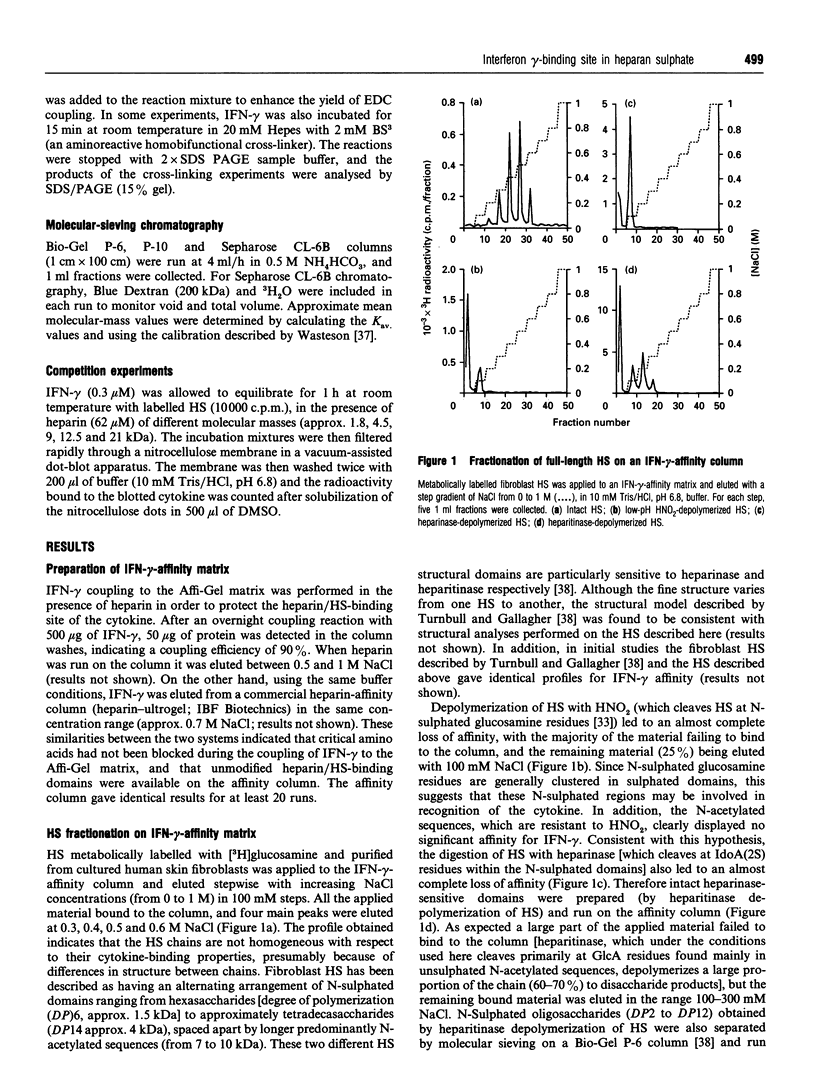
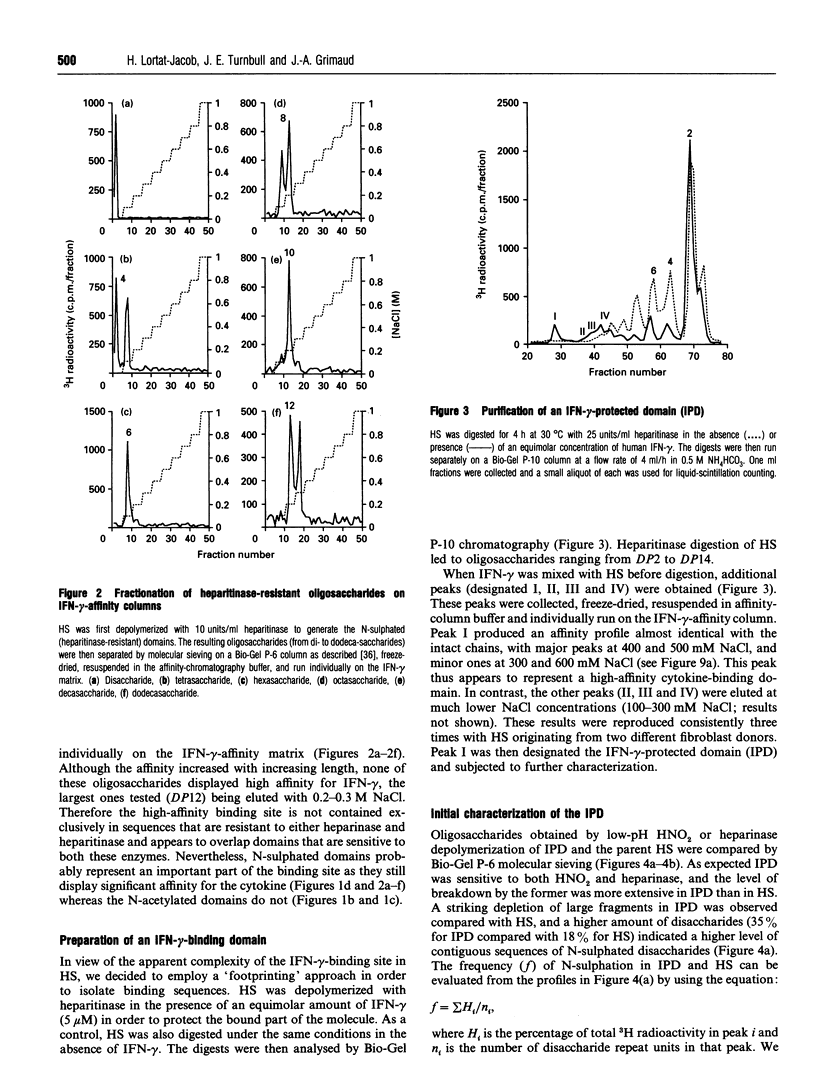
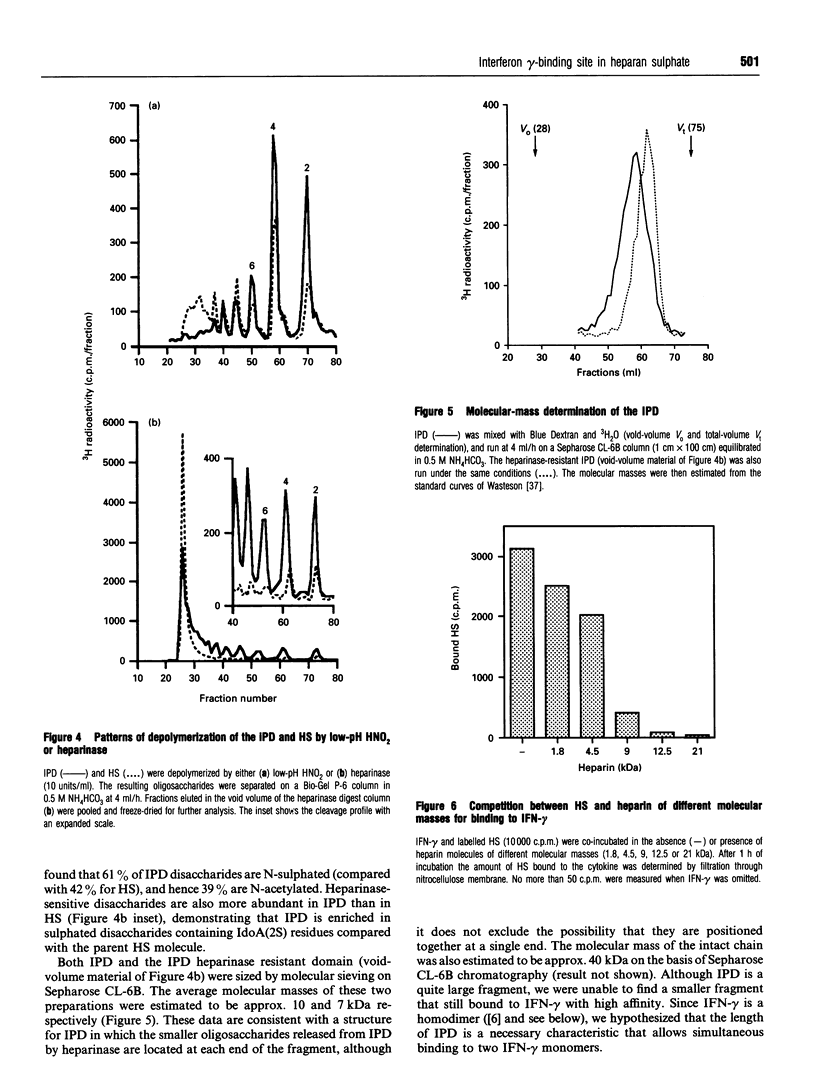
Abstract
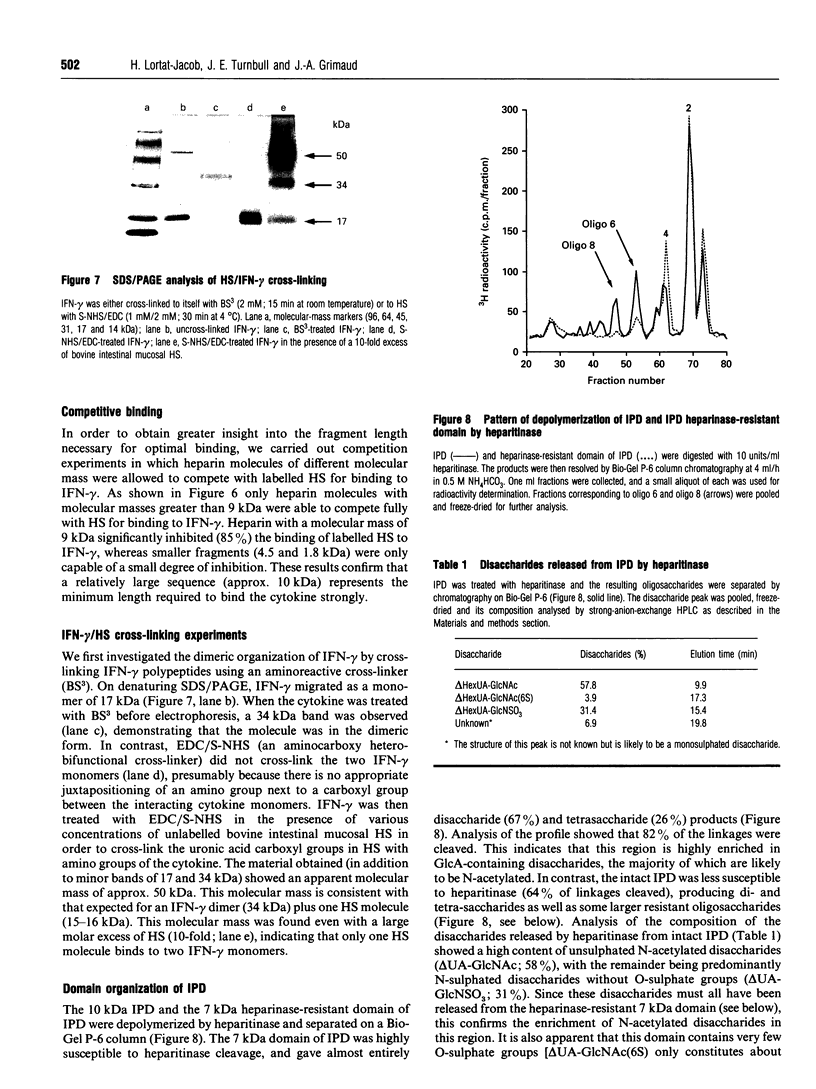
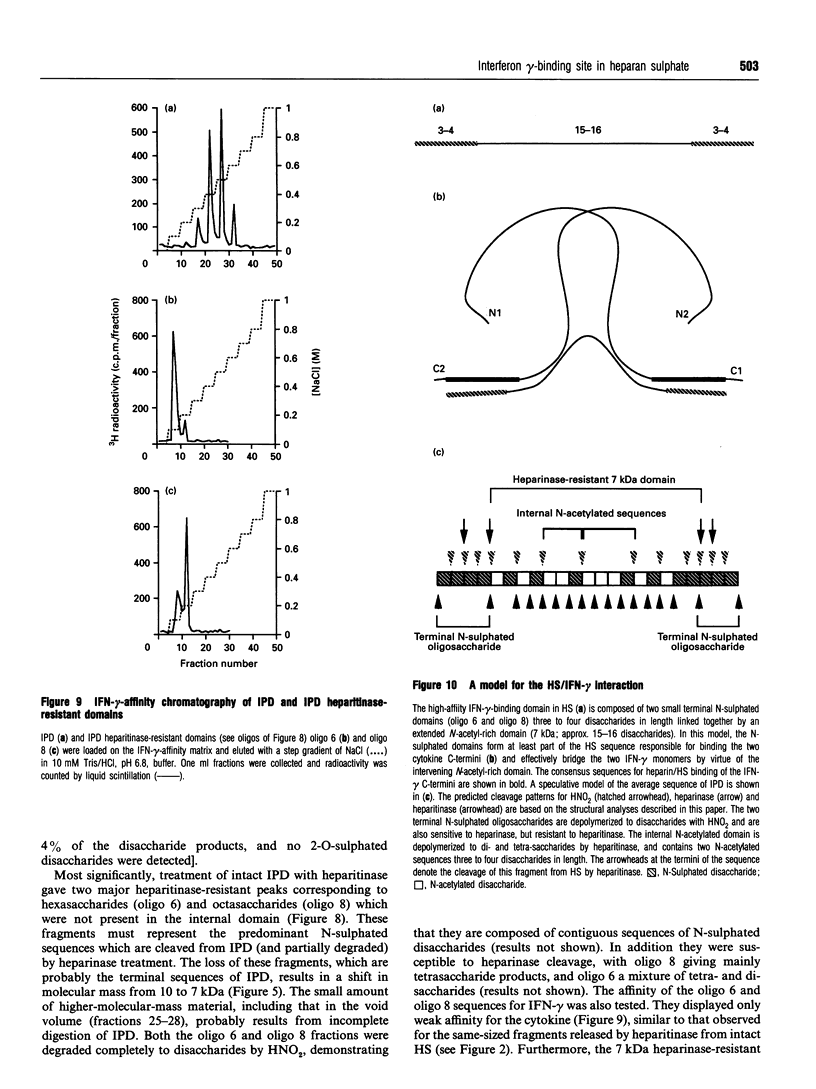
Interferon (IFN)-gamma, in common with a number of cytokines or growth factors, strongly interacts with heparan sulphate (HS). It has been shown previously that one of the C-terminal basic clusters of amino acids (a regulatory element of IFN-gamma activity) is involved in this interaction. The structural organization of the HS domain that binds to human IFN-gamma has been investigated here. IFN-gamma-affinity chromatography of HS oligosaccharides released by either enzymic or chemical cleavage showed that the binding site is not found in a domain that is resistant to either heparinase or heparitinase or exclusively N-sulphated or N-acetylated. This led us to take a 'footprinting' approach in which HS was depolymerized in the presence of IFN-gamma and the cytokine-protected sequences were separated from the digested fragments. Using this strategy we consistently isolated an IFN-gamma-protected domain (IPD; approx. 10 kDa) which displayed the same affinity as full-length HS for the cytokine. Treatment of IPD with either heparinase or heparitinase strongly reduced its affinity, confirming that the high-affinity binding site encompassed a mixture of HS structural domains. Patterns of depolymerization with either enzymic or chemical agents were consistent with IPD being composed of an extended internal domain (approx. 