Abstract
This study investigated the incidence and severity of SARS-CoV-2 breakthrough infections (BIs) and the time to swab reversion in patients with multiple sclerosis (PwMS) after the booster dose of COVID-19 mRNA vaccines. We enrolled 64 PwMS who had completed the three-dose mRNA vaccine schedule and had never experienced COVID-19 before. Among the 64 PwMS, 43.8% had BIs with a median time since the third vaccine dose of 155 days. BIs occurred more frequently in ocrelizumab-treated patients (64.7%). Patients with a relapsing-remitting MS course showed a reduced incidence of BIs compared with those with a primary-progressive disease (p = 0.002). Having anti-receptor-binding domain (RBD) antibodies represented a protective factor reducing the incidence of BIs by 60% (p = 0.042). The majority of BIs were mild, and the only severe COVID-19 cases were reported in patients with a high Expanded Disability Status Scale score (EDSS > 6). The median time for a negative swab was 11 days. Notably, fingolimod-treated patients take longer for a swab-negativization (p = 0.002). Conversely, having anti-RBD antibodies ≥ 809 BAU/mL and an IFN-γ-specific T cell response ≥ 16 pg/mL were associated with a shorter time to swab-negativization (p = 0.051 and p = 0.018, respectively). In conclusion, the immunological protection from SARS-CoV-2 infection may differ among PwMS according to DMTs.
Keywords: COVID-19 vaccination, SARS-CoV-2, multiple sclerosis, disease-modifying therapies, antibody response, T cell response, breakthrough infection
1. Introduction
Coronavirus Disease-2019 (COVID-19) is a human-to-human transmissible disease caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), that emerged in late 2019 as an outbreak and was subsequently declared a global health emergency by the World Health Organization (WHO) on 30 January 2020 [1].
The large-scale vaccination campaign starting at the beginning of 2021 has proven to be an effective measure to counteract the COVID-19 pandemic by limiting the spread of SARS-CoV-2 infections and reducing the risk of a severe disease outcome and hospitalization rate. This led the WHO to declare the end of the COVID-19 pandemic in May 2023. Nevertheless, COVID-19 still represents a threat to the public health of vulnerable categories [2,3,4], including patients with multiple sclerosis (PwMS) [5,6,7]. Besides COVID-19, other infectious diseases may have a more severe course in MS patients [8], and the COVID-19 clinical and scientific experience may represent a kind of “model” for the management of other infections as well.
Multiple sclerosis (MS) is a neurodegenerative and autoimmune disease that affects the central nervous system and represents the principal non-traumatic cause of disability among young adults in Western countries [9]. The majority of PwMS undergoes immunomodulatory or immunosuppressive disease-modifying therapies (DMTs), including ocrelizumab, fingolimod, cladribine, and interferon (IFN)-β [9]. DMTs administered in PwMS have a wide range of actions targeting the immune system and result in the potential impairment of humoral and/or cell-mediated immune responses to both infections and vaccines. For this reason, during the COVID-19 pandemic, the clinic management of PwMS was challenging and raised great concern.
Different studies reported that PwMS are more prone to contracting infections and present a higher risk of infection-related hospitalization and infection-related mortality [10,11,12,13,14].
Our and other groups showed that the majority of PwMS can mount humoral and/or T cell-specific immune responses to COVID-19 mRNA vaccines, albeit with a lower magnitude than healthy subjects [5,6,7,15,16,17]. To note, the magnitude of the immune response was different according to the ongoing DMTs. Particularly, fingolimod- and ocrelizumab-treated patients were those who presented a more compromised response to COVID-19 vaccination [5,6,7,16,18,19,20].
Despite the effectiveness of the vaccination campaign, breakthrough infections (BIs) continued to be reported owing to the emergence of SARS-CoV-2 variants escaping the host immune defense, concomitantly with the reduction of distancing measures and the decline of vaccine immunity over time [5,21,22,23,24,25]. The onset of new variants is likely favored by the ongoing replication of the virus, particularly in immunocompromised individuals who are more prone to establishing persistent infections [26].
It has been reported that the administration of the third dose of COVID-19 vaccines also contributes to protection against Omicron variants, by reducing the incidence and severity of BIs in the general population [27,28,29,30,31]. Regarding PwMS, studies reported in the literature have mainly focused on B-depleted patients [32,33,34], or have assessed the impact of clinical factors and/or antibody response on the risk of BIs without taking into account the T cell response [35,36,37,38,39,40]. Moreover, they do not investigate factors potentially associated with the time to swab reversion.
This study aimed to investigate the demographic, clinical, and immunological factors, both antibody and T cell responses, potentially associated with the risk of BIs, severity of the outcome, and time of swab negativization in the MS population treated with different DMTs after the third dose of COVID-19 mRNA vaccines. The study period overlapped with the onset of the Omicron variant, which was the predominant variant in Italy starting in January 2022 [41].
2. Materials and Methods
2.1. Study Cohort and Design
This is a prospective longitudinal single-center study. Patients diagnosed with MS according to the 2017 McDonald criteria [42] were enrolled at the MS Centre of the Department of Neurosciences of San Camillo Forlanini Hospital (Rome, Italy). We enrolled PwMS who were on treatment with ocrelizumab, fingolimod, cladribine, or IFN-β for at least 6 months from the enrolment and had received the three doses of COVID-19 mRNA vaccines (BNT162b2 or mRNA-1273). The exclusion criteria were as follows: previous SARS-CoV-2 infection based on a positive antigenic and/or molecular test by real-time polymerase chain reaction (PCR) on the swab sample and/or positive anti-nucleoprotein immunoglobulin G (anti-N-IgG) before the third vaccine dose, HIV infection, and age < 18 years.
The enrolment was initiated in March 2021, and the follow-up ended in December 2022. From a cohort previously evaluated in terms of antibody and T cell response to COVID-19 vaccination at baseline (2–4 weeks after the second dose) [6], after 24 weeks from the first dose, and 4–6 weeks after the third dose [5,7], a subgroup of 64 patients were followed up until a positive SARS-CoV-2 test or the administration of the fourth vaccine dose to evaluate the incidence rate of BIs (Figure 1). BIs were stratified based on their severity as mild, moderate, or severe [43]. Briefly, mild COVID-19 patients had any of the different signs and symptoms (i.e., fever, cough, sore throat, malaise, headache, etc.) but without shortness of breath, dyspnea, or abnormal chest imaging; moderate COVID-19 patients had lower respiratory disease and SpO2 ≥ 94% on room air, while severe COVID-19 patients required hospitalization.
Figure 1.
Timeline of COVID-19 vaccination schedule and surveillance. After 4–6 weeks from the third vaccine dose, blood samples were collected to evaluate antibody and T cell response. Abbreviations: COVID-19, Corona Virus Disease 2019. Created with BioRender.com.
Demographic and clinical data were collected at the time of the third dose, while the immunological data refer to the evaluation performed 4–6 weeks after the third dose. The laboratory procedures were carried out following the standardized protocol previously described [44].
This study was approved by the Ethical Committee of “L. Spallanzani” National Institute of Infectious Diseases (INMI)-IRCCS (approval numbers 319/2021 and 443/2021) and was conducted in agreement with the Declaration of Helsinki. A written informed consent was signed by all participants before taking part in the study.
2.2. Anti-SARS-CoV-2 Antibodies
The antibody response to COVID-19 vaccination was evaluated by assessing anti-receptor-binding domain (RBD) antibodies (Abs), which were expressed as binding antibody units (BAU)/mL, and whose cut-off to define a positive response was set at ≥ 7.1.
Concurrently, neutralizing antibodies were evaluated with a micro-neutralization assay (MNA) using the SARS-CoV-2/Human/ITA/PAVIA10734/2020 (provided by Fausto Baldanti, Pavia, Italy), as previously described [45]. The neutralization titer was expressed as the reciprocal of the highest serum dilution (MNA90) at which we observed at least 90% inhibition of the cytopathic effect. The neutralizing titer was considered positive if ≥ 10, corresponding to the first dilution tested.
The anti-nucleoprotein immunoglobulin G (Anti-N-IgG) was evaluated to screen the enrolled cohort for a previous SARS-CoV-2 infection. Subjects scored positive if index values [(sample (S)/Cutoff (CO)] ≥1.4. Both anti-RBD-IgG and anti-N-IgG were assessed following the manufacturer’s instructions (Architect® i2000sr Abbott Diagnostics, Chicago, IL, USA).
2.3. IFN-γ-Spike-Specific T cell Response
The IFN-γ-spike specific T cell response was tested using a whole blood assay. The blood was collected in lithium heparinized tubes (BD Vacutainer, Becton Dickinson, Florence, Italy, Cat. 367526) and stimulated in a 48-well plate with a mixture of spike peptides derived from the SARS-CoV-2 Wuhan spike protein (PepTivator®Prot_S1, Prot_S, and Prot_S+, Miltenyi Biotec, Bergisch Gladbach, Germany, Cat. 130–127–048, Cat. 130–126–701 and Cat. 130–127–312, respectively) at a final concentration of 0.1 µg/mL [44]. The staphylococcal enterotoxin B (SEB) (Merck Life Science, Milan, Italy, Cat. S4881) was used as a positive control (Supplementary Figure S1). After an overnight incubation at 37 °C with 5% CO2, plasma was harvested and stored at −80 °C until use. The ELLA Simple Plex Human IFN-γ (3rd Gen.) Assay (Bio-Techne, Minneapolis, MN, USA, Cat. SPCKB-PS-002574) was used to quantify IFN-γ levels that defined a positive T cell response if ≥ 16 pg/mL. The values reported are subtracted from the unstimulated control value.
