Abstract
To investigate the role of the iron-sulphur cluster in mammalian ferrochelatases, the terminal enzyme of the haem biosynthetic pathway, we examined the interaction of nitric oxide (NO) and ferrochelatase. When macrophage cell line RAW 264.7 cells were treated with interferon-gamma and lipopolysaccharide NO synthesis in the cells was stimulated, and a decrease in ferrochelatase activity was observed, with no change in the amount of ferrochelatase. The addition of NG-monomethyl-L-arginine, a selective inhibitor of NO synthesis, reduced the effect of interferon-gamma and lipopolysaccharide, while the effect of NG-monomethyl-L-arginine was suppressed by the addition of L-arginine, a substrate of NO synthase. When purified recombinant human ferrochelatase was treated with 3-morpholinosydnonimine, a NO-generating compound, ferrochelatase activity decreased with disappearance of characteristic absorbance spectra of the iron-sulphur cluster. S-Nitroso-N-acetylpenicillamine also reduced the activity, in a dose-dependent manner. These results indicate that ferrochelatase activity can be modulated by NO synthesis probably through disruption of the iron-sulphur cluster. We propose that inactivation of ferrochelatase mediated by NO (or NO-derived species) may play a role in the regulation of haem metabolism.
Full text
PDF



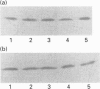
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battistini A., Marziali G., Albertini R., Habetswallner D., Bulgarini D., Coccia E. M., Fiorucci G., Romeo G., Orsatti R., Testa U. Positive modulation of hemoglobin, heme, and transferrin receptor synthesis by murine interferon-alpha and -beta in differentiating Friend cells. Pivotal role of heme synthesis. J Biol Chem. 1991 Jan 5;266(1):528–535. [PubMed] [Google Scholar]
- Beinert H. Recent developments in the field of iron-sulfur proteins. FASEB J. 1990 May;4(8):2483–2491. doi: 10.1096/fasebj.4.8.2185975. [DOI] [PubMed] [Google Scholar]
- Brenner D. A., Frasier F. Cloning of murine ferrochelatase. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):849–853. doi: 10.1073/pnas.88.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camadro J. M., Labbe P. Purification and properties of ferrochelatase from the yeast Saccharomyces cerevisiae. Evidence for a precursor form of the protein. J Biol Chem. 1988 Aug 25;263(24):11675–11682. [PubMed] [Google Scholar]
- Castro L., Rodriguez M., Radi R. Aconitase is readily inactivated by peroxynitrite, but not by its precursor, nitric oxide. J Biol Chem. 1994 Nov 25;269(47):29409–29415. [PubMed] [Google Scholar]
- Dailey H. A., Finnegan M. G., Johnson M. K. Human ferrochelatase is an iron-sulfur protein. Biochemistry. 1994 Jan 18;33(2):403–407. doi: 10.1021/bi00168a003. [DOI] [PubMed] [Google Scholar]
- Dailey H. A., Sellers V. M., Dailey T. A. Mammalian ferrochelatase. Expression and characterization of normal and two human protoporphyric ferrochelatases. J Biol Chem. 1994 Jan 7;269(1):390–395. [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J Immunol. 1988 Apr 15;140(8):2829–2838. [PubMed] [Google Scholar]
- Drapier J. C., Hirling H., Wietzerbin J., Kaldy P., Kühn L. C. Biosynthesis of nitric oxide activates iron regulatory factor in macrophages. EMBO J. 1993 Sep;12(9):3643–3649. doi: 10.1002/j.1460-2075.1993.tb06038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira G. C., Franco R., Lloyd S. G., Pereira A. S., Moura I., Moura J. J., Huynh B. H. Mammalian ferrochelatase, a new addition to the metalloenzyme family. J Biol Chem. 1994 Mar 11;269(10):7062–7065. [PubMed] [Google Scholar]
- Frustaci J. M., O'Brian M. R. Characterization of a Bradyrhizobium japonicum ferrochelatase mutant and isolation of the hemH gene. J Bacteriol. 1992 Jul;174(13):4223–4229. doi: 10.1128/jb.174.13.4223-4229.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y., Fujita H., Taketani S., Sassa S. Haem is necessary for a continued increase in ferrochelatase mRNA in murine erythroleukaemia cells during erythroid differentiation. Br J Haematol. 1993 Dec;85(4):670–675. doi: 10.1111/j.1365-2141.1993.tb03207.x. [DOI] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Harbin B. M., Dailey H. A. Orientation of ferrochelatase in bovine liver mitochondria. Biochemistry. 1985 Jan 15;24(2):366–370. doi: 10.1021/bi00323a019. [DOI] [PubMed] [Google Scholar]
- Hausladen A., Fridovich I. Superoxide and peroxynitrite inactivate aconitases, but nitric oxide does not. J Biol Chem. 1994 Nov 25;269(47):29405–29408. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Iron depletion: possible cause of tumor cell cytotoxicity induced by activated macrophages. Biochem Biophys Res Commun. 1984 Sep 17;123(2):716–723. doi: 10.1016/0006-291x(84)90288-2. [DOI] [PubMed] [Google Scholar]
