Health Organization.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/tropicalmed9080179/s1, Table S1. Database of risk factors by dwelling. Table S2. Database of serological results, characteristics and risk factors by individual.
Author Contributions
Coauthor contributions are as follows: conceptualization, F.R., A.V., J.C.A.P., S.E.R., W.A.C., A.L.B., J.A.A.A., S.M.R., F.E.F.L.J. and M.Y.W.; methodology, F.R., A.V., E.M.N.d.A., J.C.A.P., D.M.L., R.S.V., O.C., A.L.B., W.M., S.M.R., F.E.F.L.J. and M.Y.W.; software, F.R. and D.M.L.; validation, F.R. and D.M.L.; formal analysis, F.R., A.V., E.M.N.d.A., J.C.A.P., D.M.L. and S.E.R.; investigation, F.R., A.V., E.M.N.d.A., J.C.A.P., R.S.V., O.C., L.H., K.C.S.F., R.d.S.C.N., L.B.C., A.d.C.R.d.S., A.L.B., J.A.A.A., W.M. and S.M.R.; resources, J.C.A.P., M.A.N.V., O.C., A.d.C.R.d.S., F.E.F.L.J. and M.Y.W.; data curation, F.R., J.C.A.P., M.A.N.V. and D.M.L.; writing—original draft preparation, F.R., A.V. and E.M.N.d.A.; writing—review and editing, F.R., A.V., E.M.N.d.A., J.C.A.P., M.A.N.V., D.M.L., R.S.V., O.C., S.E.R., W.A.C., L.H., K.C.S.F., R.d.S.C.N., L.B.C., A.d.C.R.d.S., A.L.B., J.A.A.A., W.M., S.M.R., F.E.F.L.J. and M.Y.W.; visualization, F.R., A.V., E.M.N.d.A., J.C.A.P., M.A.N.V., D.M.L., R.S.V., O.C., S.E.R., W.A.C., L.H., K.C.S.F., R.d.S.C.N., L.B.C., A.d.C.R.d.S., A.L.B., J.A.A.A., W.M., S.M.R., F.E.F.L.J. and M.Y.W.; supervision, F.R., A.V., E.M.N.d.A., J.C.A.P., M.A.N.V., O.C., F.E.F.L.J. and M.Y.W.; project administration, F.R., A.V., E.M.N.d.A., J.C.A.P., F.E.F.L.J. and M.Y.W.; funding acquisition, A.V., E.M.N.d.A., J.C.A.P., M.A.N.V., O.C., A.d.C.R.d.S., F.E.F.L.J. and M.Y.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, was approved by the Brazilian National Commission of Ethics in Research (20311019.5.0000.5174) in November 2019, and was sustained by National Government Health Authority as a surveillance measure for health.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the serological surveillance measure for health and epidemiological survey.
Data Availability Statement
All data used in the present study are included in the Supplementary Materials, with no mention of a sensitive data origin.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by the Pan American Center for Foot-and-Mouth Disease and Veterinary Public Health (PANAFTOSA/VPH–PAHO/WHO), the Department of Transmissible Diseases of the Secretary of Surveillance in Health and Environment (Ministry of Health of Brazil), the Zoonosis State Coordination (of the Secretary of Health of the State of Pará), and the Institute Pasteur (of the Secretary of Health of the State of Sao Paulo).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hampson K., Coudeville L., Lembo T., Sambo M., Kieffer A., Attlan M., Barrat J., Blanton J.D., Briggs D.J., Cleaveland S., et al. Global Alliance for Rabies Control Partners for Rabies Prevention. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015;9:e0003786. doi: 10.1371/journal.pntd.0003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan American Health Organization REDIPRA 17, Report on the Situation of Rabies in the Americas 2017–2022. 2023. [(accessed on 22 July 2024)]. 39p. Available online: https://iris.paho.org/handle/10665.2/58958.