2.4. Statistical Analysis
The statistical analyses were performed using Stata (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX, USA: StataCorp LLC.), R Project Software (version 4.2.1), and GraphPad (version 9.3.1) (GraphPad Prism, San Diego, CA, USA). Categorical variables were expressed as absolute and relative percentages, whereas the quantitative ones were reported as medians and interquartile ranges (IQR). The following statistical tests were used: the Kruskal–Wallis test to compare groups, the Mann–Whitney test for pairwise comparison, and the Fisher Exact test to compare categorical variables.
Univariable Poisson regressions were performed to estimate the incidence rate ratio (IRR) of SARS-CoV-2 infections according to the demographic, clinical, and immunological data. Variables with p < 0.2 were entered into the multivariable model, which was chosen with the minor Bayesian information criterion (BIC). The quantile regression analysis on the median was performed to estimate the time to swab conversion according to demographic, clinical, and immunological responses. Two-tailed p-values < 0.05 were considered statistically significant. Post-hoc power analysis has been performed for the main outcomes and for univariable models, to evaluate the statistical power of our sample size using G*Power software (version 3.1.9.7) [46]. According to the post hoc sample size calculation, our study was able to detect significant differences of IRRs of at least 2.5 for the primary outcome, incidence of SARS-CoV-2 breakthrough infections, and a reduction of about 75% in the analysis of antibody and T cell-specific immune response, after sample collection, with a power of 80% and an alpha error of 0.05.
3. Results
3.1. Characteristics of the Studied Population
Within a cohort of 134 PwMS previously evaluated for the immune response to COVID-19 vaccination [5,6,7], 64 individuals were followed up to investigate the BI incidence in the MS cohort. The demographic and clinical characteristics of the enrolled subjects are reported in Table 1. The cohort had a median time since MS diagnosis of 15 years and was characterized by a female predominance of 67%, as expected in autoimmune diseases. The majority of PwMS (92.2%) showed a relapsing-remitting phenotype. All the enrolled PwMs were under DMTs: 17 (26.6%) subjects were treated with ocrelizumab, 26 (40.6%) with fingolimod, 6 (9.4%) with cladribine, and 15 (23.4%) with IFN-β. A minority of subjects (21.9%) reported having comorbidities such as metabolic or cardiovascular diseases.
Table 1.
Demographic and clinical characteristics of the 64 enrolled PwMS.
| Patients’ Characteristics | |
|---|---|
| Total | 64 (100) |
| Age, median (IQR) years | 49 (41.5–55.5) |
| Age class, n (%) | |
| 23–39 | 16 (25.0) |
| 40–49 | 19 (29.7) |
| 50–70 | 29 (45.3) |
| Gender: Female, n (%) | 43 (67.2) |
| Origin: Italian, n (%) | 62 (96.9) |
| Presence of comorbidities, n (%) | 14 (21.9) |
| BMI (kg/m2), median (IQR) | 24 (21–26) |
| Vaccination-First dose with Comirnaty, n (%) | 59 (92.2) |
| MS duration, median (IQR) years | 15 (7–25) |
| MS treatment, n (%) | |
| Cladribine | 6 (9.4) |
| Fingolimod | 26 (40.6) |
| Interferon beta | 15 (23.4) |
| Ocrelizumab | 17 (26.6) |
| MS treatment duration, median months (IQR) | 5 (2–9) |
| EDSS score, median (IQR) | 2 (0.5–4.3) |
| <3 | 37 (58.8) |
| ≥3 | 27 (42.2) |
| MS phenotype, n (%) | |
| Primary-progressive (PP) | 5 (7.8) |
| Relapsing-Remitting (RR) | 59 (92.2) |
| Lymphocytes count × 103/µL, median (IQR) | 1.3 (0.7–1.7) |
| Days from booster dose to sample, median (IQR) | 48 (43–51) |
| Among PwMS with SARS-CoV-2 breakthrough infection | 28 (43.7) |
| Days from 3rd dose to infection, median (IQR) | 155 (108–205) |
| Days to negative swab, median (IQR) | 11 (9–15) |
| COVID-19 therapy, n (%) | |
| Anti-viral therapy | 5 (17.9) |
| Monoclonal therapy | 11 (39.3) |
| Non-steroidal anti-inflammatory drugs or paracetamol or no therapy * | 12 (42.9) |
| Severity of COVID-19, n (%) | |
| Mild disease | 21 (75.0) |
| Moderate disease | 5 (17.9) |
| Severe disease | 2 (7.1) |
IQR: interquartile range; BMI: body mass index; EDSS: Expanded Disability Status Scale; PwMS: patients with multiple sclerosis; * two patients reported not having taken any therapy. In bold are reported the patient’s characteristics.
3.2. Incidence of Breakthrough Infections and Clinical Features
During the study period, out of 64 PwMS, 28 (43.8%) experienced BIs with a median time since the third vaccine dose of 155 days (Table 1). BIs occurred more frequently among PwMS treated with ocrelizumab (11/17, 64.7%) compared to those under fingolimod (10/26, 38.5%), IFN-β (6/15, 40%), and cladribine (1/6, 16.7%). Indeed, 39.3% of the total BIs were reported in subjects treated with ocrelizumab, 35.7% with fingolimod, 21.4% with IFN-β, and 3.6% with cladribine (Table 2). A different incidence of BIs was also observed according to sex; in the male population, 61.9% of subjects had a SARS-CoV-2 infection compared with only 35% of females. A significantly lower BI incidence rate was observed in PwMS with higher MS severity, reported as Expanded Disability Status Scale score (EDSS ≥ 3 IRR: 0.22, 95%CI: 0.08–0.66, p = 0.006). Moreover, PwMS with a relapsing-remitting disease showed an 88% lower rate of BI incidence (IRR: 0.12, 95%CI: 0.03–0.46, p = 0.002) compared to those with a primary-progressive disease (Table 2).
Table 2.
Incidence of SARS-CoV-2 breakthrough infections according to the demographic and clinical characteristics of PwMS after the booster dose of COVID-19 vaccination.
| Patients’ Characteristics | SARS-CoV-2 BIs | Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | Total | IRR | 95%CI | p | IRR | 95%CI | p | |
| Total, n (%) | 36 (56.3) | 28 (43.8) | 64 (100) | ||||||
| Age, median (IQR) years | 50 (45.5–58) | 47.5 (38–53.5) | 49 (41.5–55.5) | ||||||
| 23–39 | 8 (22.2) | 8 (28.6) | 16 (25.0) | Ref. | |||||
| 40–49 | 10 (27.8) | 9 (32.1) | 19 (29.7) | 1.01 | 0.39–2.63 | 0.979 | |||
| 50–70 | 18 (50.0) | 11 (39.3) | 29 (45.3) | 0.66 | 0.27–1.64 | 0.371 | |||
| Gender, n (%) | |||||||||
| Female | 28 (77.8) | 15 (53.6) | 43 (67.2) | Ref. | |||||
| Male | 8 (22.2) | 13 (46.4) | 21 (32.8) | 2.19 | 1.04–4.6 | 0.038 | |||
| Country of birth, n (%) | |||||||||
| Italy | 34 (94.4) | 28 (100) | 62 (96.9) | - | - | - | |||
| Abroad | 2 (5.6) | 0 (0) | 2 (3.1) | - | - | - | |||
| Presence of comorbidities, n (%) | |||||||||
| No | 27 (75.0) | 23 (82.1) | 50 (78.1) | Ref. | |||||
| Yes | 9 (25.0) | 5 (17.9) | 14 (21.9) | 0.69 | 0.26–1.83 | 0.460 | |||
| BMI (kg/m2), median (IQR) | 24 (20.9–25.9) | 24 (22–26.6) | 24 (21.3–26.2) | ||||||
| ≤24 | 18 (50.0) | 14 (50.0) | 32 (50.0) | Ref. | |||||
| >24 | 18 (50.0) | 14 (50.0) | 32 (50.0) | 1.03 | 0.49–2.16 | 0.938 | |||
| Vaccination-First dose, n (%) | |||||||||
| Comirnaty | 33 (91.7) | 26 (92.9) | 59 (92.2) | Ref. | |||||
| Other | 3 (8.3) | 2 (7.1) | 5 (7.8) | 1.06 | 0.25–4.45 | 0.940 | |||
| MS duration (years), median (IQR) | 16.2 (8.5–23.1) | 12.9 (5.8–25) | 15 (7.5–24.5) | ||||||
| ≤15 | 17 (47.2) | 16 (57.1) | 33 (51.6) | Ref. | |||||
| >15 | 19 (52.8) | 12 (42.9) | 31 (48.4) | 0.79 | 0.37–1.67 | 0.539 | |||
| MS treatment, n (%) | |||||||||
| Cladribine | 5 (13.9) | 1 (3.6) | 6 (9.4) | 0.32 | 0.04–2.69 | 0.297 | |||
| Fingolimod | 16 (44.4) | 10 (35.7) | 26 (40.6) | 0.97 | 0.35–2.66 | 0.948 | |||
| Interferon beta | 9 (25.0) | 6 (21.4) | 15 (23.4) | Ref. | |||||
| Ocrelizumab | 6 (16.7) | 11 (39.3) | 17 (26.6) | 2.21 | 0.82–5.97 | 0.118 | |||
| MS treatment duration (years), median (IQR) | 6.5 (2–9.1) | 3.7 (1.8–7.9) | 5.1 (1.9–8.6) | ||||||
| ≤5 | 14 (38.9) | 17 (60.7) | 31 (48.4) | Ref. | |||||
| >5 | 22 (61.1) | 11 (39.3) | 33 (51.6) | 0.49 | 0.23–1.04 | 0.062 | |||
| EDSS score, median (IQR) | 3 (0.5–5) | 1.8 (0.5–3) | 2 (0.5–4.3) | ||||||
| <3 | 17 (47.2) | 20 (71.4) | 37 (58.8) | Ref. | Ref. | ||||
| ≥3 | 19 (52.8) | 8 (28.6) | 27 (42.2) | 0.40 | 0.18–0.9 | 0.029 | 0.22 | 0.08–0.66 | 0.006 |
| MS phenotype, n (%) | |||||||||
| Primary-progressive (PP) | 1 (2.8) | 4 (14.3) | 5 (7.8) | Ref. | Ref. | ||||
| Relapsing-Remitting (RR) | 35 (97.2) | 24 (85.7) | 59 (92.2) | 0.33 | 0.11–0.94 | 0.039 | 0.12 | 0.03–0.46 | 0.002 |
| Lymphocytes count × 103/µL, median (IQR) | 1.3 (0.7–1.6) | 1.3 (0.7–1.8) | 1.28 (0.65–1.67) | ||||||
| ≤1.28 | 18 (54.6) | 11 (45.8) | 29 (50.9) | Ref. | |||||
| >1.28 | 15 (45.4) | 13 (54.2) | 28 (49.1) | 1.35 | 0.61–3.02 | 0.461 | |||
BI: breakthrough infection; PwMS: patients with multiple sclerosis; IQR: interquartile range; BMI: body mass index; EDSS: Expanded Disability Status Scale; IRR: incidence rate ratio; CI: confidence interval; Ref: reference category; univariable Poisson regression. In bold are reported the patient’s characteristics and the significant values.