7 kDa) which is predominantly N-acetylated and GlcA-rich, flanked by small N-sulphated oligosaccharides (mainly hexa- to octasaccharides). This is the first description of an HS protein-binding sequence with this type of molecular organization. Furthermore, using a cross-linking strategy, we demonstrated that one HS molecule bound to an IFN-gamma dimer. Together these results lead us to propose a novel model for the interaction of HS with a protein, in which two sulphated terminal sequences of the binding domain interact directly with the two IFN-gamma C-termini and bridge the two cytokine monomers through an internal N-acetyl-rich sequence.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa T., Horan T. P., McGinley M., Rohde M. F. Effect of amino-terminal processing by Staphylococcus aureus V-8 protease on activity and structure of recombinant human interferon-gamma. J Interferon Res. 1990 Jun;10(3):321–329. doi: 10.1089/jir.1990.10.321. [DOI] [PubMed] [Google Scholar]
- Arakawa T., Hsu Y. R., Parker C. G., Lai P. H. Role of polycationic C-terminal portion in the structure and activity of recombinant human interferon-gamma. J Biol Chem. 1986 Jun 25;261(18):8534–8539. [PubMed] [Google Scholar]
- Curling E. M., Hayter P. M., Baines A. J., Bull A. T., Gull K., Strange P. G., Jenkins N. Recombinant human interferon-gamma. Differences in glycosylation and proteolytic processing lead to heterogeneity in batch culture. Biochem J. 1990 Dec 1;272(2):333–337. doi: 10.1042/bj2720333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maeyer E., De Maeyer-Guignard J. Interferon-gamma. Curr Opin Immunol. 1992 Jun;4(3):321–326. doi: 10.1016/0952-7915(92)90083-q. [DOI] [PubMed] [Google Scholar]
- Desai U. R., Wang H. M., Linhardt R. J. Substrate specificity of the heparin lyases from Flavobacterium heparinum. Arch Biochem Biophys. 1993 Nov 1;306(2):461–468. doi: 10.1006/abbi.1993.1538. [DOI] [PubMed] [Google Scholar]