- 3.Vigilato M.A., Cosivi O., Knöbl T., Clavijo A., Silva H.M. Rabies update for Latin America and the Caribbean. Emerg. Infect. Dis. 2013;19:678–679. doi: 10.3201/eid1904.121482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vargas A., Romano A.P.M., Merchán-Hamann E. Human rabies in Brazil: A descriptive study, 2000–2017. Epidemiol. Serv. Saude. 2019;28:e2018275. doi: 10.5123/S1679-49742019000200001. (In English, Portuguese) [DOI] [PubMed] [Google Scholar]
- 5.Aguiar L.M.S. Subfamília Desmodontinae. In: Reis N.R., Peracchi A.L., Pedro W.A., Lima I.P., editors. Morcegos do Brasil. State University of Londrina; Londrina, Brazil: 2007. pp. 39–43. [Google Scholar]
- 6.Johnson N., Aréchiga-Ceballos N., Aguilar-Setien A. Vampire bat rabies: Ecology, epidemiology and control. Viruses. 2014;6:1911–1928. doi: 10.3390/v6051911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wada M.Y., Rocha S.M., Maia-Elkhoury A.N.S. Situação da Raiva no Brasil, 2000 a 2009. Epidemiol. Serv. Saúde. 2011;20:509–518. doi: 10.5123/S1679-49742011000400010. [DOI] [Google Scholar]
- 8.Fernandes D.C.S., da Silva J.M.A., de Souza B.C., Rios M.M., de Morais E.C., Gasparetto D., Miranda C.S.C., Gonçalves N.V. Spatial distribution of human rabies and primary health care: The case of the outbreak in riverine populations of the municipalities of Breves and Melgaço, Pará, Brazil. Rev. Amaz. Sci. Health. 2021;9:29–39. doi: 10.18606/2318-1419/amazonia.sci.health.v9n4p29-39. [DOI] [Google Scholar]
- 9.Medeiros R., Jusot V., Houillon G., Rasuli A., Martorelli L., Kataoka A.P., Mechlia M.B., Le Guern A.S., Rodrigues L., Assef R., et al. Persistence of Rabies Virus-Neutralizing Antibodies after Vaccination of Rural Population following Vampire Bat Rabies Outbreak in Brazil. PLoS Negl. Trop. Dis. 2016;10:e0004920. doi: 10.1371/journal.pntd.0004920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.da Rosa E.S., Kotait I., Barbosa T.F., Carrieri M.L., Brandão P.E., Pinheiro A.S., Begot A.L., Wada M.Y., de Oliveira R.C., Grisard E.C., et al. Bat-transmitted human rabies outbreaks, Brazilian Amazon. Emerg. Infect. Dis. 2006;12:1197–1202. doi: 10.3201/eid1208.050929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendes W.S., Silva A.A., Neiva R.F., Costa N.M., Assis M.S., Vidigal P.M., Branco M.R., Leite M.G., Rios J.M., Martins J.O., et al. An outbreak of bat-transmitted human rabies in a village in the Brazilian Amazon. Rev. Saude Publica. 2009;43:1075–1077. doi: 10.1590/S0034-89102009005000073. [DOI] [PubMed] [Google Scholar]
- 12.United Nations Development Programme Atlas of Human Development in Brazil. [(accessed on 22 July 2024)]. Available online: http://www.atlasbrasil.org.br/
- 13.Pereira A.L., Santos O.S., Paes M.C.F., Ikuta Y.M., Silveira R., Valentin F.N. Sociobehavioral, Biological, and Health Characteristics of Riverside People in the Xingu Region, Pará, Brazil. Int. J. Environ. Res. Public Health. 2023;20:5542. doi: 10.3390/ijerph20085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gama A.S.M., Fernandes T.G., Parente R.C.P., Secoli S.R. Inquérito de saúde em comunidades ribeirinhas do Amazonas, Brasil [A health survey in riverine communities in Amazonas State, Brazil] Cad. Saude Publica. 2018;34:e00002817. doi: 10.1590/0102-311x00002817. (In Portuguese) [DOI] [PubMed] [Google Scholar]
- 15.Gonçalves R.M., Domingos I.M. Riverside population in the Amazon and inequality in access to health. J. Const. Stud. Hermeneut. Theory Law. 2019;11:99–108. [Google Scholar]
- 16.Schneider M.C., Aron J., Santos-Burgoa C., Uieda W., Ruiz-Velazco S. Common vampire bat attacks on humans in a village of the Amazon region of Brazil. Cad. Saude Publica. 2001;17:1531–1536. doi: 10.1590/S0102-311X2001000600025. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization Rabies vaccines: WHO position paper, April 2018—Recommendations. Vaccine. 2018;36:5500–5503. doi: 10.1016/j.vaccine.2018.06.061. [DOI] [PubMed] [Google Scholar]
- 18.Coordenação-Geral de Vigilância de Zoonoses e Doenças de Transmissão Vetorial, Ministério da Saúde, Brasil, 2022. NOTA TÉCNICA Nº 8/2022-CGZV/DEIDT/SVS/MS. [(accessed on 22 July 2024)]; Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/r/raiva/prevencao.