3.3. Incidence of Breakthrough Infections and Immune Response
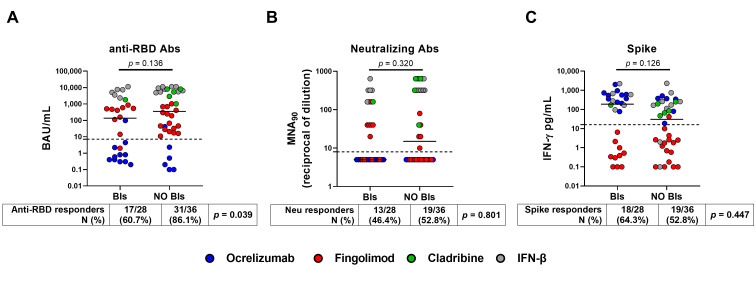
PwMS who did not have BIs showed a significantly higher seroconversion rate (31/36, 86.1%) after the third vaccine dose than those who had BIs (17/28, 60.7%) (p = 0.039). However, there were no significant differences in the magnitude of the antibody response after the third vaccine dose between PwMS who had BIs and those uninfected (135 BAU/mL, (IQR: 1–1337) vs. 352 BAU/mL, (IQR: 24–5513), p = 0.136), albeit a slightly higher antibody production was observed in those uninfected (Figure 2A). Among patients without BIs, fingolimod-treated patients were the most represented group with a frequency of 44% compared to 36% in patients with BIs (Table 2), and this likely influenced the overall result. Indeed, it is well known that fingolimod limits the ability for both T and B cell-specific responses. Therefore, although most patients receiving fingolimod therapy seroconverted, the antibody levels are low. Consequently, when comparing those without BIs with those who had BIs, even though there was a significant difference in the number of seroconverted individuals, there was no significant change in the antibody production between the two groups. Moreover, neither the neutralizing titer nor the IFN-γ T cell response significantly differed between PwMS who experienced BIs and the uninfected patients, both in terms of qualitative (p = 0.320 and p = 0.126, respectively) or quantitative response (p = 0.801 and p = 0.447, respectively) (Figure 2B,C).
Figure 2.
Antibody and IFN-γ-specific T cell responses after the third dose of COVID-19 vaccine. The enrolled PwMS (n = 64) were stratified into two groups: BIs group (n = 28), which included those who had COVID-19 after the third dose, and no BIs group (n = 36), which included those who did not have COVID-19 during the follow-up period. (A) Anti-RBD-IgG were expressed as binding antibody units (BAU)/mL, (B) neutralizing antibodies as the reciprocal of the dilution (MNA90), while (C) IFN-γ levels were subtracted from the unstimulated-control value and reported in pg/mL. The cut-off of each test is indicated by the black dashed line (anti-RBD-IgG: 7.1 BAU/mL; MNA90: 10 reciprocal dilution and spike: 16 pg/mL). Each colour dot represents a different treatment as shown in the legend. A Mann–Whitney test was performed for pairwise comparison and Fisher Exact test was to compare the proportion of responders. A p-value < 0.05 was considered significant. Abbreviations: COVID-19, Coronavirus Disease 2019; PwMS, patients with multiple sclerosis; BIs, breakthrough infections; RBD, receptor-binding domain; IgG, immunoglobulin; Abs, antibodies; IFN, interferon; N, number.
In the univariable Poisson regression, we showed that the presence of an antibody response (anti-RBD ≥ 7.1 BAU/mL) represented a protective factor, reducing the incidence rate of BIs in the MS population by 67% (IRR: 0.33, 95%CI: 0.15–0.7, p = 0.004) (Table 3). After adjusting for EDSS score and disease phenotype, having anti-RBD response ≥ 7.1 BAU/mL still conferred a 60% reduction in the BI incidence rate (IRR: 0.40, 95%CI: 0.17–0.97, p = 0.042). Instead, no significant associations were found between the incidence rate of BIs and IFN-γ levels or neutralizing antibodies (p = 0.187 and p = 0.362, respectively).
Table 3.
Incidence rate ratios for SARS-CoV-2 infection in 64 PwMS according to antibody and T cell-specific immune response evaluated after 2–4 weeks from the booster dose.
| Immune Response | No BI | BI | Total | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|---|---|---|
| 36 (56.3) | 28 (43.8) | 64 (100) | IRR | 95%CI | p | aIRR | 95%CI | p | |
| anti-RBD IgG (BAU/mL) | |||||||||
| continuous, median (IQR) | 352 (24–5513) | 135 (1–1337) | 272 (8–4222) | ||||||
| score: Negative (<7.1) | 5 (13.9) | 11 (39.3) | 16 (25.0) | Ref. | Ref. | ||||
| score: Positive (≥7.1) | 31 (86.1) | 17 (60.7) | 48 (75.0) | 0.33 | 0.15–0.7 | 0.004 | 0.40 | 0.17–0.97 | 0.042 |
| IFN-γ T cell-specific response (pg/mL) | |||||||||
| continuous, median (IQR) | 30 (1–222) | 188 (1–578) | 79 (1–349) | ||||||
| <16 | 17 (47.2) | 10 (35.7) | 27 (42.2) | Ref. | Ref. | ||||
| ≥16 | 19 (52.8) | 18 (64.3) | 37 (57.8) | 1.61 | 0.74–3.49 | 0.228 | 0.55 | 0.22–1.34 | 0.187 |
| Neutralizing antibodies | |||||||||
| continuous, median (IQR) | 15 (5–320) | 5 (5–160) | 8 (5–320) | ||||||
| <10 | 17 (47.2) | 15 (53.6) | 32 (50.0) | Ref. | Ref. | ||||
| ≥10 | 19 (52.8) | 13 (46.4) | 32 (50.0) | 0.83 | 0.39–1.74 | 0.618 | 0.68 | 0.30–1.55 | 0.362 |
PwMS: patients with multiple sclerosis; RBD: receptor-binding domain; IFN: interferon; BI: breakthrough infection; IRR: incidence rate ratio estimated with Poisson regression; aIRR: IRR adjusted for EDSS score and phenotype; CI: confidence interval; Ref: reference category. In bold are reported the patient’s immunologic variables and the significant values.
3.4. COVID-19 Severity
The majority of BIs (21/28, 75%) were mild, while 17.9% (5/28) were moderate. Only two cases of severe COVID-19 requiring hospitalization were reported and involved male subjects under fingolimod and ocrelizumab treatment (Table 4).
Table 4.
