Abstract
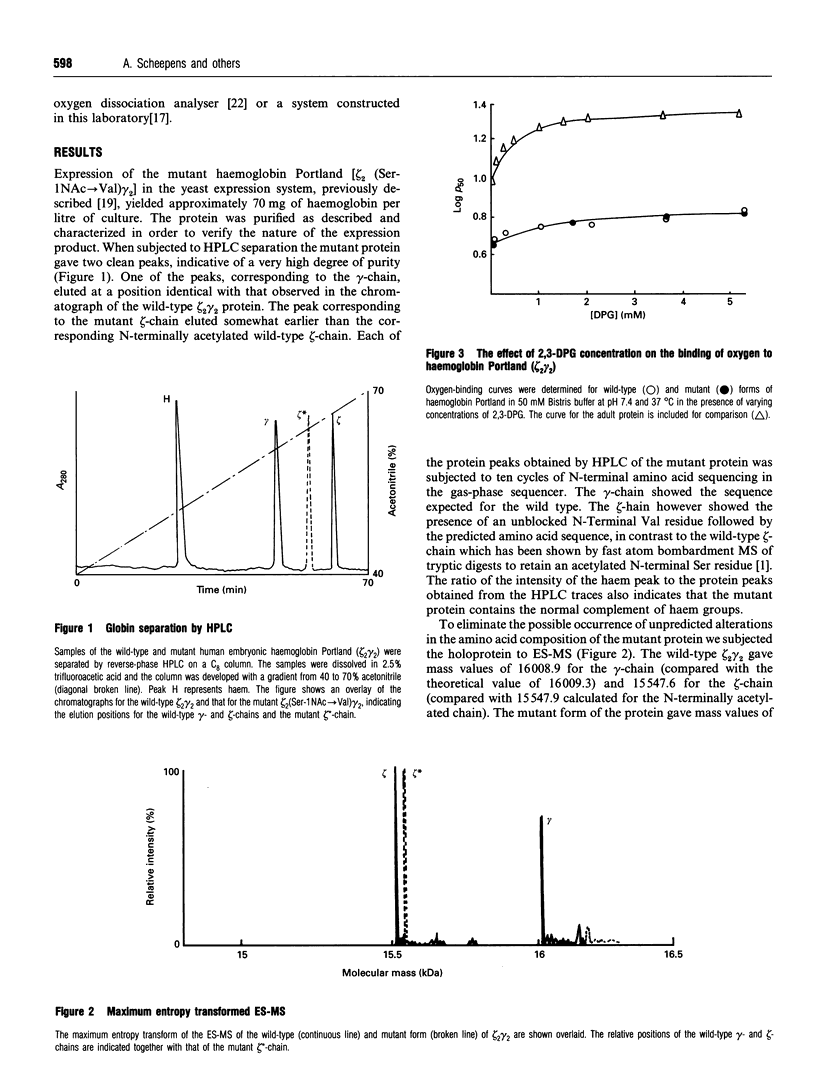
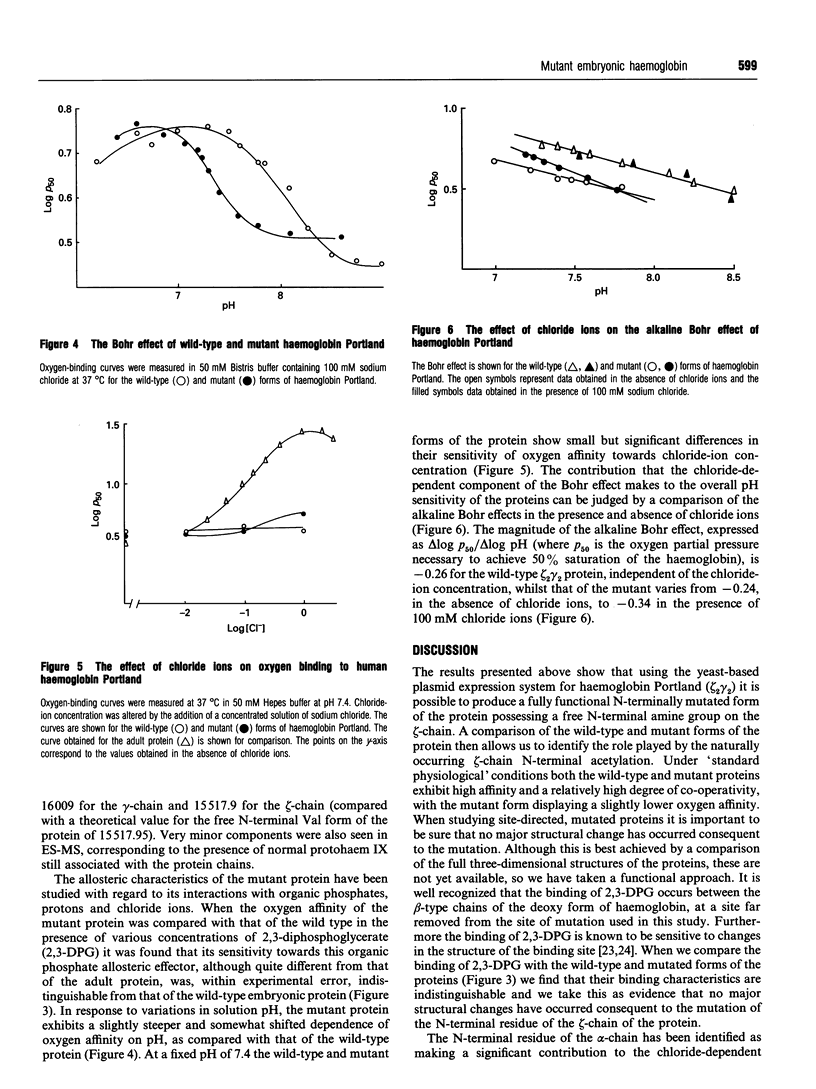
Using site-directed mutagenesis we have produced the first mutant form of a human embryonic haemoglobin. We have mutated the N-terminal Ser residue of the zeta-chain of haemoglobin Portland, zeta 2 gamma 2, (which is normally acetylated) to a Val (which possesses a free amine terminus). The protein spontaneously assembles into a fully functional tetramer which shows cooperative oxygen binding. Determination of the reactivity of the mutant protein with 2,3-diphosphoglycerate indicates that the mutation process does not lead to any major disruption of the protein structure. A comparison of the properties of the mutant and wild-type proteins identifies a significant role for the normal N-terminal acetylation of the zeta-chain with regard to the alkaline Bohr effect and the sensitivity of the oxygen affinity of the protein towards chloride ions. The possible physiological significance of this modification is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aschauer H., Schäfer W., Sanguansermsri T., Braunitzer G. Embryonale Hämoglobine des Menschen: Ac-Ser-Leu-Thr-, die N-terminale Sequenz der zeta-Ketten. Hoppe Seylers Z Physiol Chem. 1981 Dec;362(12):1657–1659. [PubMed] [Google Scholar]
- Bonaventura C., Arumugam M., Cashon R., Bonaventura J., Moo-Penn W. F. Chloride masks effects of opposing positive charges in Hb A and Hb Hinsdale (beta 139 Asn-->Lys) that can modulate cooperativity as well as oxygen affinity. J Mol Biol. 1994 Jun 17;239(4):561–568. doi: 10.1006/jmbi.1994.1395. [DOI] [PubMed] [Google Scholar]
- Bonaventura C., Bonaventura J., Amiconi G., Tentori L., Brunori M., Antonini E. Hemoglobin Abruzzo (beta143 (H21) His replaced by Arg). Consequences of altering the 2,3-diphosphoglycerate binding site. J Biol Chem. 1975 Aug 25;250(16):6273–6277. [PubMed] [Google Scholar]