- 19.Portel, Pará (Code: 1505809) Cities and States. Instituto Brasileiro de Geografia e Estatística. [(accessed on 22 July 2024)];2010 Available online: https://www.ibge.gov.br/en/cities-and-states/pa/portel.html.
- 20.Ertl H.C.J. An Opinion on Routine Rabies Pre-Exposure Prophylaxis for Children in High Endemic Areas. J. Vaccines Vaccin. 2018;9:379. doi: 10.4172/2157-7560.1000379. [DOI] [Google Scholar]
- 21.Kessels J.A., Recuenco S., Navarro-Vela A.M., Deray R., Vigilato M., Ertl H., Durrheim D., Rees H., Nel L.H., Abela-Ridder B., et al. Pre-exposure rabies prophylaxis: A systematic review. Bull. World Health Organ. 2017;95:210C–219C. doi: 10.2471/BLT.16.173039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Recuenco S., Warnock E., Osinubi M.O.V., Rupprecht C.E. A single center, open label study of intradermal administration of an inactivated purified chick embryo cell culture rabies virus vaccine in adults. Vaccine. 2017;35:4315–4320. doi: 10.1016/j.vaccine.2017.06.083. [DOI] [PubMed] [Google Scholar]
- 23.Departamento de Atenção Básica, Unidade Básica de Saúde Fluvial Especificações Técnica. Ministério da Saúde, Brasil. [(accessed on 22 July 2024)];2014 Available online: https://www.gov.br/saude/pt-br/composicao/saps/ubsf/publicacoes/especificacao_tecnica_ubs.pdf/view.
- 24.Core Team R . A Language and Environment for Statistical Computing, Version 3.5.1. R Foundation for Statistical Computing; Vienna, Austria: 2020. [Google Scholar]
- 25.Sergeant E. R Surveillance: Design and Analysis of Disease Surveillance Activities; R Package, Version 0.2.1. 2022. [(accessed on 22 July 2024)]. Available online: [DOI]
- 26.Gilbert A.T., Petersen B.W., Recuenco S., Niezgoda M., Gómez J., Laguna-Torres V.A., Rupprecht C. Evidence of rabies virus exposure among humans in the Peruvian Amazon. Am. J. Trop. Med. Hyg. 2012;87:206–215. doi: 10.4269/ajtmh.2012.11-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreira W.C., de Moura W.C., da Silva M.V., Marcovistz R. Comparison between in-house and commercial conjugates by the technique of rapid inhibition of fluorescent foci (RFFIT) in the evaluation of anti-rabic antibodies. Arq. Inst. Biol. 2005;72 doi: 10.1590/1808-1657v72p1552005. [DOI] [Google Scholar]
- 28.Smith J.S., Yager P.A., Baer G.M. A Rapid Fluorescent Focus Inhibition Test (RFFIT) for Determining Rabies Virus Neutralizing Antibody. In: Meslin F.X., Koprowsky H., Kaplan M.M., editors. Laboratory Techniques in Rabies. 4th ed. WHO; Geneva, Switzerland: 1996. [(accessed on 22 July 2024)]. pp. 181–187. Available online: http://apps.who.int/iris/bitstream/10665/38286/1/9241544791_eng.pdf. [Google Scholar]
- 29.Medeiros Caporale G.M., Rodrigues da Silva A.C., Peixoto Z.M., Chaves L.B., Carrieri M.L., Vassão R.C. First production of fluorescent anti-ribonucleoproteins conjugate for diagnostic of rabies in Brazil. J. Clin. Lab. Anal. 2009;23:7–13. doi: 10.1002/jcla.20275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization . The Rapid Fluorescent Focus Inhibition Test. In: Rupprecht C.E., Fooks A.R., Abela-Ridder B., editors. Laboratory Techniques in Rabies. 5th ed. Volume 1. World Health Organization; Geneva, Switzerland: 2018. [(accessed on 22 July 2024)]. Available online: https://iris.who.int/handle/10665/310836. [Google Scholar]
- 31.Banco Central Do Brasil Calculadora do Cidadão. Correção de Valores. Índice de Correção IGP-M (FGV) [(accessed on 22 July 2024)]; Available online: https://www3.bcb.gov.br/CALCIDADAO/publico/exibirFormCorrecaoValores.do?method=exibirFormCorrecaoValores.