Demographic and clinical characteristics of the 28 PwMS enrolled according to the COVID-19 severity.
| Patients’ Characteristics | COVID-19 Severity | ||||
|---|---|---|---|---|---|
| Mild Disease | Moderate Disease | Severe Disease | Total | p | |
| Total | 21 (75.0) | 5 (17.9) | 2 (7.1) | 28 (100) | |
| Age, median (IQR) years | 47 (39–53) | 49 (30–55) | 47.5 (46–49) | 47.5 (38–53.5) | 0.969 |
| Age class, n (%) | 0.511 | ||||
| 23–39 | 6 (28.6) | 2 (40) | 0 (0) | 8 (28.6) | |
| 40–49 | 6 (28.6) | 1 (20) | 2 (100) | 9 (32.1) | |
| 50–70 | 9 (42.9) | 2 (40) | 0 (0) | 11 (39.3) | |
| Gender, n (%) | 0.293 | ||||
| Female | 13 (61.9) | 2 (40) | 0 (0) | 15 (53.6) | |
| Male | 8 (38.1) | 3 (60) | 2 (100) | 13 (46.4) | |
| Presence of comorbidities, n (%) | 0.696 | ||||
| No | 16 (76.2) | 5 (100) | 2 (100) | 23 (82.1) | |
| Yes | 5 (23.8) | 0 (0) | 0 (0) | 5 (17.9) | |
| BMI (kg/m2), median (IQR) | 24.2 (22.8–26.8) | 22.1 (21.8–23.6) | 24.5 (23.2–25.8) | 24 (22–26.6) | 0.571 |
| Vaccination-First dose type, n (%) | 1.000 | ||||
| Comirnaty | 19 (90.5) | 5 (100) | 2 (100) | 26 (92.9) | |
| Other | 2 (9.5) | 0 (0) | 0 (0) | 2 (7.1) | |
| MS duration, median (IQR) months(?) | 15 (5.7–24.8) | 10.7 (9.1–14) | 19.1 (7.9–30.3) | 12.9 (5.8–25) | 0.763 |
| MS treatment, n (%) | 0.463 | ||||
| Cladribine | 1 (4.8) | 0 (0) | 0 (0) | 1 (3.6) | |
| Fingolimod | 8 (38.1) | 1 (20) | 1 (50) | 10 (35.7) | |
| Interferon beta | 6 (28.6) | 0 (0) | 0 (0) | 6 (21.4) | |
| Ocrelizumab | 6 (28.6) | 4 (80) | 1 (50) | 11 (39.3) | |
| MS treatment duration, median (IQR) months | 4.7 (1.9–8) | 1.9 (1.8–4.1) | 6.7 (3.4–9.9) | 3.7 (1.8–7.9) | 0.310 |
| EDSS score, median (IQR) | 2 (0–2.5) | 1.5 (1–1.5) | 6.5 (6.5–6.5) | 1.8 (0.5–3) | 0.064 |
| <3 | 16 (76.2) | 4 (80.0) | 0 (0) | 20 (71.4) | 0.089 |
| ≥3 | 5 (23.8) | 1 (20.0) | 2 (100) | 8 (28.6) | |
| MS phenotype, n (%) | 0.253 | ||||
| Primary-progressive (PP) | 2 (9.5) | 1 (20) | 1 (50) | 4 (14.3) | |
| Relapsing-Remitting (RR) | 19 (90.5) | 4 (80) | 1 (50) | 24 (85.7) | |
| Lymphocytes count × 103/µL, median (IQR) | 1.3 (0.7–1.7) | 1.5 (1.2–1.7) | 1.5 (0.2–2.7) | 1.3 (0.7–1.8) | 0.912 |
| COVID-19 therapy, n(%) | 0.098 | ||||
| Anti-viral therapy | 4 (19.1) | 1 (20.0) | 0 (0) | 5 (17.9) | |
| Monoclonal therapy | 7 (33.3) | 4 (80.0) | 0 (0) | 11 (39.3) | |
| NSAIDs, paracetamol or no therapy | 10 (47.6) | 0 (0) | 2 (100) | 12 (42.9) | |
| Days from booster dose to infection, median (IQR) | 153 (105–169) | 204 (154–204) | 263 (258–268) | 154.5 (108–204.5) | 0.129 |
| anti-RBD IgG (BAU/mL), median (IQR) | |||||
| continuous | 407 (2–2319) | 1 (0–4) | 268 (0–537) | 135 (1–1337) | 0.160 |
| <7.1, n (%) | 6 (28.6) | 4 (80) | 1 (50) | 11 (39.3) | 0.090 |
| ≥7.1, n (%) | 15 (71.4) | 1 (20) | 1 (50) | 17 (60.7) | |
| IFN-γ T cell-specific response (pg/mL), median (IQR) | |||||
| continuous | 166 (1–564) | 238 (207–353) | 365 (0–729) | 188 (1–578) | 0.957 |
| <16, n (%) | 8 (38.1) | 1 (20) | 1 (50) | 10 (35.7) | 0.823 |
| ≥16, n (%) | 13 (61.9) | 4 (80) | 1 (50) | 18 (64.3) | |
| Neutralizing antibodies, median (IQR) | |||||
| continuous | 20 (5–160) | 5 (5–5) | 23 (5–40) | 5 (5–160) | 0.323 |
| <10, n (%) | 10 (47.6) | 4 (80) | 1 (50) | 15 (53.6) | 0.655 |
| ≥10, n (%) | 11 (52.4) | 1 (20) | 1 (50) | 13 (46.4) | |
IQR: interquartile range; BMI: body mass index; NSAIDs, non-steroidal anti-inflammatory drugs; PwMS: patients with multiple sclerosis; RBD: receptor-binding domain; IFN: interferon; EDSS: Expanded Disability Status Scale. In bold are reported the patient’s characteristics.
Most PwMS who had mild COVID-19 were under fingolimod (8/21, 38.1%) therapy; differently, the majority of those with moderate COVID-19 were under ocrelizumab treatment (4/5, 80%). Regarding COVID-19 therapy, 11 PwMS with mild disease reported having assumed antiviral (n = 4) or monoclonal antibodies (n = 7), whereas 10 PwMS took non-steroidal anti-inflammatory drugs (NSAIDs) or paracetamol. Most patients with moderate COVID-19 were treated with monoclonal antibodies (Table 4). Stratifying by DMTs, we observed that monoclonal antibodies or antivirals were administered mostly to fingolimod- or ocrelizumab-treated patients, whereas patients undergoing IFN-β treatment took NSAIDs or paracetamol (Supplementary Table S1).
The two severe COVID-19 patients showed a higher EDSS score (median: 6.5, IQR: 6.5–6.5) compared to those with mild (median: 2.0, IQR: 0–2.5) or moderate disease (median: 1.5, IQR: 1.0–1.5) (Table 4).
None of the demographic, clinical, or immunological variables were significantly associated with COVID-19 severity.
3.5. Time of Swab Negativization
Among the 25 infected PwMS for whom the time of swab negativization was available, the median time to have a negative swab was 11 days (IQR: 9 –14 days) (Table 1). Despite antiviral or monoclonal antibody therapies, patients treated with fingolimod took a significantly longer time for a swab reversion (7 days, 95%CI: 2.84–11.16, p = 0.002) than PwMS under IFN-β (Table 5). A similar trend was observed also for ocrelizumab-treated patients, albeit not statistically significant (3 days, 95%CI: −1.16–7.16, p = 0.148). Instead, PwMS under cladribine and IFN-β showed similar times for swab reversion.
Table 5.
Days to SARS-CoV-2 clearance in 25 PwMS with breakthrough infections.
