Abstract
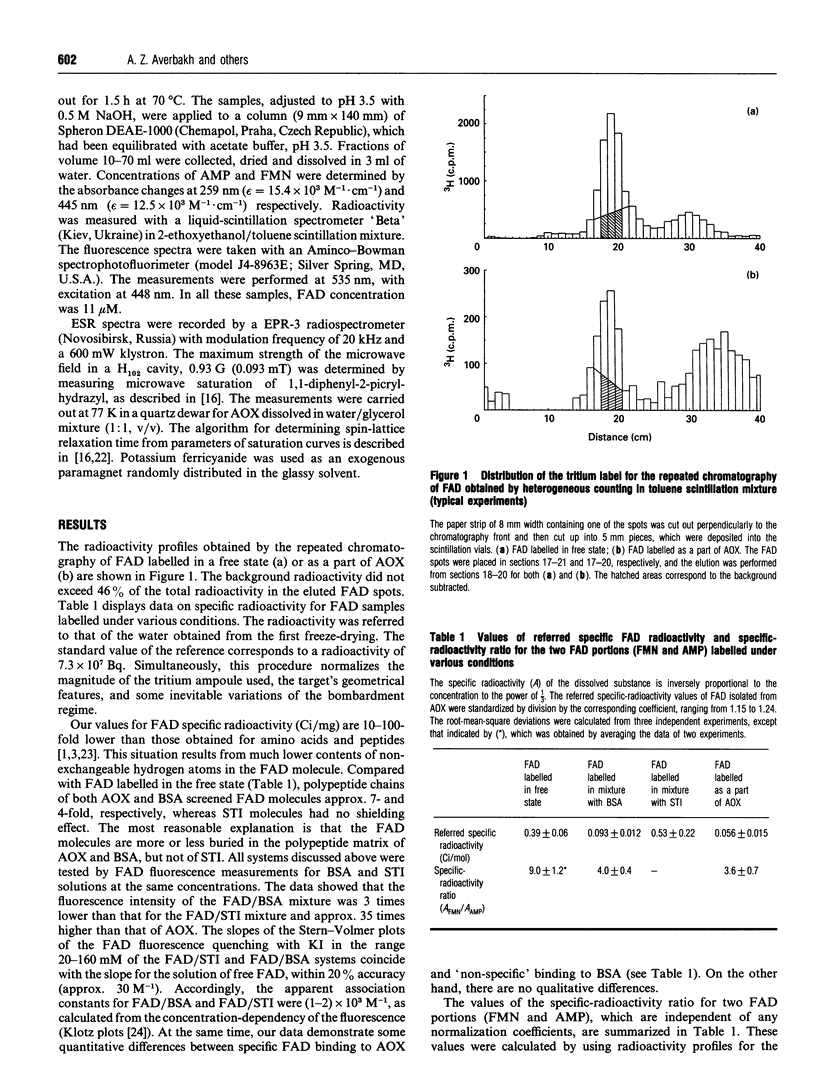
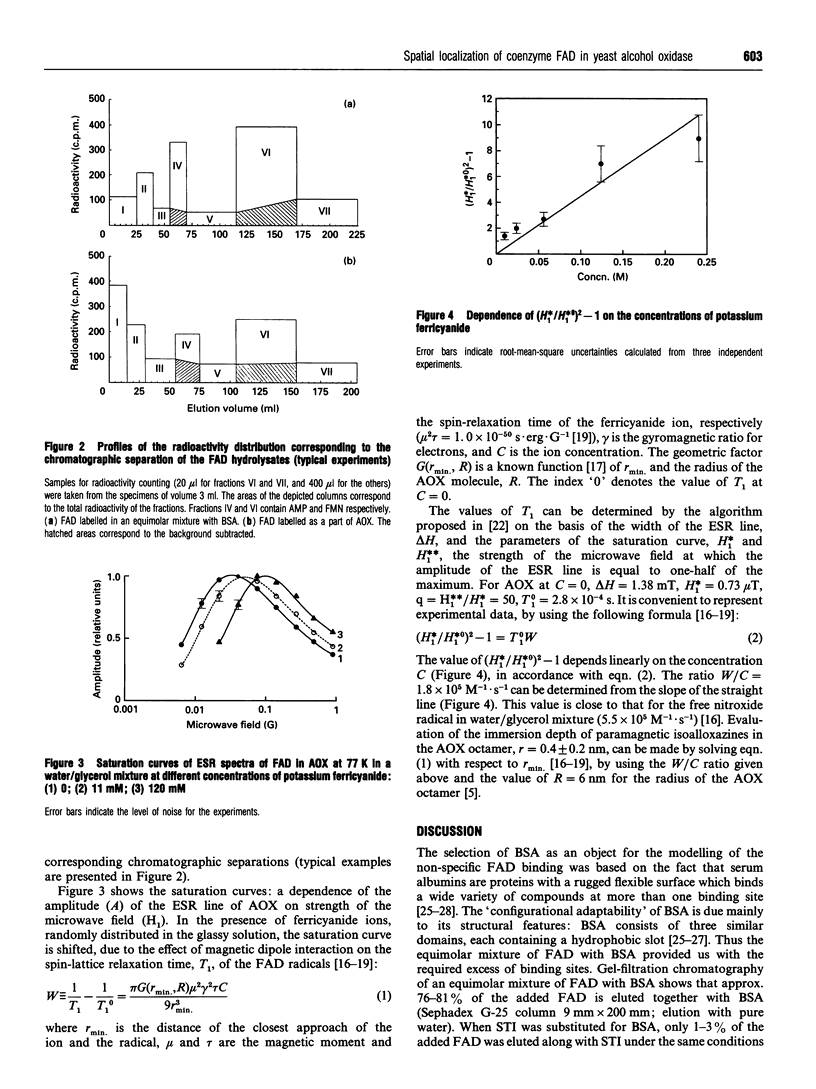
The spatial localization of the coenzyme FAD in the quaternary structure of the alcohol oxidase from the yeast Pichia pinus was studied by tritium planigraphy and ESR methods. In the present paper we measured the specific radioactivity of FAD labelled as a part of the alcohol oxidase complex. The specific-radioactivity ratio for two FAD portions (FMN and AMP) was calculated. ESR experiments show 4 A (0.4 nm) to be the depth of immersion of paramagnetic isoalloxazines into alcohol oxidase octamer molecules. It is suggested that FAD molecules are bound to the surface of the octamer, rather than to the subunit interfaces. The orientation of the prosthetic group FAD in the alcohol oxidase protein is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birk Y. The Bowman-Birk inhibitor. Trypsin- and chymotrypsin-inhibitor from soybeans. Int J Pept Protein Res. 1985 Feb;25(2):113–131. doi: 10.1111/j.1399-3011.1985.tb02155.x. [DOI] [PubMed] [Google Scholar]
- Boys C. W., Hill D. J., Stockley P. G., Woodward J. R. Crystallization of alcohol oxidase from Pichia pastoris. J Mol Biol. 1989 Jul 5;208(1):211–212. doi: 10.1016/0022-2836(89)90099-5. [DOI] [PubMed] [Google Scholar]
- Bystrykh L. V., Dijkhuizen L., Harder W. Modification of flavin adenine dinucleotide in alcohol oxidase of the yeast Hansenula polymorpha. J Gen Microbiol. 1991 Oct;137(10):2381–2386. doi: 10.1099/00221287-137-10-2381. [DOI] [PubMed] [Google Scholar]
- Carter D. C., He X. M., Munson S. H., Twigg P. D., Gernert K. M., Broom M. B., Miller T. Y. Three-dimensional structure of human serum albumin. Science. 1989 Jun 9;244(4909):1195–1198. doi: 10.1126/science.2727704. [DOI] [PubMed] [Google Scholar]
- Dixon M. The acceptor specificity of flavins and flavoproteins. I. Techniques for anaerobic spectrophotometry. Biochim Biophys Acta. 1971 Mar 2;226(2):241–258. doi: 10.1016/0005-2728(71)90092-2. [DOI] [PubMed] [Google Scholar]
- Gedrovich A., Shishkov A., Goldanskii V., Baratova L., Grebenshchikov N., Efimov A. Modelling protein three-dimensional structure using tritium planigraphy. Eur Biophys J. 1991;19(6):283–286. doi: 10.1007/BF00183316. [DOI] [PubMed] [Google Scholar]
- Geissler J., Ghisla S., Kroneck P. M. Flavin-dependent alcohol oxidase from yeast. Studies on the catalytic mechanism and inactivation during turnover. Eur J Biochem. 1986 Oct 1;160(1):93–100. doi: 10.1111/j.1432-1033.1986.tb09944.x. [DOI] [PubMed] [Google Scholar]
