Abstract
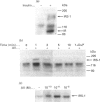
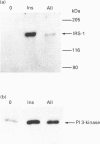
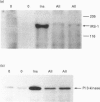
We have investigated whether angiotensin II (AII) is able to induce insulin receptor substrate 1 (IRS-1) phosphorylation and its association with phosphatidylinositol 3-kinase (PI 3-kinase) in the rat heart in vivo. The phosphorylation state of IRS-1 following infusion of insulin or AII via the vena cava was assessed after immunoprecipitation with an anti-peptide antibody to IRS-1 followed by immunoblotting with an anti-phosphotyrosine antibody and an anti-PI 3-kinase antibody. Densitometry indicated a 5.6 +/- 1.3-fold increase in IRS-1 phosphorylation after stimulation with AII and a 12.8 +/- 3.1-fold increase after insulin. The effect was maximal at an AII concentration of 10(-8) M and occurred 1 min after infusion. There was also a 6.1 +/- 1.2-fold increase in IRS-1-associated PI 3-kinase in response to AII. In the isolated perfused heart the result was similar, showing a direct effect of AII on this pathway. When the animals were pretreated for 1 h with DuP 753, a non-peptide AII-receptor 1 (AT1 receptor) antagonist, there was a marked reduction in the AII-induced tyrosine phosphorylation of IRS-1, suggesting that phosphorylation is initially mediated by the AT1 receptor. We conclude that AII stimulates tyrosine phosphorylation of IRS-1 and its association with PI 3-kinase. This pathway thus represents an additional signalling mechanism stimulated by AII in the rat heart in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backer J. M., Myers M. G., Jr, Shoelson S. E., Chin D. J., Sun X. J., Miralpeix M., Hu P., Margolis B., Skolnik E. Y., Schlessinger J. Phosphatidylinositol 3'-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 1992 Sep;11(9):3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K. M., Aceto J. F. Angiotensin II stimulation of protein synthesis and cell growth in chick heart cells. Am J Physiol. 1990 Aug;259(2 Pt 2):H610–H618. doi: 10.1152/ajpheart.1990.259.2.H610. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cheatham B., Vlahos C. J., Cheatham L., Wang L., Blenis J., Kahn C. R. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol Cell Biol. 1994 Jul;14(7):4902–4911. doi: 10.1128/mcb.14.7.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folli F., Saad M. J., Backer J. M., Kahn C. R. Insulin stimulation of phosphatidylinositol 3-kinase activity and association with insulin receptor substrate 1 in liver and muscle of the intact rat. J Biol Chem. 1992 Nov 5;267(31):22171–22177. [PubMed] [Google Scholar]
- Force T., Kyriakis J. M., Avruch J., Bonventre J. V. Endothelin, vasopressin, and angiotensin II enhance tyrosine phosphorylation by protein kinase C-dependent and -independent pathways in glomerular mesangial cells. J Biol Chem. 1991 Apr 5;266(10):6650–6656. [PubMed] [Google Scholar]
- Giorgetti S., Ballotti R., Kowalski-Chauvel A., Tartare S., Van Obberghen E. The insulin and insulin-like growth factor-I receptor substrate IRS-1 associates with and activates phosphatidylinositol 3-kinase in vitro. J Biol Chem. 1993 Apr 5;268(10):7358–7364. [PubMed] [Google Scholar]
- Hadari Y. R., Tzahar E., Nadiv O., Rothenberg P., Roberts C. T., Jr, LeRoith D., Yarden Y., Zick Y. Insulin and insulinomimetic agents induce activation of phosphatidylinositol 3'-kinase upon its association with pp185 (IRS-1) in intact rat livers. J Biol Chem. 1992 Sep 5;267(25):17483–17486. [PubMed] [Google Scholar]
- Huckle W. R., Prokop C. A., Dy R. C., Herman B., Earp S. Angiotensin II stimulates protein-tyrosine phosphorylation in a calcium-dependent manner. Mol Cell Biol. 1990 Dec;10(12):6290–6298. doi: 10.1128/mcb.10.12.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Kanai F., Ito K., Todaka M., Hayashi H., Kamohara S., Ishii K., Okada T., Hazeki O., Ui M., Ebina Y. Insulin-stimulated GLUT4 translocation is relevant to the phosphorylation of IRS-1 and the activity of PI3-kinase. Biochem Biophys Res Commun. 1993 Sep 15;195(2):762–768. doi: 10.1006/bbrc.1993.2111. [DOI] [PubMed] [Google Scholar]
- Kawahara Y., Sunako M., Tsuda T., Fukuzaki H., Fukumoto Y., Takai Y. Angiotensin II induces expression of the c-fos gene through protein kinase C activation and calcium ion mobilization in cultured vascular smooth muscle cells. Biochem Biophys Res Commun. 1988 Jan 15;150(1):52–59. doi: 10.1016/0006-291x(88)90485-8. [DOI] [PubMed] [Google Scholar]