- Jones M. S., Jones O. T. The structural organization of haem synthesis in rat liver mitochondria. Biochem J. 1969 Jul;113(3):507–514. doi: 10.1042/bj1130507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr S. R., Dailey H. A. The synthesis of murine ferrochelatase in vitro and in vivo. Biochem J. 1988 Sep 15;254(3):799–803. doi: 10.1042/bj2540799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Harford J. B. cis-trans models for post-transcriptional gene regulation. Science. 1989 Nov 17;246(4932):870–872. doi: 10.1126/science.2683086. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Rouault T. A., Harford J. B. Regulating the fate of mRNA: the control of cellular iron metabolism. Cell. 1993 Jan 15;72(1):19–28. doi: 10.1016/0092-8674(93)90046-s. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Labbe-Bois R. The ferrochelatase from Saccharomyces cerevisiae. Sequence, disruption, and expression of its structural gene HEM15. J Biol Chem. 1990 May 5;265(13):7278–7283. [PubMed] [Google Scholar]
- Lorsbach R. B., Murphy W. J., Lowenstein C. J., Snyder S. H., Russell S. W. Expression of the nitric oxide synthase gene in mouse macrophages activated for tumor cell killing. Molecular basis for the synergy between interferon-gamma and lipopolysaccharide. J Biol Chem. 1993 Jan 25;268(3):1908–1913. [PubMed] [Google Scholar]
- Melefors O., Hentze M. W. Translational regulation by mRNA/protein interactions in eukaryotic cells: ferritin and beyond. Bioessays. 1993 Feb;15(2):85–90. doi: 10.1002/bies.950150203. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Kanaya S., Morikawa K., Inokuchi H. Overproduction, purification, and characterization of ferrochelatase from Escherichia coli. J Biochem. 1994 Mar;115(3):545–551. doi: 10.1093/oxfordjournals.jbchem.a124373. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Nakahigashi K., Nishimura K., Inokuchi H. Isolation and characterization of visible light-sensitive mutants of Escherichia coli K12. J Mol Biol. 1991 Jun 5;219(3):393–398. doi: 10.1016/0022-2836(91)90180-e. [DOI] [PubMed] [Google Scholar]
- Mohr S., Stamler J. S., Brüne B. Mechanism of covalent modification of glyceraldehyde-3-phosphate dehydrogenase at its active site thiol by nitric oxide, peroxynitrite and related nitrosating agents. FEBS Lett. 1994 Jul 18;348(3):223–227. doi: 10.1016/0014-5793(94)00596-6. [DOI] [PubMed] [Google Scholar]
- Nakahashi Y., Taketani S., Okuda M., Inoue K., Tokunaga R. Molecular cloning and sequence analysis of cDNA encoding human ferrochelatase. Biochem Biophys Res Commun. 1990 Dec 14;173(2):748–755. doi: 10.1016/s0006-291x(05)80099-3. [DOI] [PubMed] [Google Scholar]
- Okuda M., Kohno H., Furukawa T., Tokunaga R., Taketani S. Overexpression in Escherichia coli, and one-step purification of the human recombinant ferrochelatase. Biochim Biophys Acta. 1994 Jul 6;1200(2):123–128. doi: 10.1016/0304-4165(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Pantopoulos K., Weiss G., Hentze M. W. Nitric oxide and the post-transcriptional control of cellular iron traffic. Trends Cell Biol. 1994 Mar;4(3):82–86. doi: 10.1016/0962-8924(94)90179-1. [DOI] [PubMed] [Google Scholar]
- Rossi E., Costin K. A., Garcia-Webb P. Ferrochelatase activity in human lymphocytes, as quantified by a new high-performance liquid-chromatographic method. Clin Chem. 1988 Dec;34(12):2481–2485. [PubMed] [Google Scholar]
- Schmidt H. H., Warner T. D., Nakane M., Förstermann U., Murad F. Regulation and subcellular location of nitrogen oxide synthases in RAW264.7 macrophages. Mol Pharmacol. 1992 Apr;41(4):615–624. [PubMed] [Google Scholar]
- Stamler J. S. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell. 1994 Sep 23;78(6):931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Taketani S., Nakahashi Y., Osumi T., Tokunaga R. Molecular cloning, sequencing, and expression of mouse ferrochelatase. J Biol Chem. 1990 Nov 15;265(32):19377–19380. [PubMed] [Google Scholar]
- Taketani S., Tokunaga R. Purification and substrate specificity of bovine liver-ferrochelatase. Eur J Biochem. 1982 Oct;127(3):443–447. doi: 10.1111/j.1432-1033.1982.tb06892.x. [DOI] [PubMed] [Google Scholar]
- Taketani S., Tokunaga R. Rat liver ferrochelatase. Purification, properties, and stimulation by fatty acids. J Biol Chem. 1981 Dec 25;256(24):12748–12753. [PubMed] [Google Scholar]
- Tsukamoto I., Yoshinaga T., Sano S. The role of zinc with special reference to the essential thiol groups in delta-aminolevulinic acid dehydratase of bovine liver. Biochim Biophys Acta. 1979 Sep 12;570(1):167–178. doi: 10.1016/0005-2744(79)90211-0. [DOI] [PubMed] [Google Scholar]
- Weiss G., Goossen B., Doppler W., Fuchs D., Pantopoulos K., Werner-Felmayer G., Wachter H., Hentze M. W. Translational regulation via iron-responsive elements by the nitric oxide/NO-synthase pathway. EMBO J. 1993 Sep;12(9):3651–3657. doi: 10.1002/j.1460-2075.1993.tb06039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton M., Granger D. L., Durack D. T. Mitochondrial iron loss from leukemia cells injured by macrophages. A possible mechanism for electron transport chain defects. J Immunol. 1988 Aug 15;141(4):1311–1317. [PubMed] [Google Scholar]