Abstract
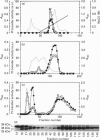
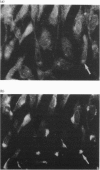
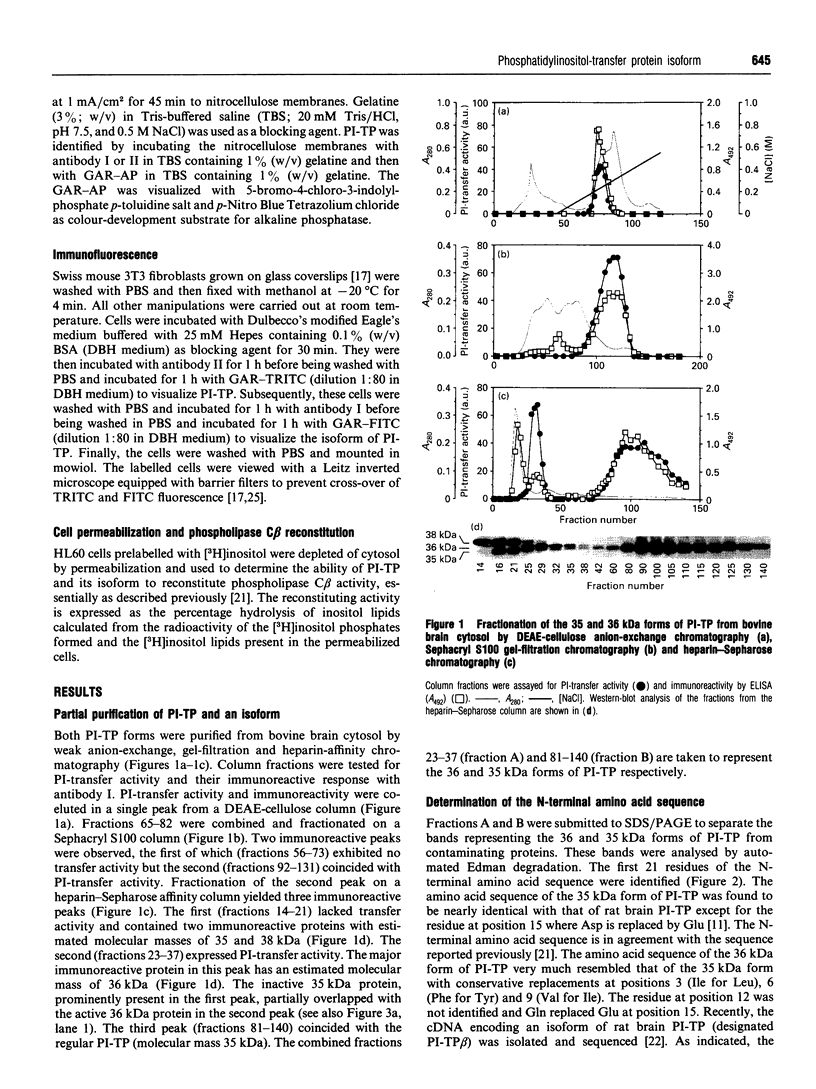
An isoform of the phosphatidylinositol-transfer protein (PI-TP) was identified in the cytosol fraction of bovine brain. This protein, designated PI-TP beta, has an apparent molecular mass of 36 kDa and an isoelectric point of 5.4. The N-terminal amino acid sequence (21 residues) is 90% similar to that of bovine brain PI-TP, henceforth designated PI-TP alpha (molecular mass 35 kDa and pI 5.5). As observed for PI-TP alpha, PI-TP beta has a distinct preference for phosphatidylinositol over phosphatidylcholine. In addition, it expresses a high transfer activity towards sphingomyelin. PI-TP alpha lacks this activity completely. By indirect immunofluorescence we demonstrated that, in Swiss mouse 3T3 fibroblasts, PI-TP beta is preferentially associated with the Golgi system whereas PI-TP alpha is predominantly present in the cytoplasm and the nucleus. In cytosol-depleted HL60 cells, both PI-TP alpha and PI-TP beta were equally effective at reconstituting guanosine 5'-[gamma-thio]triphosphate-mediated phospholipase C beta activity.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken J. F., van Heusden G. P., Temkin M., Dowhan W. The gene encoding the phosphatidylinositol transfer protein is essential for cell growth. J Biol Chem. 1990 Mar 15;265(8):4711–4717. [PubMed] [Google Scholar]
- Bankaitis V. A., Aitken J. R., Cleves A. E., Dowhan W. An essential role for a phospholipid transfer protein in yeast Golgi function. Nature. 1990 Oct 11;347(6293):561–562. doi: 10.1038/347561a0. [DOI] [PubMed] [Google Scholar]
- Bankaitis V. A., Malehorn D. E., Emr S. D., Greene R. The Saccharomyces cerevisiae SEC14 gene encodes a cytosolic factor that is required for transport of secretory proteins from the yeast Golgi complex. J Cell Biol. 1989 Apr;108(4):1271–1281. doi: 10.1083/jcb.108.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleves A. E., Bankaitis V. A. Secretory pathway function in Saccharomyces cerevisiae. Adv Microb Physiol. 1992;33:73–144. doi: 10.1016/s0065-2911(08)60216-7. [DOI] [PubMed] [Google Scholar]
- Cleves A. E., McGee T. P., Whitters E. A., Champion K. M., Aitken J. R., Dowhan W., Goebl M., Bankaitis V. A. Mutations in the CDP-choline pathway for phospholipid biosynthesis bypass the requirement for an essential phospholipid transfer protein. Cell. 1991 Feb 22;64(4):789–800. doi: 10.1016/0092-8674(91)90508-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCorleto P. E., Warach J. B., Zilversmit D. B. Purification and characterization of two phospholipid exchange proteins from bovine heart. J Biol Chem. 1979 Aug 25;254(16):7795–7802. [PubMed] [Google Scholar]
- Dickeson S. K., Helmkamp G. M., Jr, Yarbrough L. R. Sequence of a human cDNA encoding phosphatidylinositol transfer protein and occurrence of a related sequence in widely divergent eukaryotes. Gene. 1994 May 16;142(2):301–305. doi: 10.1016/0378-1119(94)90279-8. [DOI] [PubMed] [Google Scholar]
- Dickeson S. K., Lim C. N., Schuyler G. T., Dalton T. P., Helmkamp G. M., Jr, Yarbrough L. R. Isolation and sequence of cDNA clones encoding rat phosphatidylinositol transfer protein. J Biol Chem. 1989 Oct 5;264(28):16557–16564. [PubMed] [Google Scholar]