- Ealick S. E., Cook W. J., Vijay-Kumar S., Carson M., Nagabhushan T. L., Trotta P. P., Bugg C. E. Three-dimensional structure of recombinant human interferon-gamma. Science. 1991 May 3;252(5006):698–702. doi: 10.1126/science.1902591. [DOI] [PubMed] [Google Scholar]
- Farrar M. A., Schreiber R. D. The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- Favre C., Wijdenes J., Cabrillat H., Djossou O., Banchereau J., de Vries J. E. Epitope mapping of recombinant human gamma interferon using monoclonal antibodies. Mol Immunol. 1989 Jan;26(1):17–25. doi: 10.1016/0161-5890(89)90015-1. [DOI] [PubMed] [Google Scholar]
- Flaumenhaft R., Rifkin D. B. The extracellular regulation of growth factor action. Mol Biol Cell. 1992 Oct;3(10):1057–1065. doi: 10.1091/mbc.3.10.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J. T. The extended family of proteoglycans: social residents of the pericellular zone. Curr Opin Cell Biol. 1989 Dec;1(6):1201–1218. doi: 10.1016/s0955-0674(89)80072-9. [DOI] [PubMed] [Google Scholar]
- Gallagher J. T., Turnbull J. E., Lyon M. Patterns of sulphation in heparan sulphate: polymorphism based on a common structural theme. Int J Biochem. 1992 Apr;24(4):553–560. doi: 10.1016/0020-711x(92)90326-v. [DOI] [PubMed] [Google Scholar]
- Gallagher J. T., Walker A. Molecular distinctions between heparan sulphate and heparin. Analysis of sulphation patterns indicates that heparan sulphate and heparin are separate families of N-sulphated polysaccharides. Biochem J. 1985 Sep 15;230(3):665–674. doi: 10.1042/bj2300665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs N. D., Jarpe M. A., Pace J. L., Russell S. W., Johnson H. M. The N-terminus and C-terminus of IFN-gamma are binding domains for cloned soluble IFN-gamma receptor. J Immunol. 1992 Jul 15;149(2):517–520. [PubMed] [Google Scholar]