| Quantile Regression Model | ||||||
|---|---|---|---|---|---|---|
| Patient’s Characteristic | Univariable | Multivariable | ||||
| Coefficient * | 95%CI | p | Coefficient ** | 95%CI | p | |
| Age class, years n (%) | ||||||
| 23–39 | Ref. | |||||
| 40–49 | 3.00 | −3.07; 9.07 | 0.317 | |||
| 50–70 | 0.00 | −5.78; 5.78 | 1.000 | |||
| Gender, n (%) | ||||||
| Female | Ref. | |||||
| Male | 0.00 | −5.03; 5.03 | 1.000 | |||
| Presence of comorbidities, n (%) | ||||||
| No | Ref. | |||||
| Yes | −5.00 | −10.6; 0.6 | 0.078 | |||
| BMI (kg/m2), median (IQR) | −0.21 | −0.79; 0.37 | 0.459 | |||
| Vaccination-First dose, n (%) | ||||||
| Comirnaty | Ref. | |||||
| Other | −3.00 | −11.63; 5.63 | 0.479 | |||
| MS duration, median (IQR) years | 0.00 | −0.29; 0.29 | 1.000 | |||
| MS treatment, n (%) | ||||||
| Cladribine | 0.00 | −8.52; 8.52 | 1.000 | |||
| Fingolimod | 7.00 | 2.84; 11.16 | 0.002 | |||
| Interferon beta | Ref. | |||||
| Ocrelizumab | 3.00 | −1.16; 7.16 | 0.148 | |||
| MS treatment duration, median (IQR) years | −0.41 | −0.76; −0.05 | 0.028 | |||
| EDSS score, median (IQR) | 0.00 | −1.32; 1.32 | 1.000 | |||
| <3 | Ref. | |||||
| ≥3 | 0.00 | −5.13; 5.13 | 1.000 | |||
| MS phenotype, n (%) | ||||||
| Primary-progressive (PP) | Ref. | |||||
| Relapsing-Remitting (RR) | 0.00 | −7.2; 7.2 | 1.000 | |||
| Lymphocytes count × 103/µL, median (IQR) | −2.36 | −6.46; 1.75 | 0.244 | |||
| COVID-19 therapy, n (%) | ||||||
| Anti-viral therapy | Ref. | |||||
| Monoclonal therapy | 4.00 | −2.3; 10.34 | 0.204 | |||
| NSAIDs, paracetamol or no therapy | −1.00 | −7.13; 6.13 | 0.738 | |||
| Severity of COVID-19, n (%) | ||||||
| Mild illness | Ref. | |||||
| Moderate illness | 4 | −1.90; 9.90 | 0.174 | |||
| Severe illness | 1 | −11.0; 13.0 | 0.865 | |||
| Days from booster dose to sample, median (IQR) | 0.04 | −0.3; 0.39 | 0.796 | |||
| Anti-RBD IgG (BAU/mL), median (IQR) | ||||||
| continuous | −0.0004 | −0.001; 0.0003 | 0.245 | −0.001 | −0.001; 0.0002 | 0.163 |
| score: Negative (<7.1), n (%) | Ref. | Ref. | ||||
| score: Positive (≥7.1), n (%) | −4.00 | −8.31; 0.31 | 0.067 | −2.73 | −7.27; 1.81 | 0.225 |
| <809 | Ref. | Ref. | ||||
| ≥809 | −6.00 | −10.7; −1.3 | 0.015 | −4.39 | −8.8; 0.01 | 0.051 |
| IFN-γ T cell-specific response (pg/mL), median (IQR) | ||||||
| continuous | −0.0018 | −0.006; 0.003 | 0.422 | −0.002 | −0.006; 0.003 | 0.446 |
| <16, n (%) | Ref. | Ref. | ||||
| ≥16, n (%) | −5.00 | −9.77; −0.23 | 0.041 | −5.88 | −10.65; −1.10 | 0.018 |
| Neutralizing antibodies, median (IQR) | ||||||
| continuous | −0.01 | −0.03; 0.01 | 0.178 | −0.01 | −0.03; 0 | 0.135 |
| <10, n (%) | Ref. | Ref. | ||||
| ≥10, n (%) | 0.00 | −3.71; 3.71 | 1.000 | −2.5 | −7.5; 2.5 | 0.310 |
CI: confidence interval; Ref: reference category; NSAIDs, non-steroidal anti-inflammatory drugs; PwMS: patients with multiple sclerosis; RBD: receptor-binding domain; IFN: interferon; EDSS: Expanded Disability Status Scale. * Estimated by univariable median regression model; ** estimated by median regression model after adjusting for the time elapsed from sample collection. In bold are reported the patient’s characteristics and the significant values.
Conversely, having a high anti-RBD antibody titer (≥ 809 BAU/mL) and an IFN-γ-specific T cell response (≥ 16 pg/mL) were associated with a significantly shorter time for a negative swab (−6.00 days, 95%CI: −10.7–−1.3, p = 0.015; −5.00 days, 95%CI: −9.77–−0.23, p = 0.041, respectively). This result was also confirmed after adjusting for time elapsed from sample collection (anti-RBD: −4.39 days, 95%CI: −8.8–0.01, p = 0.051; T cell: −5.88 days, 95%CI: −10.65–−1.10, p = 0.018, respectively) (Table 5). Instead, neutralizing titers did not show any impact on the time needed for SARS-CoV-2 clearance.
4. Discussion
To the best of our knowledge, this is the first longitudinal prospective study investigating the demographic, clinical, and immunological factors potentially associated with both the risk of SARS-CoV-2 infection, disease severity, and the time for swab reversion in a cohort of PwMS treated with different DMTs after the third dose of COVID-19 vaccines.
The majority of the current studies have mainly focused on B-depleted patients [32,33,34] and the role played by clinical features and/or the only antibody response on the incidence and severity of BIs [35,36,37,38,39,40]. On the other hand, evidence regarding the impact of DMTs other than anti-CD20 agents or T cell responses on the incidence and severity of BIs is limited, as is the identification of clinical or immunological factors potentially associated with the time to swab reversion.
The identification of clinical and immune markers associated with reduced incidence or severity of SARS-CoV-2 infections and the timing of negativization are needed to improve the clinical management of PwMS under immunosuppressive therapies.
In this study, we found that 43.8% (28/64) of PwMS experienced a BI after three doses of COVID-19 mRNA vaccine. This relatively high incidence rate of SARS-CoV-2 infection is likely due to the spread of the Omicron variant in Italy, which coincided with the follow-up period of the study [41]. Omicron variants are known to be less pathogenic than the previous ones; however, they are highly transmissible despite vaccination [27,29,47]. The first-generation mRNA vaccines were designed against the spike protein of the Wuhan strain; thus, it is conceivable that they offered reduced protection against the Omicron variant that is highly mutated in the spike protein compared to any others [48,49].
The majority of BIs (75%) were mild, thus confirming the less pathogenic character of the Omicron variant and the protective role of vaccination against the severe disease. These results corroborate what was observed in larger cohorts of PwMS [40,50,51]. Only two cases of severe COVID-19 were reported in our cohort, specifically in two PwMS showing high disability levels. One patient was undergoing fingolimod therapy while the other one was treated with ocrelizumab.
Interestingly, the male population of our MS cohort seems to be more prone to BIs than females (61.9% males vs. 35% females). This greater susceptibility to COVID-19 may be ascribed to biological and genetic differences, particularly to the different innate and adaptive immune responses reported between males and females [52].
Among the clinical factors, the phenotype and severity of MS disease were associated with greater protection against infection. Having a relapsing-remitting phenotype significantly reduced the BI incidence rate in PwMS by 88% compared to those with a primary-progressive disease, which is in agreement with previous data [53]. Unlike the primary-progressive MS that progresses more gradually with the slow accumulation of neurological disability, the relapsing-remitting disease is characterized by relapses, which are exacerbations or attacks of neurological dysfunction, followed by remissions, which are periods without obvious disease activity [54].
Moreover, we found a significantly lower incidence rate of BIs in patients with greater MS severity (EDSS ≥ 3), although a higher EDSS, in those infected with SARS-CoV-2, was associated with an increased risk of severe COVID-19 [53,55,56]. Our results may be explained by the nature of the MS itself. MS is a central nervous system autoimmune disease in which damage to myelin causes symptoms including muscle weakness, thus resulting in walking difficulties and problems of coordination [54]. Therefore, it is reasonable to think that PwMS with a higher degree of disability have maintained greater social distancing measures, thus limiting the possibility of being infected. Consequently, this unexpected correlation between lower incidence of BIs and MS severity could be considered a reverse causality as the severity of the disease does not represent a protective factor for SARS-CoV-2 infection.
To note, SARS-CoV-2 infections in PwMS occurred after about 5 months from the third dose, a time corresponding to the initial waning of the humoral response as previously reported in several longitudinal studies [5,7,22,23,25,39]. A protective role of SARS-CoV-2 antibody levels against the Delta variant (HR = 0.57) and lower protection for the Omicron cases (HR = 1.40) was observed by Sormani and colleagues [35].
In this study, we observed a significantly lower seroconversion rate among subjects who had BI than uninfected subjects. Having an anti-RBD response ≥ 7.1 BAU/mL still conferred a 60% reduction of the BI incidence rate after adjusting for EDSS score and disease phenotype. Anti-RBD antibodies can be directed against different epitopes in the RBD of the spike protein, but not all of them can neutralize the infectious capacity of the virus [57]. Non-neutralizing antibodies are antibodies that can bind to viruses but cannot neutralize them. They can mediate protection against viruses through different strategies other than classical neutralization assays. For instance, non-neutralizing antibodies can mediate antibody-dependent cellular cytotoxicity, antibody-dependent cellular phagocytosis, and complement activation, and their binding can expose epitopes for neutralizing antibody binding possibly increasing the virus neutralization efficacy [58]. Our data highlight the important role of the antibody response in COVID-19 protection as supported by the higher frequency of BIs observed in ocrelizumab-treated patients (64.7%). Ocrelizumab is an anti-CD20 monoclonal antibody that acts by depleting B lymphocytes. This mechanism prevents PwMS treated with ocrelizumab from mounting an appropriate humoral response to vaccines or infections [5,6,7,33,37,39,59,60]. The lack of the antibody response makes ocrelizumab-treated patients a high-risk category for COVID-19 and longer persistence of SARS-CoV-2 infection, despite having a T cell-specific response [61].
Our results confirm what was reported by Novak and colleagues, who showed a rate of 59% in BIs after the third SARS-CoV-2 mRNA vaccination in ocrelizumab-treated patients without resulting in a severe outcome [33]. Moreover, it has been reported that patients treated with anti-CD20 agents showed a risk almost four times higher of having SARS-CoV-2 infection compared to patients under different DMTs with a hazard ratio (HR) of 3.72 [50].
As far as we know, this study showed for the first time an association between antibody and T cell-specific responses with a more rapid swab reversion. Specifically, having high anti-RBD antibody titers (≥ 809 BAU/mL) and an IFN-γ-specific T cell response significantly reduced the time required to obtain a negative swab by about 4.39 and 5.88 days, respectively, as confirmed by the multivariable analysis. On the contrary, the treatment with fingolimod significantly delayed the swab reversion by about 7 days. This result agrees with the mechanism of action of this immunosuppressive drug. Indeed, fingolimod is a sphingosine-1-phosphate receptor modulator that retains lymphocytes in the lymph nodes, thereby reducing the number of T and B cells in circulation [62]. Consequently, patients treated with fingolimod are incapable of mounting an appropriate T cell response to vaccines and infections [6,63].
