Abstract
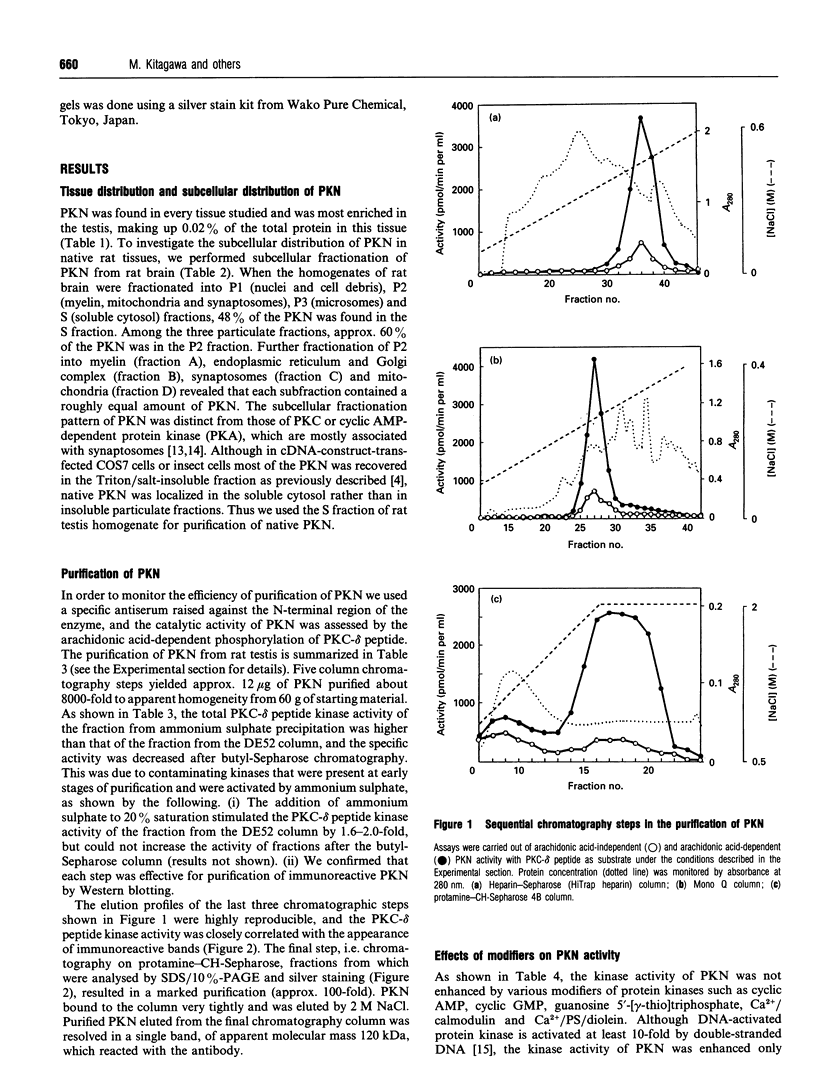
PKN, a novel protein kinase with a catalytic domain homologous to that of the protein kinase C (PKC) family and unique N-terminal leucine-zipper-like sequences, was identified by molecular cloning from a human hippocampus cDNA library [Mukai and Ono (1994) Biochem. Biophys. Res. Commun. 199, 897-904]. Recently we partially purified recombinant PKN from COS7 cells transfected with the cDNA construct encoding human PKN, and demonstrated that the recombinant PKN was activated by unsaturated fatty acids and limited proteolysis [Mukai, Kitagawa, Shibata et al. (1994) Biochem. Biophys. Res. Commun. 204, 348-356]. The present work has focused on the further purification and characterization of PKN from native rat tissue. Immunochemical measurement revealed that PKN was found in every tissue, and was especially abundant in testis, spleen and brain; subcellular fractionation of rat brain showed that half of the PKN was localized in the soluble cytosolic fraction. PKN was purified approx. 8000-fold to apparent homogeneity from the cytosolic fraction of rat testis by DEAE-cellulose chromatography, ammonium sulphate fractionation and chromatography on butyl-Sepharose, heparin-Sepharose, Mono Q and protamine-CH-Sepharose. The enzyme migrates as a band of apparent molecular mass 120 kDa. Using serine-containing peptides based on the pseudosubstrate sequence of PKC-delta as phosphate acceptors, the kinase activity was stimulated several-fold by 40 microM unsaturated fatty acids or by detergents such as 0.04% sodium deoxycholate and 0.004% SDS. In the absence of modifiers, protamine sulphate, myelin basic protein and synthetic peptides based on the pseudosubstrate site of PKCs or ribosomal S6 protein were good substrates for phosphorylation by the kinase. In the presence of 40 microM arachidonic acid the kinase activity of PKN for these phosphate acceptors was increased 2-18-fold. The autophosphorylation activity of purified PKN was partially inhibited by pretreatment with alkaline phosphatase. These properties appear to distinguish PKN from many protein kinases isolated previously.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S. B., Leszczynska-Piziak J., Weissmann G. Arachidonic acid as a second messenger. Interactions with a GTP-binding protein of human neutrophils. J Immunol. 1991 Jul 1;147(1):231–236. [PubMed] [Google Scholar]
- Band A. M., Jones P. M., Howell S. L. Arachidonic acid-induced insulin secretion from rat islets of Langerhans. J Mol Endocrinol. 1992 Apr;8(2):95–101. doi: 10.1677/jme.0.0080095. [DOI] [PubMed] [Google Scholar]
- Basudev H., Jones P. M., Persaud S. J., Howell S. L. Arachidonic acid induces phosphorylation of an 18 kDa protein in electrically permeabilised rat islets of Langerhans. FEBS Lett. 1992 Jan 13;296(1):69–72. doi: 10.1016/0014-5793(92)80405-6. [DOI] [PubMed] [Google Scholar]
- Harrison D. E., Ashcroft S. J., Christie M. R., Lord J. M. Protein phosphorylation in the pancreatic B-cell. Experientia. 1984 Oct 15;40(10):1075–1084. doi: 10.1007/BF01971454. [DOI] [PubMed] [Google Scholar]
- Hunter T. Protein kinase classification. Methods Enzymol. 1991;200:3–37. doi: 10.1016/0076-6879(91)00125-g. [DOI] [PubMed] [Google Scholar]