- Bonaventura J., Bonaventura C., Sullivan B., Ferruzzi G., McCurdy P. R., Fox J., Moo-Penn W. F. Hemoglobin providence. Functional consequences of two alterations of the 2,3-diphosphoglycerate binding site at position beta 82. J Biol Chem. 1976 Dec 10;251(23):7563–7571. [PubMed] [Google Scholar]
- Clegg J. B., Gagnon J. Structure of the zeta chain of human embryonic hemoglobin. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6076–6080. doi: 10.1073/pnas.78.10.6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann O., Carrucan G., Robson N., Brittain T. The chloride effect in the human embryonic haemoglobins. Biochem J. 1995 Aug 1;309(Pt 3):959–962. doi: 10.1042/bj3090959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann O., Mould R., Brittain T. Allosteric modulation of oxygen binding to the three human embryonic haemoglobins. Biochem J. 1995 Mar 1;306(Pt 2):367–370. doi: 10.1042/bj3060367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann O., Mould R., Brittain T. Allosteric modulation of oxygen binding to the three human embryonic haemoglobins. Biochem J. 1995 Mar 1;306(Pt 2):367–370. doi: 10.1042/bj3060367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh J. S., Rogers P. H., Case D. A., Arnone A. High-resolution X-ray study of deoxyhemoglobin Rothschild 37 beta Trp----Arg: a mutation that creates an intersubunit chloride-binding site. Biochemistry. 1992 Apr 28;31(16):4111–4121. doi: 10.1021/bi00131a030. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Breen J. J., Roberts G. C., Ho C. Direct measurement of the pK values of an alkaline Bohr group in human hemoglobin. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1246–1249. doi: 10.1073/pnas.70.4.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin J. V., Rossi-Bernardi L. Inhibition of CO2 combination and reduction of the Bohr effect in haemoglobin chemically modified at its alpha-amino groups. Nature. 1969 Jun 28;222(5200):1243–1246. doi: 10.1038/2221243a0. [DOI] [PubMed] [Google Scholar]