Considering the pivotal role played by the cell-mediated immune response in fighting SARS-CoV-2 infections by removing infected cells [64], it is reasonable to think that the lack of T cell response in fingolimod-treated patients is responsible for the longer time taken for swab reversion.
Some limitations of the study need to be acknowledged. The small sample size of the cohort may have limited the emergence of significant associations. Moreover, the reduced number of cladribine-treated patients may have restricted their ability to demonstrate significant differences compared with other DMTs. However, the robustness of the reported data was confirmed by the multivariable analysis. The small number of PwMS with severe COVID-19 may have limited our ability to identify clinical and immunological factors associated with disease severity. The collected data were rigorously checked by the investigators and by those who carried out the analyses; however, we cannot completely rule out the presence of reporting bias. Furthermore, it is reasonable to think that precautions such as social distancing taken by patients with greater MS severity may have influenced the incidence of infection in our cohort.
On the other hand, this work has some strengths. This is a longitudinal and prospective study with a long-term follow-up. The cohort was well characterized both immunologically and clinically, allowing an in-depth analysis of SARS-CoV-2 incidence, severity, and timing of swab negativization. Moreover, we used standardized methods to detect antibody and T cell-specific responses.
5. Conclusions
In conclusion, this study provides evidence of the real-world impact of clinical and immunological data on both the incidence and severity of SARS-CoV-2 infection and on the timing of negativization in a cohort of PwMS undergoing DMTs after the third dose of COVID-19 mRNA vaccine. Our results showed that having a high antibody titer and T cell response favor a more rapid viral clearance, whereas treatment with fingolimod is associated with a delayed timing of swab reversion. These findings suggest different immunological protection from SARS-CoV-2 infection according to DMTs and, if confirmed in larger-scale population studies, they would be relevant to optimize the clinical management of PwMS.
Acknowledgments
The authors gratefully acknowledge the nurses of the MS Centre of the San Camillo Forlanini Hospital and the patients who provided their consent to generate this study.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/vaccines12080926/s1, Figure S1: IFN-γ-specific T cell response after stimulation with staphylococcal enterotoxin B (SEB), Table S1: COVID-19 therapy.
Author Contributions
Conceptualization, D.G., and C.G.; methodology, V.V., S.M., G.M., A.S., A.M.G.A., and F.M.; formal analysis, A.A., and A.N.; enrolling patients and collecting clinical data: S.R., C.T., S.H., L.P., G.C., M.E.Q., S.V., S.G., E.N., and C.G.; writing—original draft preparation, A.A., S.R., and D.G.; writing—review and editing, A.A., S.R., A.N., C.T., V.V., S.H., L.P., G.C., A.S., M.E.Q., A.M.G.A., S.M., G.M., S.V., S.G., F.M., E.N., C.G., and D.G.; funding acquisition, D.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethical Committee of “L. Spallanzani” National Institute of Infectious Diseases (INMI)-IRCCS (approval numbers 319/2021 and 443/2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, and further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
S.R. has received honoraria from Biogen, Merck Serono, Novartis, and Teva for consulting services, speaking, and/or travel support. C.T. and C.G. received honoraria for speaking, manuscript writing, or educational events from Merck, Biogen, Roche, Novartis Sanofi, Celgene, and Almirall. L.P. received consulting fees and/or speaker honoraria from Biogen, Celgene, Genzyme, Merck-Serono, Novartis, and Teva, travel grants from Biogen, Genzyme, Novartis, and Teva, and research grants from the Italian MS Society (Associazione Italiana Sclerosi Multipla) and Genzyme. S.H. received travel funding and/or speaker honoraria from Biogen, Roche, Genzyme, Novartis, and CSL Behring. S.M. has nothing to disclose in relation to this manuscript. She received relevant financial activities outside the submitted work: a research grant and a supply of kits from Abbott s.r.l. S.G. received honoraria for speaking and travel grants from Biogen, Sanofi-Aventis, Merck Serono, Bayer-Schering, Teva, Genzyme, Almirall, and Novartis. E.N. participates on a data safety monitoring board or advisory board and receives fees for educational training from Gilead, Eli Lilly, G.S., SOBI, and Roche, and he has a patent pending for raloxifene use in COVID-19 with Dompè Pharmaceutical. DG is a member of the advisory board of Biomerieux and Eli Lilly and received fees for educational training or consultancy from Almirall, Biogen, Celgene, Diasorin, Janssen, Qiagen, and Quidel. All the other authors declare that they have no conflicts of interest. The funders had no role in the design of the study, in the collection, analyses or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
This research was supported by INMI “Lazzaro Spallanzani” Ricerca Corrente on emerging infections funded by the Italian Ministry of Health and by generous liberal donation/funding for COVID-19 research from Camera di Commercio, Industria e Artigianato di Roma (resolution number 395 on 25 May 2021).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Coronavirus Disease (COVID-19)—World Health Organization. [(accessed on 2 May 2023)]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- 2.Grifoni A., Alonzi T., Alter G., Noonan D.M., Landay A.L., Albini A., Goletti D. Impact of Aging on Immunity in the Context of COVID-19, HIV, and Tuberculosis. Front. Immunol. 2023;14:1146704. doi: 10.3389/fimmu.2023.1146704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y., Choudhary M.C., Regan J., Boucau J., Nathan A., Speidel T., Liew M.Y., Edelstein G.E., Kawano Y., Uddin R., et al. SARS-CoV-2 Viral Clearance and Evolution Varies by Type and Severity of Immunodeficiency. Sci. Transl. Med. 2024;16:eadk1599. doi: 10.1126/scitranslmed.adk1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrone L., Tortorella C., Aiello A., Farroni C., Ruggieri S., Castilletti C., Meschi S., Cuzzi G., Vanini V., Palmieri F., et al. Humoral and Cellular Response to Spike of Delta SARS-CoV-2 Variant in Vaccinated Patients with Multiple Sclerosis. Front. Neurol. 2022;13:881988. doi: 10.3389/fneur.2022.881988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruggieri S., Aiello A., Tortorella C., Navarra A., Vanini V., Meschi S., Lapa D., Haggiag S., Prosperini L., Cuzzi G., et al. Dynamic Evolution of Humoral and T-Cell Specific Immune Response to COVID-19 mRNA Vaccine in Patients with Multiple Sclerosis Followed until the Booster Dose. Int. J. Mol. Sci. 2023;24:8525. doi: 10.3390/ijms24108525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tortorella C., Aiello A., Gasperini C., Agrati C., Castilletti C., Ruggieri S., Meschi S., Matusali G., Colavita F., Farroni C., et al. Humoral- and T-Cell-Specific Immune Responses to SARS-CoV-2 mRNA Vaccination in Patients with MS Using Different Disease-Modifying Therapies. Neurology. 2022;98:e541–e554. doi: 10.1212/WNL.0000000000013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aiello A., Coppola A., Ruggieri S., Farroni C., Altera A.M.G., Salmi A., Vanini V., Cuzzi G., Petrone L., Meschi S., et al. Longitudinal Characterisation of B and T-Cell Immune Responses after the Booster Dose of COVID-19 mRNA-Vaccine in People with Multiple Sclerosis Using Different Disease-Modifying Therapies. J. Neurol. Neurosurg. Psychiatry. 2023;94:290–299. doi: 10.1136/jnnp-2022-330175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castelo-Branco A., Chiesa F., Conte S., Bengtsson C., Lee S., Minton N., Niemcryk S., Lindholm A., Rosenlund M., Piehl F., et al. Infections in Patients with Multiple Sclerosis: A National Cohort Study in Sweden. Mult. Scler. Relat. Disord. 2020;45:102420. doi: 10.1016/j.msard.2020.102420. [DOI] [PubMed] [Google Scholar]
- 9.McGinley M.P., Goldschmidt C.H., Rae-Grant A.D. Diagnosis and Treatment of Multiple Sclerosis: A Review. JAMA. 2021;325:765–779. doi: 10.1001/jama.2020.26858. [DOI] [PubMed] [Google Scholar]