- Jones P. M., Persaud S. J. Arachidonic acid as a second messenger in glucose-induced insulin secretion from pancreatic beta-cells. J Endocrinol. 1993 Apr;137(1):7–14. doi: 10.1677/joe.0.1370007. [DOI] [PubMed] [Google Scholar]
- Khan W. A., Blobe G. C., Richards A. L., Hannun Y. A. Identification, partial purification, and characterization of a novel phospholipid-dependent and fatty acid-activated protein kinase from human platelets. J Biol Chem. 1994 Apr 1;269(13):9729–9735. [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Minakuchi R., Inohara S., Nishizuka Y. Calcium-activated, phospholipid-dependent protein kinase from rat brain. Subcellular distribution, purification, and properties. J Biol Chem. 1982 Nov 25;257(22):13341–13348. [PubMed] [Google Scholar]
- Kim D., Clapham D. E. Potassium channels in cardiac cells activated by arachidonic acid and phospholipids. Science. 1989 Jun 9;244(4909):1174–1176. doi: 10.1126/science.2727703. [DOI] [PubMed] [Google Scholar]
- Kosako H., Gotoh Y., Matsuda S., Ishikawa M., Nishida E. Xenopus MAP kinase activator is a serine/threonine/tyrosine kinase activated by threonine phosphorylation. EMBO J. 1992 Aug;11(8):2903–2908. doi: 10.1002/j.1460-2075.1992.tb05359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landt M., Easom R. A., Colca J. R., Wolf B. A., Turk J., Mills L. A., McDaniel M. L. Parallel effects of arachidonic acid on insulin secretion, calmodulin-dependent protein kinase activity and protein kinase C activity in pancreatic islets. Cell Calcium. 1992 Mar;13(3):163–172. doi: 10.1016/0143-4160(92)90044-s. [DOI] [PubMed] [Google Scholar]
- Lees-Miller S. P., Chen Y. R., Anderson C. W. Human cells contain a DNA-activated protein kinase that phosphorylates simian virus 40 T antigen, mouse p53, and the human Ku autoantigen. Mol Cell Biol. 1990 Dec;10(12):6472–6481. doi: 10.1128/mcb.10.12.6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden D. J., Murakami K., Routtenberg A. A newly discovered protein kinase C activator (oleic acid) enhances long-term potentiation in the intact hippocampus. Brain Res. 1986 Aug 6;379(2):358–363. doi: 10.1016/0006-8993(86)90790-0. [DOI] [PubMed] [Google Scholar]
- Maeno H., Johnson E. M., Greengard P. Subcellular distribution of adenosine 3',5'-monophosphate-dependent protein kinase in rat brain. J Biol Chem. 1971 Jan 10;246(1):134–142. [PubMed] [Google Scholar]
- Matsuda S., Kosako H., Takenaka K., Moriyama K., Sakai H., Akiyama T., Gotoh Y., Nishida E. Xenopus MAP kinase activator: identification and function as a key intermediate in the phosphorylation cascade. EMBO J. 1992 Mar;11(3):973–982. doi: 10.1002/j.1460-2075.1992.tb05136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhail L. C., Clayton C. C., Snyderman R. A potential second messenger role for unsaturated fatty acids: activation of Ca2+-dependent protein kinase. Science. 1984 May 11;224(4649):622–625. doi: 10.1126/science.6231726. [DOI] [PubMed] [Google Scholar]
- Metz S. A., Draznin B., Sussman K. E., Leitner J. W. Unmasking of arachidonate-induced insulin release by removal of extracellular calcium. Arachidonic acid mobilizes cellular calcium in rat islets of Langerhans. Biochem Biophys Res Commun. 1987 Jan 15;142(1):251–258. doi: 10.1016/0006-291x(87)90478-5. [DOI] [PubMed] [Google Scholar]
