Abstract
Hepatitis delta virus (HDV) is a satellite of hepatitis B virus (HBV), which requires the HBV surface antigen (HBsAg) for its assembly and propagation. Although countries affected by HBV infection in Africa are well identified, data on HDV infection are still scarce, like in Nigeria, where HBV infection is endemic. In this study, we aimed to determine the prevalence of HDV infection and identify the circulating genotypes/strains in the country. A nationwide study was performed on 1281 HBsAg-positive samples collected from patients across eleven sites drawn from the six geopolitical zones in Nigeria. Anti-HDV antibody (HDV-Ab) screening and HDV-RNA viral load quantification were performed using a commercial ELISA assay and real-time RT-PCR kit, respectively. HDV genotyping was performed by the Sanger sequencing of amplicons from the so-called R0 region of the viral genome, followed by phylogenetic analyses. Of the 1281 HBsAg-positive samples, 61 (4.8%) were HDV-Ab positive, among which, 12 (19.7%) were HDV-RNA positive. Genotypes were obtained for nine of them: seven “African” HDV-1, one “Asian/European” HDV-1 and one HDV-6. This study shows that Nigeria is a country of low HDV prevalence where mainly “African” genotype-1 strains are circulating.
Keywords: HDV, genotype, Nigeria, nationwide study
1. Introduction
Hepatitis D virus (HDV) is a subviral satellite of Hepatitis B virus (HBV), requiring HBV envelope proteins for its assembly and propagation. Simultaneous infection with HBV and HDV (co-infection) or HDV infection in a chronically HBV-infected patient (super-infection) can occur. Globally, an estimated 12 million individuals (4.5%) among the general population of HBsAg carriers worldwide also have serological evidence of HDV infection with geographical heterogeneity [1]. So far, at the molecular level, eight HDV genotypes (HDV-1 to -8) and several sub-genotypes (within genotypes) have been described across the globe with distinct geographic origin and distribution [2,3,4]. While HDV-1 has been reported to have a wider geographical spread, others (HDV-2 to -8) showed a more confined distribution, with HDV-2 and -4 spreading in Asia, mainly in Eastern and Northern Asia; HDV-3 in South America; and HDV 5–8 seen majorly in sub-Saharan Africa (SSA) [3].
Nigeria, a country of over 200 million individuals with distinct ethnic populations spread across its six geopolitical zones, is a country of HBV hyperendemicity [5,6]. HBV prevalence varies greatly across these zones; however, a national average prevalence of 8.1% has been recorded among the adult population [5,7]. Available HDV reports showed prevalence ranging between 2 and 15% among different population groups in particular regions, but no data on some other regions [8,9,10,11,12,13,14]. Thus, HDV burden in many parts of Nigeria remains unknown, as screening for HDV in HBV-infected individuals (even among those with worsening liver disease due to HBV) is not a routine practice in most hospitals in Nigeria. Therefore, we carried out a nationwide screen of HDV from plasma samples obtained from 11 sites (including 10 states and the federal capital territory) spread across the six geopolitical zones of Nigeria.
2. Materials and Methods
2.1. Study Area
The study sites were hospitals selected from 11 sites (located in 10 states and the Federal Capital Territory) across the six geopolitical zones of Nigeria (Figure 1). The selected hospitals included major referral and/or tertiary hospitals that provide specialized health care services to the inhabitants of the city and nearby environs in the individual states where they are located.