- 32.Banco Central do Brasil Consulta de Cotações e Boletins. [(accessed on 22 July 2024)]; Available online: https://www.bcb.gov.br/estabilidadefinanceira/historicocotacoes.
- 33.Departamento de Atenção Básica, Unidade Básica de Saúde Fluvial. Estimativas de Custo—2013. Ministério da Saúde, Brasil. [(accessed on 22 July 2024)];2013 Available online: https://www.gov.br/saude/pt-br/composicao/saps/ubsf/publicacoes/planilha_estimativa_custos_construcao.pdf/view.
- 34.Agência Nacional do Petróleo, Gás Natural e Biocombustíveis Painel Dinâmico de Preços de Combustíveis e Derivados do Petróleo. Ministério de Minas e Energia, Brasil. [(accessed on 22 July 2024)];2020 Available online: https://www.gov.br/anp/pt-br/centrais-de-conteudo/paineis-dinamicos-da-anp/painel-dinamico-de-precos-de-combustiveis-e-derivados-do-petroleo.
- 35.Pan American Health Organization (PAHO) Expanded Immunization Program. Rotational Fund, January 2019. [(accessed on 22 July 2024)]. Available online: https://www.paho.org/en/documents/paho-revolving-fund-vaccine-prices-2019.
- 36.Programa Nacional de Imunizações, Calendário Nacional de Vacinação. Ministério da Saúde, Brasil. [(accessed on 22 July 2024)]; Available online: https://www.gov.br/saude/pt-br/vacinacao/calendario.
- 37.World Health Organization Fair Allocation Mechanism for COVID-19 Vaccines through the COVAX Facility. [(accessed on 22 July 2024)]. Available online: https://www.who.int/publications/m/item/fair-allocation-mechanism-for-covid-19-vaccines-through-the-covax-facility.
- 38.Penna G., Pinto L.F., Soranz D., Glatt R. High incidence of diseases endemic to the Amazon region of Brazil, 2001–2006. Emerg. Infect. Dis. 2009;15:626–632. doi: 10.3201/eid1504.081329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bobrowiec P.E.D., Lemes M.R., Gribel R. Prey preference of the common vampire bat (Desmodus rotundus, Chiroptera) using molecular analysis. J. Mammal. 2015;96:54–63. doi: 10.1093/jmammal/gyu002. [DOI] [Google Scholar]
- 40.Freitas M.B., Welker A.F., Millan S.F., Pinheiro E.C. Metabolic responses induced by fasting in the common vampire bat Desmodus rotundus. J. Comp. Physiol. B. 2003;173:703–707. doi: 10.1007/s00360-003-0383-3. [DOI] [PubMed] [Google Scholar]
- 41.Medina A., Harvey C.A., Merlo D.S., Vílchez S., Hernández B. Bat Diversity and Movement in an Agricultural Landscape in Matiguás, Nicaragua. Biotropica. 2007;39:120–128. doi: 10.1111/j.1744-7429.2006.00240.x. [DOI] [Google Scholar]
- 42.Pan American Health Organization REDIPRA 17, Implementation of National Policies for Pre-Exposure Prophylaxis of Human Rabies Transmitted by the D. rotundus in at-Risk Populations. 17th Meeting of Rabies Program Directors of the Americas: Final Report 2023; 44p. [(accessed on 22 July 2024)]. Available online: https://iris.paho.org/handle/10665.2/59306.
- 43.Silva J.G.N., Silva S.S., Gomes T.C.M., Nascimento G.S., Valentim L.A., Quaresma T.C., Fernandes F.P., Oliveira S.M.S., Moraes W.P. Empowering Riverine Communities in the Amazon: Strategies for Preventing Rabies. Int. J. Environ. Res. Public Health. 2024;21:117. doi: 10.3390/ijerph21010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in the present study are included in the Supplementary Materials, with no mention of a sensitive data origin.