- Metz S. A. Perspectives in diabetes. Is protein kinase C required for physiologic insulin release? Diabetes. 1988 Jan;37(1):3–7. doi: 10.2337/diab.37.1.3. [DOI] [PubMed] [Google Scholar]
- Miller B., Sarantis M., Traynelis S. F., Attwell D. Potentiation of NMDA receptor currents by arachidonic acid. Nature. 1992 Feb 20;355(6362):722–725. doi: 10.1038/355722a0. [DOI] [PubMed] [Google Scholar]
- Morrice N. A., Gabrielli B., Kemp B. E., Wettenhall R. E. A cardiolipin-activated protein kinase from rat liver structurally distinct from the protein kinases C. J Biol Chem. 1994 Aug 5;269(31):20040–20046. [PubMed] [Google Scholar]
- Mukai H., Kitagawa M., Shibata H., Takanaga H., Mori K., Shimakawa M., Miyahara M., Hirao K., Ono Y. Activation of PKN, a novel 120-kDa protein kinase with leucine zipper-like sequences, by unsaturated fatty acids and by limited proteolysis. Biochem Biophys Res Commun. 1994 Oct 14;204(1):348–356. doi: 10.1006/bbrc.1994.2466. [DOI] [PubMed] [Google Scholar]
- Mukai H., Kuno T., Chang C. D., Lane B., Luly J. R., Tanaka C. FKBP12-FK506 complex inhibits phosphatase activity of two mammalian isoforms of calcineurin irrespective of their substrates or activation mechanisms. J Biochem. 1993 Mar;113(3):292–298. doi: 10.1093/oxfordjournals.jbchem.a124041. [DOI] [PubMed] [Google Scholar]
- Mukai H., Ono Y. A novel protein kinase with leucine zipper-like sequences: its catalytic domain is highly homologous to that of protein kinase C. Biochem Biophys Res Commun. 1994 Mar 15;199(2):897–904. doi: 10.1006/bbrc.1994.1313. [DOI] [PubMed] [Google Scholar]
- Murakami K., Routtenberg A. Direct activation of purified protein kinase C by unsaturated fatty acids (oleate and arachidonate) in the absence of phospholipids and Ca2+. FEBS Lett. 1985 Nov 18;192(2):189–193. doi: 10.1016/0014-5793(85)80105-8. [DOI] [PubMed] [Google Scholar]
- Naor Z., Catt K. J. Mechanism of action of gonadotropin-releasing hormone. Involvement of phospholipid turnover in luteinizing hormone release. J Biol Chem. 1981 Mar 10;256(5):2226–2229. [PubMed] [Google Scholar]
- Naor Z. Is arachidonic acid a second messenger in signal transduction? Mol Cell Endocrinol. 1991 Sep;80(1-3):C181–C186. doi: 10.1016/0303-7207(91)90135-f. [DOI] [PubMed] [Google Scholar]
- Naor Z., Shearman M. S., Kishimoto A., Nishizuka Y. Calcium-independent activation of hypothalamic type I protein kinase C by unsaturated fatty acids. Mol Endocrinol. 1988 Nov;2(11):1043–1048. doi: 10.1210/mend-2-11-1043. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Ono Y., Fujii T., Igarashi K., Kikkawa U., Ogita K., Nishizuka Y. Nucleotide sequences of cDNAs for alpha and gamma subspecies of rat brain protein kinase C. Nucleic Acids Res. 1988 Jun 10;16(11):5199–5200. doi: 10.1093/nar/16.11.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y., Fujii T., Ogita K., Kikkawa U., Igarashi K., Nishizuka Y. Protein kinase C zeta subspecies from rat brain: its structure, expression, and properties. Proc Natl Acad Sci U S A. 1989 May;86(9):3099–3103. doi: 10.1073/pnas.86.9.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y., Fujii T., Ogita K., Kikkawa U., Igarashi K., Nishizuka Y. The structure, expression, and properties of additional members of the protein kinase C family. J Biol Chem. 1988 May 15;263(14):6927–6932. [PubMed] [Google Scholar]