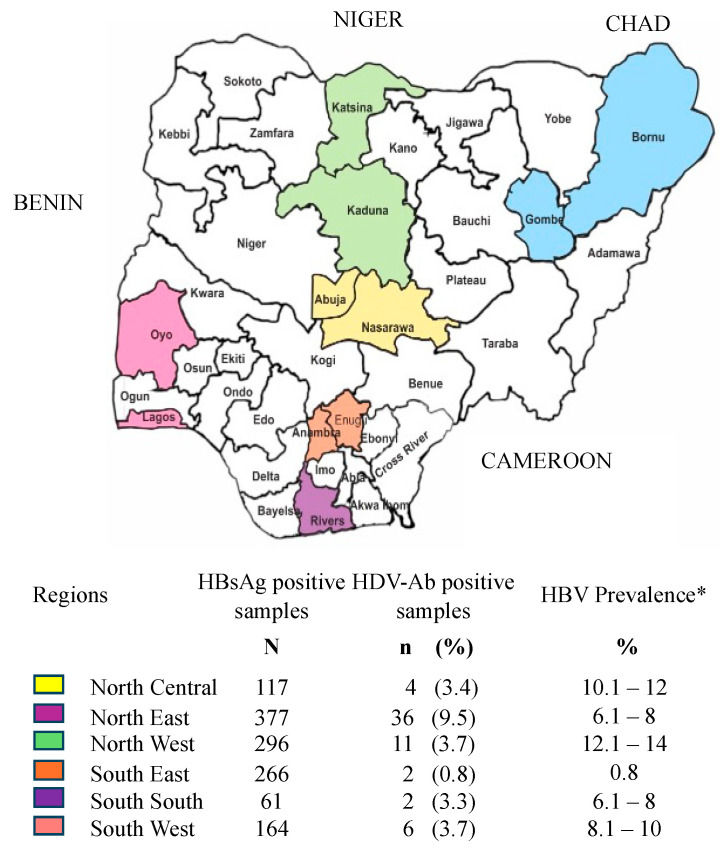
Figure 1.
Prevalence of HDV-Ab in HBsAg-positive samples over the whole country. * HBV Prevalence as previously documented by Ajuwon et al. [5].
2.2. Patients and Samples
A cross-sectional nationwide study was carried out among 1281 consenting chronic HBV patients attending the medical outpatient clinic (MOP) of the various selected health facilities between October 2017 and January 2019. The participants included 706 males and 575 females aged between 2 and 73 years (median age 34.6). The participants were asymptomatic HBsAg carriers who were referred to the specialist clinics on account of an initial positive HBsAg screen from either the general outpatient department (GOPD) within the same hospital or primary health care centers and other health care facilities around the major referral hospitals (study sites). Parental consent and assent (where applicable) were sought and obtained for underage participants before enrolment into the study. Consenting participants were rescreened onsite for HBsAg using a rapid HBsAg test kit (Acumen labs and diagnostic center, Bangalore, India) according to the manufacturer’s instructions. Thereafter, blood samples were collected by elbow venipuncture into EDTA tubes. Plasma was separated into appropriately labeled cryovials and stored at −20 degrees Celsius in the hospital laboratories. At the end of recruitment, the stored cryovials were sent to the Institute for Advanced Medical Research and Training (IAMRAT) University of Ibadan Nigeria where they were kept at −80 degrees Celsius until they were shipped to the French National Reference Laboratory for HDV (FNRL-HDV) for HDV antibody and RNA viral load quantification and genotyping.
2.3. HDV-Antibody, HDV-RNA Viral Load and HDV Genotyping
HDV-Ab positivity testing was performed using the LIAISON® XL fully automated chemiluminescence analyzer from Diasorin (Saluggia, Italy), and HDV-RNA viral load was quantified in all positive samples using the CE marked Eurobio HDV kit EBX-004 (Courtaboeuf, Les Ulis, France) [15]. HDV genotyping was performed on all replicating strains using direct sequencing by the Sanger method of amplicons of the so-called R0 region of the genome exactly as described earlier [3]. Phylogenetic trees were performed using well-characterized strains of all genotypes from our collection, including the ubiquitous HDV-1 from Asia, Europe and Africa.