- 10.Iyer R.B., Raghavendra S., Nooraine J., Jaychandran R. COVID-19 Outcomes in Persons with Multiple Sclerosis Treated with Rituximab. Mult. Scler. Relat. Disord. 2022;57:103371. doi: 10.1016/j.msard.2021.103371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foerch C., Friedauer L., Bauer B., Wolf T., Adam E.H. Severe COVID-19 Infection in a Patient with Multiple Sclerosis Treated with Fingolimod. Mult. Scler. Relat. Disord. 2020;42:102180. doi: 10.1016/j.msard.2020.102180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simpson-Yap S., De Brouwer E., Kalincik T., Rijke N., Hillert J.A., Walton C., Edan G., Moreau Y., Spelman T., Geys L., et al. Associations of Disease-Modifying Therapies With COVID-19 Severity in Multiple Sclerosis. Neurology. 2021;97:e1870–e1885. doi: 10.1212/WNL.0000000000012753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaloum S.A., Wood C.H., Tank P., Upcott M., Vickaryous N., Anderson V., Baker D., Chance R., Evangelou N., George K., et al. Risk of COVID-19 in People with Multiple Sclerosis Who Are Seronegative Following Vaccination. Mult. Scler. J. 2023;29:979–989. doi: 10.1177/13524585231185247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prosperini L., Tortorella C., Haggiag S., Ruggieri S., Galgani S., Gasperini C. Increased Risk of Death from COVID-19 in Multiple Sclerosis: A Pooled Analysis of Observational Studies. J. Neurol. 2022;269:1114–1120. doi: 10.1007/s00415-021-10803-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Achiron A., Mandel M., Dreyer-Alster S., Harari G., Magalashvili D., Sonis P., Dolev M., Menascu S., Flechter S., Falb R., et al. Humoral Immune Response to COVID-19 mRNA Vaccine in Patients with Multiple Sclerosis Treated with High-Efficacy Disease-Modifying Therapies. Ther. Adv. Neurol. Disord. 2021;14:17562864211012835. doi: 10.1177/17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabatino J.J., Mittl K., Rowles W.M., McPolin K., Rajan J.V., Laurie M.T., Zamecnik C.R., Dandekar R., Alvarenga B.D., Loudermilk R.P., et al. Multiple Sclerosis Therapies Differentially Affect SARS-CoV-2 Vaccine–Induced Antibody and T Cell Immunity and Function. JCI Insight. 2022;7:e156978. doi: 10.1172/jci.insight.156978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sainz de la Maza S., Walo-Delgado P.E., Rodríguez-Domínguez M., Monreal E., Rodero-Romero A., Chico-García J.L., Pariente R., Rodríguez-Jorge F., Ballester-González R., Villarrubia N., et al. Short- and Long-Term Humoral and Cellular Immune Responses to SARS-CoV-2 Vaccination in Patients with Multiple Sclerosis Treated with Disease-Modifying Therapies. Vaccines. 2023;11:786. doi: 10.3390/vaccines11040786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apostolidis S.A., Kakara M., Painter M.M., Goel R.R., Mathew D., Lenzi K., Rezk A., Patterson K.R., Espinoza D.A., Kadri J.C., et al. Cellular and Humoral Immune Responses Following SARS-CoV-2 mRNA Vaccination in Patients with Multiple Sclerosis on Anti-CD20 Therapy. Nat. Med. 2021;27:1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Habek M., Željko C., Savić Mlakar A., Bendelja K., Rogić D., Adamec I., Barun B., Gabelić T., Krbot Skorić M. Humoral and Cellular Immunity in Convalescent and Vaccinated COVID-19 People with Multiple Sclerosis: Effects of Disease Modifying Therapies. Mult. Scler. Relat. Disord. 2022;59:103682. doi: 10.1016/j.msard.2022.103682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer-Arndt L., Braun J., Fauchere F., Vanshylla K., Loyal L., Henze L., Kruse B., Dingeldey M., Jürchott K., Mangold M., et al. SARS-CoV-2 mRNA Vaccinations Fail to Elicit Humoral and Cellular Immune Responses in Patients with Multiple Sclerosis Receiving Fingolimod. J. Neurol. Neurosurg. Psychiatry. 2022;93:960–971. doi: 10.1136/jnnp-2022-329395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shrotri M., Navaratnam A.M.D., Nguyen V., Byrne T., Geismar C., Fragaszy E., Beale S., Fong W.L.E., Patel P., Kovar J., et al. Spike-Antibody Waning after Second Dose of BNT162b2 or ChAdOx1. Lancet. 2021;398:385–387. doi: 10.1016/S0140-6736(21)01642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bajwa H.M., Novak F., Nilsson A.C., Nielsen C., Holm D.K., Østergaard K., Witt A.H., Byg K.-E., Johansen I.S., Mittl K., et al. Persistently Reduced Humoral and Sustained Cellular Immune Response from First to Third SARS-CoV-2 mRNA Vaccination in Anti-CD20-Treated Multiple Sclerosis Patients. Mult. Scler. Relat. Disord. 2022;60:103729. doi: 10.1016/j.msard.2022.103729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., Doolman R., Asraf K., Mendelson E., Ziv A., et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N. Engl. J. Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillot C., Bayart J.-L., Closset M., Cabo J., Maloteau V., Dogné J.-M., Douxfils J., Favresse J. Peri-Infection Titers of Neutralizing and Binding Antibodies as a Predictor of COVID-19 Breakthrough Infections in Vaccinated Healthcare Professionals: Importance of the Timing. Clin. Chem. Lab. Med. 2023;61:1670–1675. doi: 10.1515/cclm-2023-0134. [DOI] [PubMed] [Google Scholar]
- 25.Menegale F., Manica M., Zardini A., Guzzetta G., Marziano V., d’Andrea V., Trentini F., Ajelli M., Poletti P., Merler S. Evaluation of Waning of SARS-CoV-2 Vaccine-Induced Immunity: A Systematic Review and Meta-Analysis. JAMA Netw. Open. 2023;6:e2310650. doi: 10.1001/jamanetworkopen.2023.10650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Machkovech H.M., Hahn A.M., Garonzik Wang J., Grubaugh N.D., Halfmann P.J., Johnson M.C., Lemieux J.E., O’Connor D.H., Piantadosi A., Wei W., et al. Persistent SARS-CoV-2 Infection: Significance and Implications. Lancet Infect. Dis. 2024;24:e453–e462. doi: 10.1016/S1473-3099(23)00815-0. [DOI] [PubMed] [Google Scholar]
- 27.Accorsi E.K., Britton A., Fleming-Dutra K.E., Smith Z.R., Shang N., Derado G., Miller J., Schrag S.J., Verani J.R. Association Between 3 Doses of mRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants. JAMA. 2022;327:639–651. doi: 10.1001/jama.2022.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ioannou G.N., Bohnert A.S.B., O’Hare A.M., Boyko E.J., Maciejewski M.L., Smith V.A., Bowling C.B., Viglianti E., Iwashyna T.J., Hynes D.M., et al. Effectiveness of mRNA COVID-19 Vaccine Boosters Against Infection, Hospitalization, and Death: A Target Trial Emulation in the Omicron (B.1.1.529) Variant Era. Ann. Intern. Med. 2022;175:1693–1706. doi: 10.7326/M22-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrews N., Stowe J., Kirsebom F., Toffa S., Rickeard T., Gallagher E., Gower C., Kall M., Groves N., O’Connell A.-M., et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsang N.N.Y., So H.C., Cowling B.J., Leung G.M., Ip D.K.M. Effectiveness of BNT162b2 and CoronaVac COVID-19 Vaccination against Asymptomatic and Symptomatic Infection of SARS-CoV-2 Omicron BA.2 in Hong Kong: A Prospective Cohort Study. Lancet Infect. Dis. 2023;23:421–434. doi: 10.1016/S1473-3099(22)00732-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nyberg T., Ferguson N.M., Nash S.G., Webster H.H., Flaxman S., Andrews N., Hinsley W., Bernal J.L., Kall M., Bhatt S., et al. Comparative Analysis of the Risks of Hospitalisation and Death Associated with SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) Variants in England: A Cohort Study. Lancet. 2022;399:1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calabrese C.M., Kirchner E., Husni E.M., Moss B.P., Fernandez A.P., Jin Y., Calabrese L.H. Breakthrough SARS-CoV-2 Infections in Patients with Immune-Mediated Disease Undergoing B Cell-Depleting Therapy: A Retrospective Cohort Analysis. Arthritis Rheumatol. 2022;74:1906–1915. doi: 10.1002/art.42287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novak F., Bajwa H.M., Coia J.E., Nilsson A.C., Nielsen C., Holm D.K., Østergaard K., Hvidt M.V.M., Byg K.-E., Johansen I.S., et al. Low Protection from Breakthrough SARS-CoV-2 Infection and Mild Disease Course in Ocrelizumab-Treated Patients with Multiple Sclerosis after Three mRNA Vaccine Doses. J. Neurol. Neurosurg. Psychiatry. 2023;94:934–937. doi: 10.1136/jnnp-2022-330757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cross A.H., Delgado S., Habek M., Davydovskaya M., Ward B.J., Cree B.A.C., Totolyan N., Pingili R., Mancione L., Hu X., et al. Correction to: COVID-19 Outcomes and Vaccination in People with Relapsing Multiple Sclerosis Treated with Ofatumumab. Neurol. Ther. 2022;11:759–762. doi: 10.1007/s40120-022-00346-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sormani M.P., Schiavetti I., Inglese M., Carmisciano L., Laroni A., Lapucci C., Visconti V., Serrati C., Gandoglia I., Tassinari T., et al. Breakthrough SARS-CoV-2 Infections after COVID-19 mRNA Vaccination in MS Patients on Disease Modifying Therapies during the Delta and the Omicron Waves in Italy. EBioMedicine. 