- Ordway R. W., Walsh J. V., Jr, Singer J. J. Arachidonic acid and other fatty acids directly activate potassium channels in smooth muscle cells. Science. 1989 Jun 9;244(4909):1176–1179. doi: 10.1126/science.2471269. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Piomelli D., Greengard P. Lipoxygenase metabolites of arachidonic acid in neuronal transmembrane signalling. Trends Pharmacol Sci. 1990 Sep;11(9):367–373. doi: 10.1016/0165-6147(90)90182-8. [DOI] [PubMed] [Google Scholar]
- Posada J., Cooper J. A. Requirements for phosphorylation of MAP kinase during meiosis in Xenopus oocytes. Science. 1992 Jan 10;255(5041):212–215. doi: 10.1126/science.1313186. [DOI] [PubMed] [Google Scholar]
- Sekiguchi K., Tsukuda M., Ogita K., Kikkawa U., Nishizuka Y. Three distinct forms of rat brain protein kinase C: differential response to unsaturated fatty acids. Biochem Biophys Res Commun. 1987 Jun 15;145(2):797–802. doi: 10.1016/0006-291x(87)91035-7. [DOI] [PubMed] [Google Scholar]
- Shearman M. S., Naor Z., Kikkawa U., Nishizuka Y. Differential expression of multiple protein kinase C subspecies in rat central nervous tissue. Biochem Biophys Res Commun. 1987 Sep 30;147(3):911–919. doi: 10.1016/s0006-291x(87)80157-2. [DOI] [PubMed] [Google Scholar]
- Shearman M. S., Naor Z., Sekiguchi K., Kishimoto A., Nishizuka Y. Selective activation of the gamma-subspecies of protein kinase C from bovine cerebellum by arachidonic acid and its lipoxygenase metabolites. FEBS Lett. 1989 Jan 30;243(2):177–182. doi: 10.1016/0014-5793(89)80125-5. [DOI] [PubMed] [Google Scholar]
- Tsai M. H., Yu C. L., Stacey D. W. A cytoplasmic protein inhibits the GTPase activity of H-Ras in a phospholipid-dependent manner. Science. 1990 Nov 16;250(4983):982–985. doi: 10.1126/science.2237442. [DOI] [PubMed] [Google Scholar]
- Tsai M. H., Yu C. L., Wei F. S., Stacey D. W. The effect of GTPase activating protein upon ras is inhibited by mitogenically responsive lipids. Science. 1989 Jan 27;243(4890):522–526. doi: 10.1126/science.2536192. [DOI] [PubMed] [Google Scholar]
- Ueda T., Greengard P., Berzins K., Cohen R. S., Blomberg F., Grab D. J., Siekevitz P. Subcellular distribution in cerebral cortex of two proteins phosphorylated by a cAMP-dependent protein kinase. J Cell Biol. 1979 Nov;83(2 Pt 1):308–319. doi: 10.1083/jcb.83.2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa M., Sakuma M., Yagi K. Calmodulins from muscles of marine invertebrates, scallop and sea anemone. J Biochem. 1980 May;87(5):1313–1320. doi: 10.1093/oxfordjournals.jbchem.a132869. [DOI] [PubMed] [Google Scholar]
- Zeitler P., Handwerger S. Arachidonic acid stimulates phosphoinositide hydrolysis and human placental lactogen release in an enriched fraction of placental cells. Mol Pharmacol. 1985 Dec;28(6):549–554. [PubMed] [Google Scholar]
- van den Bosch H. Intracellular phospholipases A. Biochim Biophys Acta. 1980 Sep 30;604(2):191–246. doi: 10.1016/0005-2736(80)90574-x. [DOI] [PubMed] [Google Scholar]