3. Results
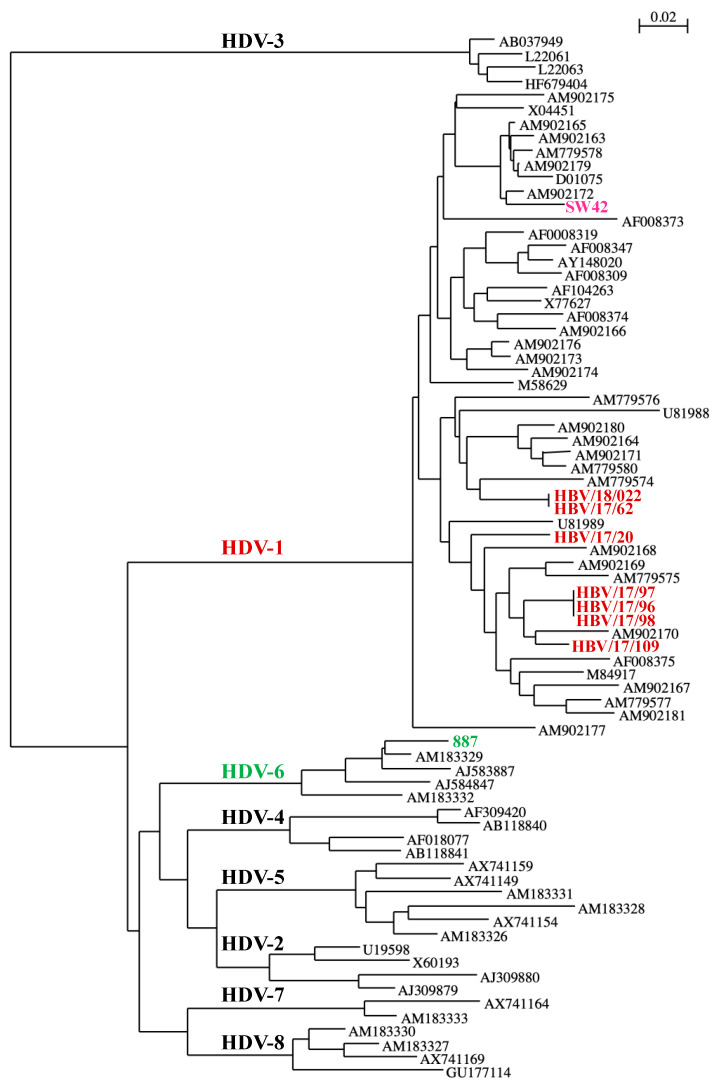
In total, 61 (4.8%) of the 1281 HBsAg-positive samples screened were found to be positive for anti-HDV antibodies (HDV-Ab). Nigeria can be classified as a low to very low country for HDV infection, ranging from 0.8 to 9.5%, with regional disparities. Indeed, a prevalence of nearly 10% was observed in the northeast region compared to 3 to 4% in other regions of the country (Figure 1). Only 12 patients (19.7%) were viremic, with a mean viral load of 4.4 log IU/mL, ranging from 2.8 to 7.4 log IU/mL. Due to the volume of samples available, genotypes could only be obtained from nine of the twelve viremic patients, among which, seven were the African HDV-1 genotype; one a non-African (European/Asian) HDV-1 {(sample SW42)-possessing alanine instead of serine at amino acid position 202 of the large delta protein and the remainder were HDV-6 (Figure 2). Of note, three HDV-1-strains infecting patients who were siblings were identical, indicating an intrafamilial transmission of the infection.
Figure 2.
Phylogenetic tree showing genotype distribution of HDV strains from this study. Study isolates are indicated in red (African HDV-1), pink (non-African HDV-1) and green (HVD-6).
4. Discussion
The pooled anti-HDV prevalence of 4.8% from this study suggests that Nigeria is a country of low endemicity for HDV infection among individuals with asymptomatic chronic HBV infection, albeit with regional variations. Similar observations have been noted in previous studies, with reported prevalence ranging from 0 to 5.6% in similar cohorts [9,11,16,17,18]. However, higher anti-HDV prevalences of 12.5% and 16.7% have also been reported in similar cohorts in Nigeria [19,20]. This observed difference in rates reported by these other studies is probably due to variations in sample size, notable regional variations in HDV prevalence, assay variation or other factors.
In addition, the prevalence of viremic patients was low, as only 19.7% of the infected patients actively replicated the virus even while they were not on any treatment for HDV. This low HDV viremia among HDV-infected persons in sub-Saharan Africa (SSA) has been noted by Stockdale et al. [10] in a systematic review and meta- analysis describing the global prevalence of hepatitis Delta infection. Indeed, other studies have documented low HDV RNA among HDV-Ab-positive samples [8,9,21]. Factors that may be responsible for the low level of HDV active replication in the SSA population are still not clear. However, one could hypothesize earlier infection at birth or in the perinatal period with the immune tolerance phenomenon. Regional disparities, especially in the northeast, are seen and are very likely related to the common border with Cameroon, a country of high HDV endemicity. As expected, African genotypes, mainly HDV-1, are spreading in the country. However, the main results of this remarkable nationwide study will need to be consolidated with larger sampling because of the low prevalence of HDV infection. In this setting, the HDV-Ab reflex testing of all HBV-infected patients should be implemented. The development of the newly described rapid diagnostic test [22] would be of great interest.