- Mould R. M., Hofmann O. M., Brittain T. Production of human embryonic haemoglobin (Gower II) in a yeast expression system. Biochem J. 1994 Mar 15;298(Pt 3):619–622. doi: 10.1042/bj2980619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell S., Mandaro R., Schuster T. M., Arnone A. X-ray diffraction and solution studies of specifically carbamylated human hemoglobin A. Evidence for the location of a proton- and oxygen-linked chloride binding site at valine 1 alpha. J Biol Chem. 1979 Dec 10;254(23):12204–12208. [PubMed] [Google Scholar]
- Perutz M. F., Fermi G., Poyart C., Pagnier J., Kister J. A novel allosteric mechanism in haemoglobin. Structure of bovine deoxyhaemoglobin, absence of specific chloride-binding sites and origin of the chloride-linked Bohr effect in bovine and human haemoglobin. J Mol Biol. 1993 Oct 5;233(3):536–545. doi: 10.1006/jmbi.1993.1530. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Mazzarella L., Crowther R. A., Greer J., Kilmartin J. V. Identification of residues responsible for the alkaline Bohr effect in haemoglobin. Nature. 1969 Jun 28;222(5200):1240–1243. doi: 10.1038/2221240a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Shih D. T., Williamson D. The chloride effect in human haemoglobin. A new kind of allosteric mechanism. J Mol Biol. 1994 Jun 17;239(4):555–560. doi: 10.1006/jmbi.1994.1394. [DOI] [PubMed] [Google Scholar]
- Shaanan B. Structure of human oxyhaemoglobin at 2.1 A resolution. J Mol Biol. 1983 Nov 25;171(1):31–59. doi: 10.1016/s0022-2836(83)80313-1. [DOI] [PubMed] [Google Scholar]
- Stegink L. D., Meyer P. D., Brummel M. C. Human fetal hemoglobin F 1. Acetylation status. J Biol Chem. 1971 May 10;246(9):3001–3007. [PubMed] [Google Scholar]
- Wagenbach M., O'Rourke K., Vitez L., Wieczorek A., Hoffman S., Durfee S., Tedesco J., Stetler G. Synthesis of wild type and mutant human hemoglobins in Saccharomyces cerevisiae. Biotechnology (N Y) 1991 Jan;9(1):57–61. doi: 10.1038/nbt0191-57. [DOI] [PubMed] [Google Scholar]