- Geissler J., Hemmerich P. Yeast methanol oxidases: an unusual type of flavoprotein. FEBS Lett. 1981 Apr 20;126(2):152–156. doi: 10.1016/0014-5793(81)80229-3. [DOI] [PubMed] [Google Scholar]
- Gol'danskii V. I., Rumiantsev Iu M., Shishkov A. V., Baratova L. A., Belianova L. P. Issledovanie prostranstvennoi struktury belkov pri pomoshchi tritievoi metki. II. Vnutrimolekuliarnoe raspredelenie tritiia v N-kontsevoi chasti mioglobina i tretichnaia struktura belka. Mol Biol (Mosk) 1982 May-Jun;16(3):528–534. [PubMed] [Google Scholar]
- Goldanskii V. I., Kashirin I. A., Shishkov A. V., Baratova L. A., Grebenshchikov N. I. The use of thermally activated tritium atoms for structural-biological investigations: the topography of the TMV protein-accessible surface of the virus. J Mol Biol. 1988 Jun 5;201(3):567–574. doi: 10.1016/0022-2836(88)90638-9. [DOI] [PubMed] [Google Scholar]
- Johanson K. O., Wetlaufer D. B., Reed R. G., Peters T., Jr Refolding of bovine serum albumin and its proteolytic fragments. Regain of disulfide bonds, secondary structure, and ligand-binding ability. J Biol Chem. 1981 Jan 10;256(1):445–450. [PubMed] [Google Scholar]
- Kalb V. F., Jr, Bernlohr R. W. A new spectrophotometric assay for protein in cell extracts. Anal Biochem. 1977 Oct;82(2):362–371. doi: 10.1016/0003-2697(77)90173-7. [DOI] [PubMed] [Google Scholar]
- Kato N., Omori Y., Tani Y., Ogata K. Alcohol oxidases of Kloeckera sp. and Hansenula polymorpha. Catalytic properties and subunit structures. Eur J Biochem. 1976 May 1;64(2):341–350. doi: 10.1111/j.1432-1033.1976.tb10307.x. [DOI] [PubMed] [Google Scholar]
- Kulikov A. V., Cherepanova E. S., Bogatyrenko V. R., Nasonova T. A., Fisher V. R. Opredelenie glubiny pogruzheniia radikalov v biologicheskie matritsy metodom EPR. Izv Akad Nauk SSSR Biol. 1987 Sep-Oct;(5):762–769. [PubMed] [Google Scholar]
- Likhtenstein G. I., Kulikov A. V., Kotelnikov A. I., Levchenko L. A. Methods of physical labels--a combined approach to the study of microstructure and dynamics in biological systems. J Biochem Biophys Methods. 1986 Jan;12(1-2):1–28. doi: 10.1016/0165-022x(86)90047-3. [DOI] [PubMed] [Google Scholar]
- Sahm H., Wagner F. Microbial assimilation of methanol. The ethanol- and methanol-oxidizing enzymes of the yeast Candida boidinii. Eur J Biochem. 1973 Jul 2;36(1):250–256. doi: 10.1111/j.1432-1033.1973.tb02907.x. [DOI] [PubMed] [Google Scholar]