Author Contributions
Conceptualization, I.M.I. and E.G.; methodology and formal analysis, A.G., F.L.G. and S.B.; writing—original draft preparation, I.M.I. and E.G.; writing—review and editing, S.A.B., G.A., S.O.K., S.D., C.A., P.-A.B.G. and O.M.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of participating centers with the following approval numbers: UI/EC/18/0314; UMTH/REC/17/119; FHREC/2017/01/24/06-08-17; FMCNHREC.REG.N003/082012.
Informed Consent Statement
All subjects gave their informed consent for inclusion before they participated in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
Emmanuel Gordien declares that he has attended speaking engagements, has received invitations to scientific meetings and has provided consulting and scientific assistance for Gilead Sciences and Eurobio.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Stockdale A.J., Kreuels B., Henrion M.Y.R., de Martel C., Hutin Y., Geretti A.M. The global prevalence of hepatitis D virus infection: Systematic review and meta-analysis. J. Hepatol. 2020;73:523–532. doi: 10.1016/j.jhep.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ivaniushina V., Radjef N., Alexeeva M., Gault E., Semenov S., Salhi M., Kiselev O., Dény P. Hepatitis delta virus genotypes I and II cocirculate in an endemic area of Yakutia, Russia. J. Gen. Virol. 2001;82:2709–2718. doi: 10.1099/0022-1317-82-11-2709. [DOI] [PubMed] [Google Scholar]
- 3.Le Gal F., Brichler S., Drugan T., Alloui C., Roulot D., Pawlotsky J., Dény P., Gordien E. Genetic diversity and worldwide distribution of the deltavirus genus: A study of 2,152 clinical strains. Hepatology. 2017;66:1826–1841. doi: 10.1002/hep.29574. [DOI] [PubMed] [Google Scholar]
- 4.Radjef N., Gordien E., Ivaniushina V., Gault E., Anaïs P., Drugan T., Trinchet J.-C., Roulot D., Tamby M., Milinkovitch M.C., et al. Molecular Phylogenetic Analyses Indicate a Wide and Ancient Radiation of African Hepatitis Delta Virus, Suggesting a Deltavirus Genus of at Least Seven Major Clades. J. Virol. 2004;78:2537–2544. doi: 10.1128/JVI.78.5.2537-2544.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ajuwon B.I., Yujuico I., Roper K., Richardson A., Sheel M., Lidbury B.A. Hepatitis B virus infection in Nigeria: A systematic review and meta-analysis of data published between 2010 and 2019. BMC Infect. Dis. 2021;21:1120. doi: 10.1186/s12879-021-06800-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Bureau of Statistics Demographic Statistics Bulletin. Abuja Dec. [(accessed on 2 July 2024)];2023 Available online: https://nigerianstat.gov.ng/elibrary/read/1241422.
- 7.Federal Ministry of Health Nigeria Nigeria HIV/AIDS Indicator and Impact Survey (NAIIS) 2018: Technical Report. Abuja Oct. [(accessed on 2 July 2024)];2019 Available online: www.health.gov.ng.
- 8.Opaleye O.O., Akanbi O.A., Osundare F.A., Wang B., Adesina O., Oluremi A.S., Sunday S.T., Akindele A.A., Klink P., Bock C.T. Prevalence and characteristics of hepatitis B and D virus infections among HIV-positive individuals in Southwestern Nigeria. Virol. J. 2021;18:20. doi: 10.1186/s12985-021-01493-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Opaleye O.O., Japhet O.M., Adewumi O.M., Omoruyi E.C., Akanbi O.A., Oluremi A.S., Wang B., van Tong H., Velavan T.P., Bock C.-T. Molecular epidemiology of hepatitis D virus circulating in Southwestern Nigeria. Virol. J. 2016;13:61. doi: 10.1186/s12985-016-0514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stockdale A.J., Chaponda M., Beloukas A., Phillips R.O., Matthews P.C., Papadimitropoulos A., King S., Bonnett L., Geretti A.M. Prevalence of hepatitis D virus infection in sub-Saharan Africa: A systematic review and meta-analysis. Lancet Glob. Health. 2017;5:e992–e1003. doi: 10.1016/S2214-109X(17)30298-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nwokediuko S.C., Ijeoma U. Seroprevalence of antibody to HDV in Nigerians with hepatitis B virus-related liver diseases. Niger. J. Clin. Pract. 2009;12:439–442. [PubMed] [Google Scholar]
