Abstract
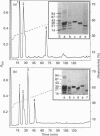
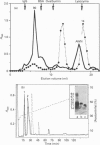
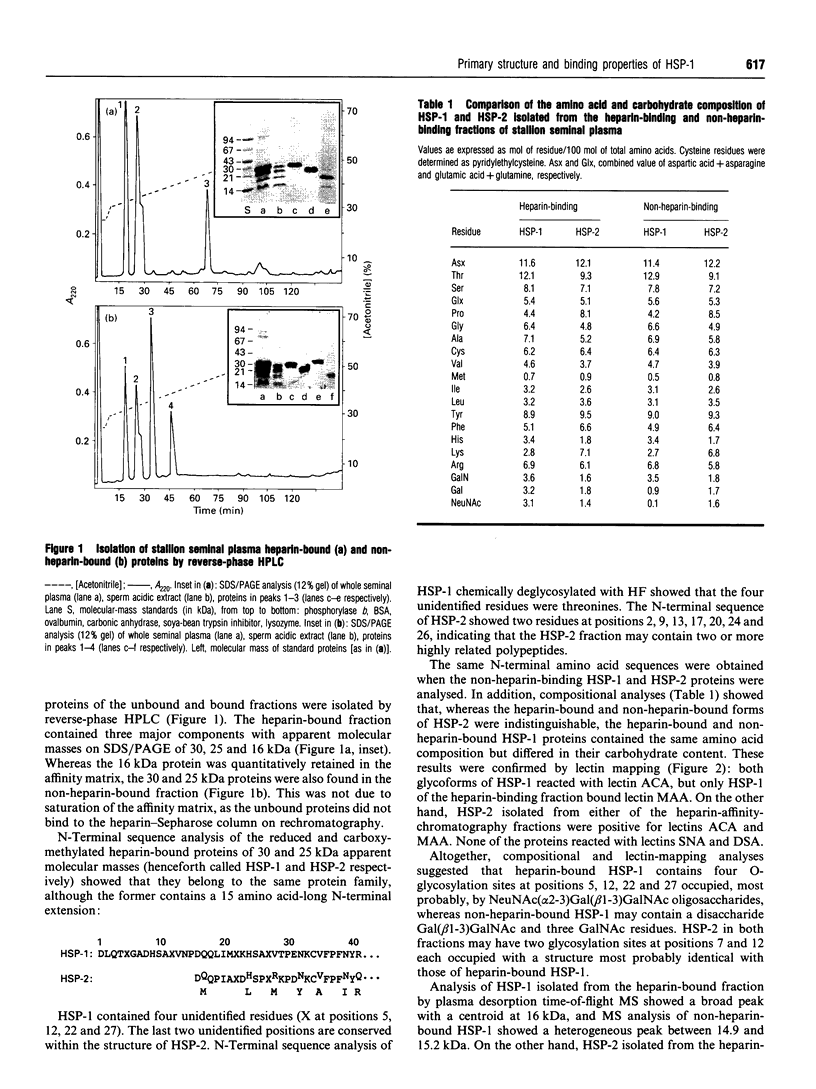
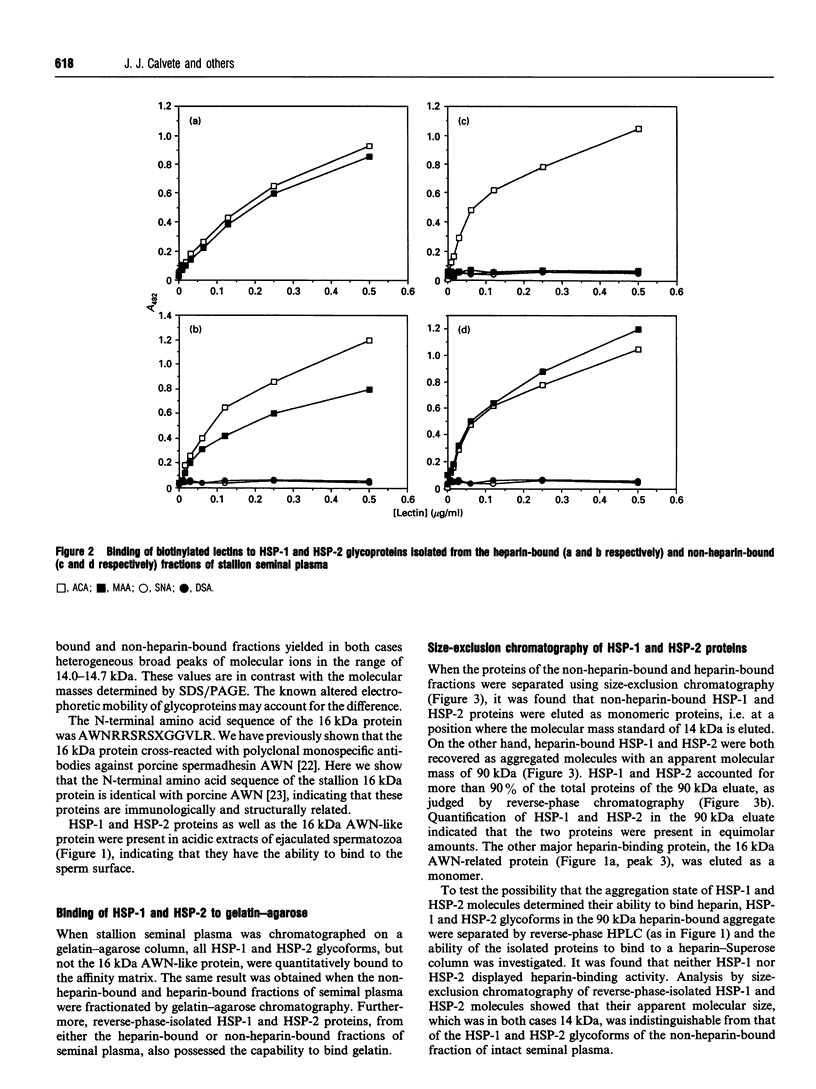
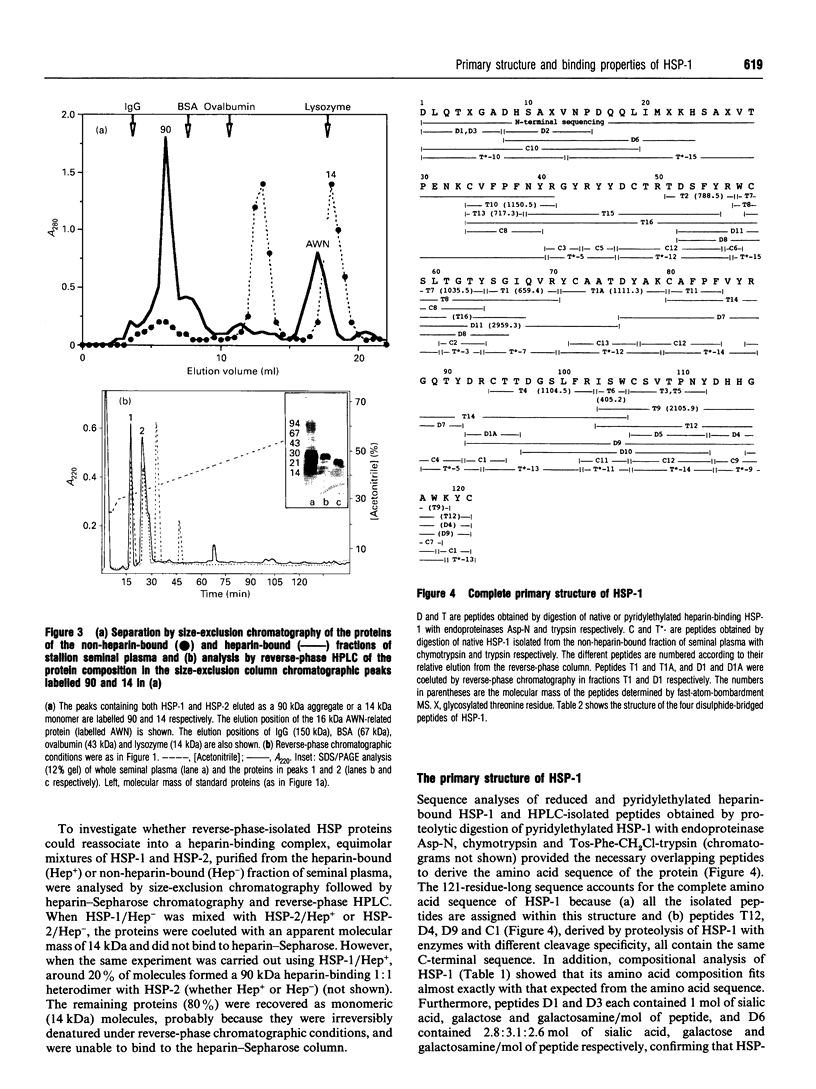
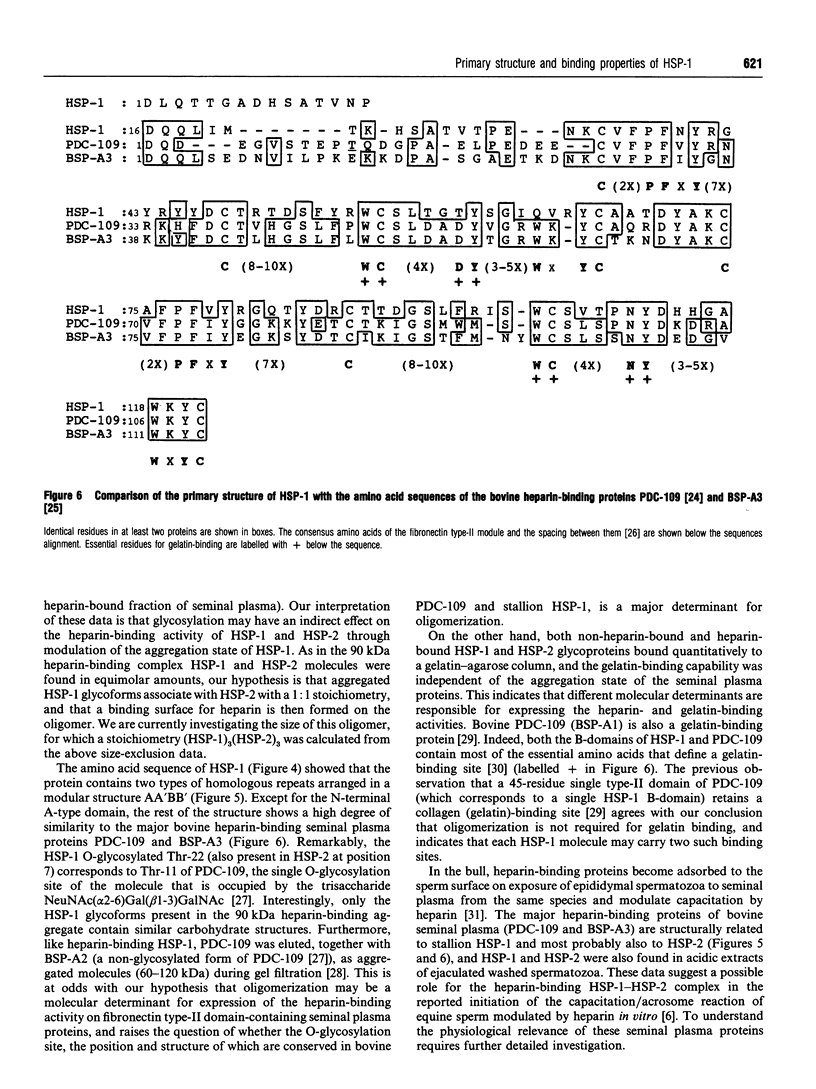
We report the complete amino acid sequence of HSP-1, a major protein isolated from stallion seminal plasma or acid extracts of ejaculated spermatozoa. The protein consists of 121 amino acids organized in two types of homologous repeats arranged in the pattern AA'BB'. Each of the 13-15-residue A-type repeats contains two O-linked oligosaccharide chains. The B-type repeats span 44-47 amino acids each, are not glycosylated, and have the consensus pattern of the gelatin-binding fibronectin type-II module. This domain also occurs in the major bovine seminal plasma heparin-binding proteins PDC-109 (BSP-A1/A2) and BSP-A3. However, unlike the bovine proteins which bind quantitatively to a heparin-Sepharose column, stallion HSP-1 was recovered in both the flow-through and the heparin-bound fractions. Structural analysis showed that the two HSP-1 forms contain identical polypeptide chains which are differently glycosylated. Moreover, size-exclusion chromatography showed that heparin-bound HSP-1 associates with HSP-2, another major seminal plasma protein, into a 90 kDa product, whereas the non-heparin-bound glycoform of HSP-1 is eluted as a monomeric (14 kDa) protein. This suggests that glycosylation may have an indirect effect on the heparin-binding ability of HSP-1 through modulation of its aggregation state. On the other hand, both glycoforms of HSP-1 displayed gelatin-binding activity, indicating that the molecular determinants for binding heparin and gelatin are different.