- Tsetlin V. I., Alyonycheva T. N., Shemyakin V. V., Neiman L. A., Ivanov V. T. Tritium thermal activation study of bacteriorhodopsin topography. Eur J Biochem. 1988 Dec 1;178(1):123–129. doi: 10.1111/j.1432-1033.1988.tb14437.x. [DOI] [PubMed] [Google Scholar]
- Tsuge H., Mitsuda H. Studies on the molecular complex of flavins. V. Possible role of free sulfhydryl group in apoprotein of glucose oxidase and 6-amino group in adenine moiety of FAD. J Biochem. 1974 Feb;75(2):399–406. doi: 10.1093/oxfordjournals.jbchem.a130406. [DOI] [PubMed] [Google Scholar]
- Tykarska E., Lebioda L., Marchut E., Steczko J., Stec B. Crystallization of alcohol oxidase from Pichia pastoris. Secondary structure predictions indicate a domain with the eightfold beta/alpha-barrel fold. J Protein Chem. 1990 Feb;9(1):83–86. doi: 10.1007/BF01024988. [DOI] [PubMed] [Google Scholar]
- Van der Klei I. J., Lawson C. L., Rozeboom H., Dijkstra B. W., Veenhuis M., Harder W., Hol W. G. Use of electron microscopy in the examination of lattice defects in crystals of alcohol oxidase. FEBS Lett. 1989 Feb 13;244(1):213–216. doi: 10.1016/0014-5793(89)81195-0. [DOI] [PubMed] [Google Scholar]
- Vonck J., van Bruggen E. F. Electron microscopy and image analysis of two-dimensional crystals and single molecules of alcohol oxidase from Hansenula polymorpha. Biochim Biophys Acta. 1990 Mar 29;1038(1):74–79. doi: 10.1016/0167-4838(90)90012-5. [DOI] [PubMed] [Google Scholar]
- Wilton D. C. The fatty acid analogue 11-(dansylamino)undecanoic acid is a fluorescent probe for the bilirubin-binding sites of albumin and not for the high-affinity fatty acid-binding sites. Biochem J. 1990 Aug 15;270(1):163–166. doi: 10.1042/bj2700163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusupov M. M., Spirin A. S. Are there proteins between the ribosomal subunits? Hot tritium bombardment experiments. FEBS Lett. 1986 Mar 3;197(1-2):229–233. doi: 10.1016/0014-5793(86)80332-5. [DOI] [PubMed] [Google Scholar]
- Yusupov M. M., Spirin A. S. Hot tritium bombardment technique for ribosome surface topography. Methods Enzymol. 1988;164:426–439. doi: 10.1016/s0076-6879(88)64059-6. [DOI] [PubMed] [Google Scholar]
- van der Klei I. J., Bystrykh L. V., Harder W. Alcohol oxidase from Hansenula polymorpha CBS 4732. Methods Enzymol. 1990;188:420–427. doi: 10.1016/0076-6879(90)88067-k. [DOI] [PubMed] [Google Scholar]