- Grzesiek S., Döbeli H., Gentz R., Garotta G., Labhardt A. M., Bax A. 1H, 13C, and 15N NMR backbone assignments and secondary structure of human interferon-gamma. Biochemistry. 1992 Sep 8;31(35):8180–8190. doi: 10.1021/bi00150a009. [DOI] [PubMed] [Google Scholar]
- Guimond S., Maccarana M., Olwin B. B., Lindahl U., Rapraeger A. C. Activating and inhibitory heparin sequences for FGF-2 (basic FGF). Distinct requirements for FGF-1, FGF-2, and FGF-4. J Biol Chem. 1993 Nov 15;268(32):23906–23914. [PubMed] [Google Scholar]
- Hogrefe H. H., McPhie P., Bekisz J. B., Enterline J. C., Dyer D., Webb D. S., Gerrard T. L., Zoon K. C. Amino terminus is essential to the structural integrity of recombinant human interferon-gamma. J Biol Chem. 1989 Jul 25;264(21):12179–12186. [PubMed] [Google Scholar]
- Jackson R. L., Busch S. J., Cardin A. D. Glycosaminoglycans: molecular properties, protein interactions, and role in physiological processes. Physiol Rev. 1991 Apr;71(2):481–539. doi: 10.1152/physrev.1991.71.2.481. [DOI] [PubMed] [Google Scholar]
- Jimenez S. A., Freundlich B., Rosenbloom J. Selective inhibition of human diploid fibroblast collagen synthesis by interferons. J Clin Invest. 1984 Sep;74(3):1112–1116. doi: 10.1172/JCI111480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinikki P. O., Calderon J., Luquette M. H., Schreiber R. D. Reduced receptor binding by a human interferon-gamma fragment lacking 11 carboxyl-terminal amino acids. J Immunol. 1987 Nov 15;139(10):3360–3366. [PubMed] [Google Scholar]