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anumula K. R., Taylor P. B. Rapid characterization of asparagine-linked oligosaccharides isolated from glycoproteins using a carbohydrate analyzer. Eur J Biochem. 1991 Jan 1;195(1):269–280. doi: 10.1111/j.1432-1033.1991.tb15703.x. [DOI] [PubMed] [Google Scholar]
- Aumüller G., Vesper M., Seitz J., Kemme M., Scheit K. H. Binding of a major secretory protein from bull seminal vesicles to bovine spermatozoa. Cell Tissue Res. 1988 May;252(2):377–384. doi: 10.1007/BF00214380. [DOI] [PubMed] [Google Scholar]
- Bányai L., Trexler M., Koncz S., Gyenes M., Sipos G., Patthy L. The collagen-binding site of type-II units of bovine seminal fluid protein PDC-109 and fibronectin. Eur J Biochem. 1990 Nov 13;193(3):801–806. doi: 10.1111/j.1432-1033.1990.tb19403.x. [DOI] [PubMed] [Google Scholar]
- Calvete J. J., Raida M., Sanz L., Wempe F., Scheit K. H., Romero A., Töpfer-Petersen E. Localization and structural characterization of an oligosaccharide O-linked to bovine PDC-109. Quantitation of the glycoprotein in seminal plasma and on the surface of ejaculated and capacitated spermatozoa. FEBS Lett. 1994 Aug 22;350(2-3):203–206. doi: 10.1016/0014-5793(94)00768-3. [DOI] [PubMed] [Google Scholar]
- Chandonnet L., Roberts K. D., Chapdelaine A., Manjunath P. Identification of heparin-binding proteins in bovine seminal plasma. Mol Reprod Dev. 1990 Aug;26(4):313–318. doi: 10.1002/mrd.1080260404. [DOI] [PubMed] [Google Scholar]