- Divecha N., Banfić H., Irvine R. F. The polyphosphoinositide cycle exists in the nuclei of Swiss 3T3 cells under the control of a receptor (for IGF-I) in the plasma membrane, and stimulation of the cycle increases nuclear diacylglycerol and apparently induces translocation of protein kinase C to the nucleus. EMBO J. 1991 Nov;10(11):3207–3214. doi: 10.1002/j.1460-2075.1991.tb04883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek T. B., de Groot E., van Baal J., Brunink F., Westerman J., Snoek G. T., Wirtz K. W. Characterization of mouse phosphatidylinositol transfer protein expressed in Escherichia coli. Biochim Biophys Acta. 1994 Aug 4;1213(3):309–318. doi: 10.1016/0005-2760(94)00063-8. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A. The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem. 1994 Feb 4;269(5):3125–3128. [PubMed] [Google Scholar]
- Hay J. C., Martin T. F. Phosphatidylinositol transfer protein required for ATP-dependent priming of Ca(2+)-activated secretion. Nature. 1993 Dec 9;366(6455):572–575. doi: 10.1038/366572a0. [DOI] [PubMed] [Google Scholar]
- Hay J. C., Martin T. F. Resolution of regulated secretion into sequential MgATP-dependent and calcium-dependent stages mediated by distinct cytosolic proteins. J Cell Biol. 1992 Oct;119(1):139–151. doi: 10.1083/jcb.119.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmkamp G. M., Jr, Harvey M. S., Wirtz K. W., Van Deenen L. L. Phospholipid exchange between membranes. Purification of bovine brain proteins that preferentially catalyze the transfer of phosphatidylinositol. J Biol Chem. 1974 Oct 25;249(20):6382–6389. [PubMed] [Google Scholar]
- Helmkamp G. M., Jr Phosphatidylinositol transfer proteins: structure, catalytic activity, and physiological function. Chem Phys Lipids. 1985 Aug 30;38(1-2):3–16. doi: 10.1016/0009-3084(85)90053-2. [DOI] [PubMed] [Google Scholar]
- Helmkamp G. M., Jr Transport and metabolism of phosphatidylinositol in eukaryotic cells. Subcell Biochem. 1990;16:129–174. doi: 10.1007/978-1-4899-1621-1_6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linardic C. M., Hannun Y. A. Identification of a distinct pool of sphingomyelin involved in the sphingomyelin cycle. J Biol Chem. 1994 Sep 23;269(38):23530–23537. [PubMed] [Google Scholar]
- Martelli A. M., Gilmour R. S., Bertagnolo V., Neri L. M., Manzoli L., Cocco L. Nuclear localization and signalling activity of phosphoinositidase C beta in Swiss 3T3 cells. Nature. 1992 Jul 16;358(6383):242–245. doi: 10.1038/358242a0. [DOI] [PubMed] [Google Scholar]
- McGee T. P., Skinner H. B., Whitters E. A., Henry S. A., Bankaitis V. A. A phosphatidylinositol transfer protein controls the phosphatidylcholine content of yeast Golgi membranes. J Cell Biol. 1994 Feb;124(3):273–287. doi: 10.1083/jcb.124.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama S. R., Cleves A. E., Malehorn D. E., Whitters E. A., Bankaitis V. A. Cloning and characterization of Kluyveromyces lactis SEC14, a gene whose product stimulates Golgi secretory function in Saccharomyces cerevisiae. J Bacteriol. 1990 Aug;172(8):4510–4521. doi: 10.1128/jb.172.8.4510-4521.