- Lindahl U., Thunberg L., Bäckström G., Riesenfeld J., Nordling K., Björk I. Extension and structural variability of the antithrombin-binding sequence in heparin. J Biol Chem. 1984 Oct 25;259(20):12368–12376. [PubMed] [Google Scholar]
- Lohse D. L., Linhardt R. J. Purification and characterization of heparin lyases from Flavobacterium heparinum. J Biol Chem. 1992 Dec 5;267(34):24347–24355. [PubMed] [Google Scholar]
- Lortat-Jacob H., Grimaud J. A. Binding of interferon-gamma to heparan sulfate is restricted to the heparin-like domains and involves carboxylic--but not N-sulfated--groups. Biochim Biophys Acta. 1992 Sep 15;1117(2):126–130. doi: 10.1016/0304-4165(92)90069-7. [DOI] [PubMed] [Google Scholar]
- Lortat-Jacob H., Grimaud J. A. Interferon-gamma C-terminal function: new working hypothesis. Heparan sulfate and heparin, new targets for IFN-gamma, protect, relax the cytokine and regulate its activity. Cell Mol Biol. 1991;37(3):253–260. [PubMed] [Google Scholar]
- Lortat-Jacob H., Grimaud J. A. Interferon-gamma binds to heparan sulfate by a cluster of amino acids located in the C-terminal part of the molecule. FEBS Lett. 1991 Mar 11;280(1):152–154. doi: 10.1016/0014-5793(91)80225-r. [DOI] [PubMed] [Google Scholar]
- Lortat-Jacob H., Kleinman H. K., Grimaud J. A. High-affinity binding of interferon-gamma to a basement membrane complex (matrigel). J Clin Invest. 1991 Mar;87(3):878–883. doi: 10.1172/JCI115093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk S. K., Jay E., Jay F. T. Structure-function analysis of the human interferon gamma. The COOH terminus is not essential for functional activity. J Biol Chem. 1990 Aug 5;265(22):13314–13319. [PubMed] [Google Scholar]