- Collier I. E., Krasnov P. A., Strongin A. Y., Birkedal-Hansen H., Goldberg G. I. Alanine scanning mutagenesis and functional analysis of the fibronectin-like collagen-binding domain from human 92-kDa type IV collagenase. J Biol Chem. 1992 Apr 5;267(10):6776–6781. [PubMed] [Google Scholar]
- Desnoyers L., Manjunath P. Major proteins of bovine seminal plasma exhibit novel interactions with phospholipid. J Biol Chem. 1992 May 15;267(14):10149–10155. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einspanier R., Krause I., Calvete J. J., Töfper-Petersen E., Klostermeyer H., Karg H. Bovine seminal plasma aSFP: localization of disulfide bridges and detection of three different isoelectric forms. FEBS Lett. 1994 May 9;344(1):61–64. doi: 10.1016/0014-5793(94)00362-9. [DOI] [PubMed] [Google Scholar]
- Esch F. S., Ling N. C., Böhlen P., Ying S. Y., Guillemin R. Primary structure of PDC-109, a major protein constituent of bovine seminal plasma. Biochem Biophys Res Commun. 1983 Jun 29;113(3):861–867. doi: 10.1016/0006-291x(83)91078-1. [DOI] [PubMed] [Google Scholar]
- Haselbeck A., Schickaneder E., von der Eltz H., Hösel W. Structural characterization of glycoprotein carbohydrate chains by using diagoxigenin-labeled lectins on blots. Anal Biochem. 1990 Nov 15;191(1):25–30. doi: 10.1016/0003-2697(90)90381-i. [DOI] [PubMed] [Google Scholar]
- Jonákova V., Sanz L., Calvete J. J., Henschen A., Cechová D., Töpfer-Petersen E. Isolation and biochemical characterization of a zona pellucida-binding glycoprotein of boar spermatozoa. FEBS Lett. 1991 Mar 11;280(1):183–186. doi: 10.1016/0014-5793(91)80233-s. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leblond E., Desnoyers L., Manjunath P. Phosphorylcholine-binding proteins from the seminal fluids of different species share antigenic determinants with the major proteins of bovine seminal plasma. Mol Reprod Dev. 1993 Apr;34(4):443–449. doi: 10.1002/mrd.1080340414. [DOI] [PubMed] [Google Scholar]
- Manjunath P., Chandonnet L., Leblond E., Desnoyers L. Major proteins of bovine seminal vesicles bind to spermatozoa. Biol Reprod. 1994 Jan;50(1):27–37. doi: 10.1095/biolreprod50.1.27. [DOI] [PubMed] [Google Scholar]
- Manjunath P., Sairam M. R. Purification and biochemical characterization of three major acidic proteins (BSP-A1, BSP-A2 and BSP-A3) from bovine seminal plasma. Biochem J. 1987 Feb 1;241(3):685–692. doi: 10.1042/bj2410685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. J., Winer M. A., Ax R. L. Heparin-binding proteins from seminal plasma bind to bovine spermatozoa and modulate capacitation by heparin. Biol Reprod. 1990 May-Jun;42(5-6):899–915. doi: 10.1095/biolreprod42.6.899. [DOI] [PubMed] [Google Scholar]
- Parrish J. J., Susko-Parrish J. L., Handrow R. R., Ax R. L., First N. L. Effect of sulfated glycoconjugates on capacitation and the acrosome reaction of bovine and hamster spermatozoa. Gamete Res. 1989 Dec;24(4):403–413. doi: 10.1002/mrd.1120240407. [DOI] [PubMed] [Google Scholar]
- Sanz L., Calvete J. J., Mann K., Gabius H. J., Töpfer-Petersen E. Isolation and biochemical characterization of heparin-binding proteins from boar seminal plasma: a dual role for spermadhesins in fertilization. Mol Reprod Dev. 1993 May;35(1):37–43. doi: 10.1002/mrd.1080350107. [DOI] [PubMed] [Google Scholar]