2022;80:104042. doi: 10.1016/j.ebiom.2022.104042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Disanto G., Galante A., Cantu’ M., Sacco R., Mele F., Eisler J.J., Keller F., Bernasconi E., Sallusto F., Zecca C., et al. Longitudinal Postvaccine SARS-CoV-2 Immunoglobulin G Titers, Memory B-Cell Responses, and Risk of COVID-19 in Multiple Sclerosis Over 1 Year. Neurol. Neuroimmunol. Neuroinflamm. 2023;10:e200043. doi: 10.1212/NXI.0000000000200043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holroyd K.B., Healy B.C., Conway S., Houtchens M., Bakshi R., Bhattacharyya S., Bose G., Galetta K., Kaplan T., Severson C., et al. Humoral Response to COVID-19 Vaccination in MS Patients on Disease Modifying Therapy: Immune Profiles and Clinical Outcomes. Mult. Scler. Relat. Disord. 2022;67:104079. doi: 10.1016/j.msard.2022.104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klineova S., Farber R.S., DeAngelis T., Leung T., Smith T., Blanck R., Zhovtis-Ryerson L., Harel A. Vaccine-Breakthrough SARS-CoV-2 Infections in People with Multiple Sclerosis and Related Conditions: An Observational Study by the New York COVID-19 Neuro-Immunology Consortium (NYCNIC-2) Mult. Scler. J. 2023;29:990–1000. doi: 10.1177/13524585231185246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jakimovski D., Zakalik K., Awan S., Kavak K.S., Pennington P., Hojnacki D., Kolb C., Lizarraga A.A., Eckert S.P., Sarrosa R., et al. COVID-19 Vaccination in Multiple Sclerosis and Inflammatory Diseases: Effects from Disease-Modifying Therapy, Long-Term Seroprevalence and Breakthrough Infections. Vaccines. 2022;10:695. doi: 10.3390/vaccines10050695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Kempen Z.L.E., Stalman E.W., Steenhuis M., Kummer L.Y.L., van Dam K.P.J., Wilbrink M.F., Ten Brinke A., van Ham S.M., Kuijpers T., Rispens T., et al. SARS-CoV-2 Omicron Breakthrough Infections in Patients with Multiple Sclerosis. J. Neurol. Neurosurg. Psychiatry. 2023;94:280–283. doi: 10.1136/jnnp-2022-330100. [DOI] [PubMed] [Google Scholar]
- 41.Stefanelli P., Trentini F., Petrone D., Mammone A., Ambrosio L., Manica M., Guzzetta G., d’Andrea V., Marziano V., Zardini A., et al. Tracking the Progressive Spread of the SARS-CoV-2 Omicron Variant in Italy, December 2021 to January 2022. Eurosurveillance. 2022;27:2200125. doi: 10.2807/1560-7917.ES.2022.27.45.2200125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson A.J., Banwell B.L., Barkhof F., Carroll W.M., Coetzee T., Comi G., Correale J., Fazekas F., Filippi M., Freedman M.S., et al. Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria. Lancet Neurol. 2018;17:162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 43.Clinical Spectrum. [(accessed on 3 March 2024)]; Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
- 44.Aiello A., Najafi Fard S., Petruccioli E., Petrone L., Vanini V., Farroni C., Cuzzi G., Navarra A., Gualano G., Mosti S., et al. Spike Is the Most Recognized Antigen in the Whole-Blood Platform in Both Acute and Convalescent COVID-19 Patients. Int. J. Infect. Dis. 2021;106:338–347. doi: 10.1016/j.ijid.2021.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matusali G., Colavita F., Lapa D., Meschi S., Bordi L., Piselli P., Gagliardini R., Corpolongo A., Nicastri E., Antinori A., et al. SARS-CoV-2 Serum Neutralization Assay: A Traditional Tool for a Brand-New Virus. Viruses. 2021;13:655. doi: 10.3390/v13040655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faul F., Erdfelder E., Buchner A., Lang A.-G. Statistical Power Analyses Using G*Power 3.1: Tests for Correlation and Regression Analyses. Behav. Res. Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki R., Yamasoba D., Kimura I., Wang L., Kishimoto M., Ito J., Morioka Y., Nao N., Nasser H., Uriu K., et al. Attenuated Fusogenicity and Pathogenicity of SARS-CoV-2 Omicron Variant. Nature. 2022;603:700–705. doi: 10.1038/s41586-022-04462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao Y., Wang J., Jian F., Xiao T., Song W., Yisimayi A., Huang W., Li Q., Wang P., An R., et al. Omicron Escapes the Majority of Existing SARS-CoV-2 Neutralizing Antibodies. Nature. 2022;602:657–663. doi: 10.1038/s41586-021-04385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu L., Iketani S., Guo Y., Chan J.F.-W., Wang M., Liu L., Luo Y., Chu H., Huang Y., Nair M.S., et al. Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2. Nature. 2022;602:676–681. doi: 10.1038/s41586-021-04388-0. [DOI] [PubMed] [Google Scholar]
- 50.Immovilli P., Schiavetti I., Franceschini A., De Mitri P., Gelati L., Rota E., Guidetti D. Breakthrough COVID-19 in People with Multiple Sclerosis on Disease Modifying Treatments: Is It Still a Severe Disease? Mult. Scler. Relat. Disord. 2024;85:105547. doi: 10.1016/j.msard.2024.105547. [DOI] [PubMed] [Google Scholar]
- 51.Spierer R., Lavi I., Bloch S., Mazar M., Golan D. Risk of Breakthrough COVID-19 after Vaccination among People with Multiple Sclerosis on Disease-Modifying Therapies. J. Neurol. 2023;270:4632–4639. doi: 10.1007/s00415-023-11935-4. [DOI] [PubMed] [Google Scholar]
- 52.Plebani M., Lippi G. Sex and gender differences in COVID-19: A narrative review. J. Sex Gend. Specif. Med. 2022;8:105–111. [Google Scholar]
- 53.Chaudhry F., Bulka H., Rathnam A.S., Said O.M., Lin J., Lorigan H., Bernitsas E., Rube J., Korzeniewski S.J., Memon A.B., et al. COVID-19 in Multiple Sclerosis Patients and Risk Factors for Severe Infection. J. Neurol. Sci. 2020;418:117147. doi: 10.1016/j.jns.2020.117147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dobson R., Giovannoni G. Multiple Sclerosis—A Review. Eur. J. Neurol. 2019;26:27–40. doi: 10.1111/ene.13819. [DOI] [PubMed] [Google Scholar]
- 55.Louapre C., Collongues N., Stankoff B., Giannesini C., Papeix C., Bensa C., Deschamps R., Créange A., Wahab A., Pelletier J., et al. Clinical Characteristics and Outcomes in Patients with Coronavirus Disease 2019 and Multiple Sclerosis. JAMA Neurol. 2020;77:1–10. doi: 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Immovilli P., Schiavetti I., Cordioli C., De Mitri P., Grazioli S., Guidetti D., Sormani M.P. Lung Involvement Correlates with Disability in MS Patients with COVID-19 Pneumonia. Neurol. Sci. 2022;43:6657–6659. doi: 10.1007/s10072-022-06333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan M., Liu H., Wu N.C., Lee C.-C.D., Zhu X., Zhao F., Huang D., Yu W., Hua Y., Tien H., et al. Structural Basis of a Shared Antibody Response to SARS-CoV-2. Science. 2020;369:1119–1123. doi: 10.1126/science.abd2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chandler T.L., Yang A., Otero C.E., Permar S.R., Caddy S.L. Protective Mechanisms of Nonneutralizing Antiviral Antibodies. PLoS Pathog. 2023;19:e1011670. doi: 10.1371/journal.ppat.1011670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Katz J.D., Bouley A.J., Jungquist R.M., Douglas E.A., O’Shea I.L., Lathi E.S. Humoral and T-Cell Responses to SARS-CoV-2 Vaccination in Multiple Sclerosis Patients Treated with Ocrelizumab. Mult. Scler. Relat. Disord. 2022;57:103382. doi: 10.1016/j.msard.2021.103382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Räuber S., Korsen M., Huntemann N., Rolfes L., Müntefering T., Dobelmann V., Hermann A.M., Kölsche T., Lipinski K., von Wnuck Lipinski K., et al. Immune Response to SARS-CoV-2 Vaccination in Relation to Peripheral Immune Cell Profiles among Patients with Multiple Sclerosis Receiving Ocrelizumab. J. Neurol. Neurosurg. Psychiatry. 2022;93:978–985. doi: 10.1136/jnnp-2021-328197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Januel E., Hajage D., Labauge P., Maillart E., De Sèze J., Zephir H., Pelletier J., Guilloton L., Bensa C., Heinzlef O., et al. Association Between Anti-CD20 Therapies and COVID-19 Severity Among Patients with Relapsing-Remitting and Progressive Multiple Sclerosis. JAMA Netw. Open. 2023;6:e2319766. doi: 10.1001/jamanetworkopen.2023.19766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chun J., Hartung H.-P. Mechanism of Action of Oral Fingolimod (FTY720) in Multiple Sclerosis. Clin. Neuropharmacol. 2010;33:91–101. doi: 10.1097/WNF.0b013e3181cbf825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Palomares Cabeza V., Kummer L.Y.L., Wieske L., Hagen R.R., Duurland M., Konijn V.A.L., van Dam K.P.J., Stalman E.W., van de Sandt C.E., Boekel L., et al. Longitudinal T-Cell Responses after a Third SARS-CoV-2 Vaccination in Patients with Multiple Sclerosis on Ocrelizumab or Fingolimod. Neurol. Neuroimmunol. Neuroinflamm. 2022;9:e1178. doi: 10.1212/NXI.0000000000001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Petrone L., Sette A., de Vries R.D., Goletti D. The Importance of Measuring SARS-CoV-2-Specific T-Cell Responses in an Ongoing Pandemic. Pathogens. 2023;12:862. doi: 10.3390/pathogens12070862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, and further inquiries can be directed to the corresponding author/s.