- Lundell D., Lunn C., Dalgarno D., Fossetta J., Greenberg R., Reim R., Grace M., Narula S. The carboxyl-terminal region of human interferon gamma is important for biological activity: mutagenic and NMR analysis. Protein Eng. 1991 Feb;4(3):335–341. doi: 10.1093/protein/4.3.335. [DOI] [PubMed] [Google Scholar]
- Magazine H. I., Carter J. M., Russell J. K., Torres B. A., Dunn B. M., Johnson H. M. Use of synthetic peptides to identify an N-terminal epitope on mouse gamma interferon that may be involved in function. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1237–1241. doi: 10.1073/pnas.85.4.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinderknecht E., O'Connor B. H., Rodriguez H. Natural human interferon-gamma. Complete amino acid sequence and determination of sites of glycosylation. J Biol Chem. 1984 Jun 10;259(11):6790–6797. [PubMed] [Google Scholar]
- Rose K., Simona M. G., Offord R. E., Prior C. P., Otto B., Thatcher D. R. A new mass-spectrometric C-terminal sequencing technique finds a similarity between gamma-interferon and alpha 2-interferon and identifies a proteolytically clipped gamma-interferon that retains full antiviral activity. Biochem J. 1983 Nov 1;215(2):273–277. doi: 10.1042/bj2150273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell. 1991 Mar 8;64(5):867–869. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]
- Sakaguchi M., Honda S., Ozawa M., Nishimura O. Human interferon-gamma lacking 23 COOH-terminal amino acids is biologically active. FEBS Lett. 1988 Mar 28;230(1-2):201–204. doi: 10.1016/0014-5793(88)80671-9. [DOI] [PubMed] [Google Scholar]