- Sanz L., Calvete J. J., Mann K., Schäfer W., Schmid E. R., Amselgruber W., Sinowatz F., Ehrhard M., Töpfer-Petersen E. The complete primary structure of the spermadhesin AWN, a zona pellucida-binding protein isolated from boar spermatozoa. FEBS Lett. 1992 Apr 6;300(3):213–218. doi: 10.1016/0014-5793(92)80848-b. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Manjunath P., Rochemont J., Sairam M. R., Chrétien M. Complete amino acid sequence of BSP-A3 from bovine seminal plasma. Homology to PDC-109 and to the collagen-binding domain of fibronectin. Biochem J. 1987 Apr 1;243(1):195–203. doi: 10.1042/bj2430195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorstengaard K., Jensen M. S., Sahl P., Petersen T. E., Magnusson S. Complete primary structure of bovine plasma fibronectin. Eur J Biochem. 1986 Dec 1;161(2):441–453. doi: 10.1111/j.1432-1033.1986.tb10464.x. [DOI] [PubMed] [Google Scholar]
- Valencia A., Wens M. A., Merchant H., Reyes R., Delgado N. M. Capacitation of human spermatozoa by heparin. Arch Androl. 1984;12 (Suppl):109–113. [PubMed] [Google Scholar]
- Varner D. D., Bowen J. A., Johnson L. Effect of heparin on capacitation/acrosome reaction of equine sperm. Arch Androl. 1993 Nov-Dec;31(3):199–207. doi: 10.3109/01485019308988400. [DOI] [PubMed] [Google Scholar]
- Wieland F., Heitzer R., Schaefer W. Asparaginylglucose: Novel type of carbohydrate linkage. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5470–5474. doi: 10.1073/pnas.80.18.5470. [DOI] [PMC free article] [PubMed] [Google Scholar]