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Skinner H. B., Alb J. G., Jr, Whitters E. A., Helmkamp G. M., Jr, Bankaitis V. A. Phospholipid transfer activity is relevant to but not sufficient for the essential function of the yeast SEC14 gene product. EMBO J. 1993 Dec;12(12):4775–4784. doi: 10.1002/j.1460-2075.1993.tb06166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoek G. T., Westerman J., Wouters F. S., Wirtz K. W. Phosphorylation and redistribution of the phosphatidylinositol-transfer protein in phorbol 12-myristate 13-acetate- and bombesin-stimulated Swiss mouse 3T3 fibroblasts. Biochem J. 1993 Apr 15;291(Pt 2):649–656. doi: 10.1042/bj2910649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoek G. T., de Wit I. S., van Mourik J. H., Wirtz K. W. The phosphatidylinositol transfer protein in 3T3 mouse fibroblast cells is associated with the Golgi system. J Cell Biochem. 1992 Aug;49(4):339–348. doi: 10.1002/jcb.240490404. [DOI] [PubMed] [Google Scholar]
- Somerharju P. J., Virtanen J. A., Eklund K. K., Vainio P., Kinnunen P. K. 1-Palmitoyl-2-pyrenedecanoyl glycerophospholipids as membrane probes: evidence for regular distribution in liquid-crystalline phosphatidylcholine bilayers. Biochemistry. 1985 May 21;24(11):2773–2781. doi: 10.1021/bi00332a027. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Hosaka K. Cloning of a cDNA encoding a second phosphatidylinositol transfer protein of rat brain by complementation of the yeast sec14 mutation. J Biochem. 1994 May;115(5):981–984. doi: 10.1093/oxfordjournals.jbchem.a124448. [DOI] [PubMed] [Google Scholar]
- Thomas G. M., Cunningham E., Fensome A., Ball A., Totty N. F., Truong O., Hsuan J. J., Cockcroft S. An essential role for phosphatidylinositol transfer protein in phospholipase C-mediated inositol lipid signaling. Cell. 1993 Sep 10;74(5):919–928. doi: 10.1016/0092-8674(93)90471-2. [DOI] [PubMed] [Google Scholar]
- Van Paridon P. A., Gadella T. W., Jr, Somerharju P. J., Wirtz K. W. On the relationship between the dual specificity of the bovine brain phosphatidylinositol transfer protein and membrane phosphatidylinositol levels. Biochim Biophys Acta. 1987 Sep 18;903(1):68–77. doi: 10.1016/0005-2736(87)90156-8. [DOI] [PubMed] [Google Scholar]
- Van Paridon P. A., Visser A. J., Wirtz K. W. Binding of phospholipids to the phosphatidylinositol transfer protein from bovine brain as studied by steady-state and time-resolved fluorescence spectroscopy. Biochim Biophys Acta. 1987 Apr 9;898(2):172–180. doi: 10.1016/0005-2736(87)90035-6. [DOI] [PubMed] [Google Scholar]
- Wirtz K. W., Jolles J., Westerman J., Neys F. Phospholipid exchange proteins in synaptosome and myelin fraction from rat brain. Nature. 1976 Mar 25;260(5549):354–355. doi: 10.1038/260354a0. [DOI] [PubMed] [Google Scholar]
- Wirtz K. W. Phospholipid transfer proteins. Annu Rev Biochem. 1991;60:73–99. doi: 10.1146/annurev.bi.60.070191.000445. [DOI] [PubMed] [Google Scholar]
- de Vries K. J., Momchilova-Pankova A., Snoek G. T., Wirtz K. W. A novel acidic form of the phosphatidylinositol transfer protein is preferentially retained in permeabilized Swiss mouse 3T3 fibroblasts. Exp Cell Res. 1994 Nov;215(1):109–113. doi: 10.1006/excr.1994.1321. [DOI] [PubMed] [Google Scholar]
- van den Akker W. M., Westerman J., Gadella T. W., Jr, Wirtz K. W., Snoek G. T. Proteolytic activation of a bovine brain protein with phosphatidylinositol transfer activity. FEBS Lett. 1990 Dec 10;276(1-2):123–126. doi: 10.1016/0014-5793(90)80523-l. [DOI] [PubMed] [Google Scholar]