- Seelig G. F., Wijdenes J., Nagabhushan T. L., Trotta P. P. Evidence for a polypeptide segment at the carboxyl terminus of recombinant human gamma interferon involved in expression of biological activity. Biochemistry. 1988 Mar 22;27(6):1981–1987. doi: 10.1021/bi00406a026. [DOI] [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976 Sep 7;15(18):3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- Turnbull J. E., Fernig D. G., Ke Y., Wilkinson M. C., Gallagher J. T. Identification of the basic fibroblast growth factor binding sequence in fibroblast heparan sulfate. J Biol Chem. 1992 May 25;267(15):10337–10341. [PubMed] [Google Scholar]
- Turnbull J. E., Gallagher J. T. Distribution of iduronate 2-sulphate residues in heparan sulphate. Evidence for an ordered polymeric structure. Biochem J. 1991 Feb 1;273(Pt 3):553–559. doi: 10.1042/bj2730553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A., Turnbull J. E., Gallagher J. T. Specific heparan sulfate saccharides mediate the activity of basic fibroblast growth factor. J Biol Chem. 1994 Jan 14;269(2):931–935. [PubMed] [Google Scholar]
- Wasteson A. A method for the determination of the molecular weight and molecular-weight distribution of chondroitin sulphate. J Chromatogr. 1971 Jul 8;59(1):87–97. doi: 10.1016/s0021-9673(01)80009-1. [DOI] [PubMed] [Google Scholar]
- Wetzel R., Perry L. J., Veilleux C., Chang G. Mutational analysis of the C-terminus of human interferon-gamma. Protein Eng. 1990 Jul;3(7):611–623. doi: 10.1093/protein/3.7.611. [DOI] [PubMed] [Google Scholar]
- Wheelock E. F. Interferon-like virus-inhibitor induced in human leukocytes by phytohemagglutinin. Science. 1965 Jul 16;149(3681):310–311. [PubMed] [Google Scholar]