- 12.Ifeorah I.M., Bakarey A.S., Adeniji J.A., Onyemelukwe F.N. Seroprevalence of hepatitis B and delta viruses among HIV-infected population attending anti-retroviral clinic in selected health facilities in Abuja, Nigeria. J. Immunoass. Immunochem. 2017;38:608–619. doi: 10.1080/15321819.2017.1372474. [DOI] [PubMed] [Google Scholar]
- 13.Ifeorah I.M., Faleye T.O.C., Bakarey A.S., Adewumi O.M., Gerber A., Le Gal F., Adeniji J.A., Gordien E., Onyemelukwe N.F. Characterization of hepatitis delta virus strains spreading in Abuja, Nigeria. J. Med. Virol. 2019;91:1688–1692. doi: 10.1002/jmv.25503. [DOI] [PubMed] [Google Scholar]
- 14.Sobajo O.A., George U.E., Osasona O.G., Eromon P., Aborisade O.Y., Ajayi O.D., Folarin O.A., Komolafe I.O.O. Seroprevalence, co-infection and risk of transmission of Hepatitis B and D virus among hospital attendees in two South-western states in Nigeria. J. Immunoass. Immunochem. 2022;44:133–146. doi: 10.1080/15321819.2022.2141578. [DOI] [PubMed] [Google Scholar]
- 15.Le Gal F., Dziri S., Gerber A., Alloui C., Ben Abdesselam Z., Roulot D., Brichler S., Gordien E. Performance Characteristics of a New Consensus Commercial Kit for Hepatitis D Virus RNA Viral Load Quantification. J. Clin. Microbiol. 2017;55:431–441. doi: 10.1128/JCM.02027-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adeleye O.O., Lesi O.A., Onyekewere C.A. Prevalence of Hepatitis Delta in Nigerian Subjects with Chronic Hepatitis B Virus Infection. [(accessed on 1 July 2024)];Niger. Med. Pract. 2015 68:3–6. Available online: https://www.ajol.info/index.php/nmp/article/view/192147. [Google Scholar]
- 17.Okonkwo U.C., Okpara H.C., Inaku K., Aluka T.M., Chukwudike E.S., Ogarekpe Y., Emin E.J., Hodo O., Otu A.A. Prevalence and risk factors of Hepatitis D virus antibody among asymptomatic carriers of Hepatitis B virus: A community survey. Afr. Health Sci. 2022;22:504–510. doi: 10.4314/ahs.v22i1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onyekwere C.A., Audu R.A., Duro-Emmanuel F., Ige F.A. Hepatitis D infection in Nigeria. Indian J. Gastroenterol. 2012;31:34–35. doi: 10.1007/s12664-011-0158-9. [DOI] [PubMed] [Google Scholar]
- 19.Abdulkareem L.O., Ndububa D.A., Uhunmwangho A.O., Yunusa T. Hepatitis D virus antibodies and liver function profile among patients with chronic hepatitis B infection in Abuja, Nigeria. J. Infect. Dev. Ctries. 2021;15:141–146. doi: 10.3855/jidc.13127. [DOI] [PubMed] [Google Scholar]
- 20.Akande K.O., Fowotade A., Adekanmbi O. The effect of Hepatitis D co-infection on the immunologic and molecular profile of Hepatitis B in asymptomatic Chronic Hepatitis B patients in southwest Nigeria. J. Immunoass. Immunochem. 2020;41:272–280. doi: 10.1080/15321819.2020.1728542. [DOI] [PubMed] [Google Scholar]
- 21.Tassachew Y., Belyhun Y., Abebe T., Mihret A., Teffera T., Ababi G., Shewaye A., Desalegn H., Aseffa A., Mulu A., et al. Magnitude and genotype of hepatitis delta virus among chronic hepatitis B carriers with a spectrum of liver diseases in Ethiopia. Ann. Hepatol. 2023;28:100770. doi: 10.1016/j.aohep.2022.100770. [DOI] [PubMed] [Google Scholar]
- 22.Lempp F.A., Roggenbach I., Nkongolo S., Sakin V., Schlund F., Schnitzler P., Wedemeyer H., Le Gal F., Gordien E., Yurdaydin C., et al. A rapid point-of-care test for the serodiagnosis of hepatitis delta virus infection. Viruses. 2021;13:2371. doi: 10.3390/v13122371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.