- Keegan A. D., Nelms K., White M., Wang L. M., Pierce J. H., Paul W. E. An IL-4 receptor region containing an insulin receptor motif is important for IL-4-mediated IRS-1 phosphorylation and cell growth. Cell. 1994 Mar 11;76(5):811–820. doi: 10.1016/0092-8674(94)90356-5. [DOI] [PubMed] [Google Scholar]
- Keller S. R., Kitagawa K., Aebersold R., Lienhard G. E., Garner C. W. Isolation and characterization of the 160,000-Da phosphotyrosyl protein, a putative participant in insulin signaling. J Biol Chem. 1991 Jul 15;266(20):12817–12820. [PubMed] [Google Scholar]
- Kelly K. L., Ruderman N. B. Insulin-stimulated phosphatidylinositol 3-kinase. Association with a 185-kDa tyrosine-phosphorylated protein (IRS-1) and localization in a low density membrane vesicle. J Biol Chem. 1993 Feb 25;268(6):4391–4398. [PubMed] [Google Scholar]
- Kromer E. P., Riegger G. A. Effects of long-term angiotensin converting enzyme inhibition on myocardial hypertrophy in experimental aortic stenosis in the rat. Am J Cardiol. 1988 Jul 1;62(1):161–163. doi: 10.1016/0002-9149(88)91389-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lavan B. E., Kuhné M. R., Garner C. W., Anderson D., Reedijk M., Pawson T., Lienhard G. E. The association of insulin-elicited phosphotyrosine proteins with src homology 2 domains. J Biol Chem. 1992 Jun 5;267(16):11631–11636. [PubMed] [Google Scholar]
- MORGAN H. E., HENDERSON M. J., REGEN D. M., PARK C. R. Regulation of glucose uptake in muscle. I. The effects of insulin and anoxia on glucose transport and phosphorylation in the isolated, perfused heart of normal rats. J Biol Chem. 1961 Feb;236:253–261. [PubMed] [Google Scholar]
- Madoff D. H., Martensen T. M., Lane M. D. Insulin and insulin-like growth factor 1 stimulate the phosphorylation on tyrosine of a 160 kDa cytosolic protein in 3T3-L1 adipocytes. Biochem J. 1988 May 15;252(1):7–15. doi: 10.1042/bj2520007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy C. J., Taylor D. S., Weber H. Angiotensin II stimulation of rapid protein tyrosine phosphorylation and protein kinase activation in rat aortic smooth muscle cells. J Biol Chem. 1993 Apr 5;268(10):7338–7345. [PubMed] [Google Scholar]
- Morris A. D., Petrie J. R., Ueda S., Connell J. M., Elliott H. L., Small M., Donnelly R. Pressor and subpressor doses of angiotensin II increase insulin sensitivity in NIDDM. Dissociation of metabolic and blood pressure effects. Diabetes. 1994 Dec;43(12):1445–1449. doi: 10.2337/diab.43.12.1445. [DOI] [PubMed] [Google Scholar]
- Murphy T. J., Alexander R. W., Griendling K. K., Runge M. S., Bernstein K. E. Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature. 1991 May 16;351(6323):233–236. doi: 10.1038/351233a0. [DOI] [PubMed] [Google Scholar]
- Myers M. G., Jr, Sun X. J., Cheatham B., Jachna B. R., Glasheen E. M., Backer J. M., White M. F. IRS-1 is a common element in insulin and insulin-like growth factor-I signaling to the phosphatidylinositol 3'-kinase. Endocrinology. 1993 Apr;132(4):1421–1430. doi: 10.1210/endo.132.4.8384986. [DOI] [PubMed] [Google Scholar]
- Naftilan A. J., Gilliland G. K., Eldridge C. S., Kraft A. S. Induction of the proto-oncogene c-jun by angiotensin II. Mol Cell Biol. 1990 Oct;10(10):5536–5540. doi: 10.1128/mcb.10.10.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftilan A. J., Pratt R. E., Dzau V. J. Induction of platelet-derived growth factor A-chain and c-myc gene expressions by angiotensin II in cultured rat vascular smooth muscle cells. J Clin Invest. 1989 Apr;83(4):1419–1424. doi: 10.1172/JCI114032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely J. R., Rovetto M. J. Techniques for perfusing isolated rat hearts. Methods Enzymol. 1975;39:43–60. doi: 10.1016/s0076-6879(75)39008-3. [DOI] [PubMed] [Google Scholar]
- Peach M. J., Dostal D. E. The angiotensin II receptor and the actions of angiotensin II. J Cardiovasc Pharmacol. 1990;16 (Suppl 4):S25–S30. doi: 10.1097/00005344-199016004-00007. [DOI] [PubMed] [Google Scholar]
- Quon M. J., Butte A. J., Zarnowski M. J., Sesti G., Cushman S. W., Taylor S. I. Insulin receptor substrate 1 mediates the stimulatory effect of insulin on GLUT4 translocation in transfected rat adipose cells. J Biol Chem. 1994 Nov 11;269(45):27920–27924. [PubMed] [Google Scholar]
- Rose D. W., Saltiel A. R., Majumdar M., Decker S. J., Olefsky J. M. Insulin receptor substrate 1 is required for insulin-mediated mitogenic signal transduction. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):797–801. doi: 10.1073/pnas.91.2.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg P. L., Lane W. S., Karasik A., Backer J., White M., Kahn C. R. Purification and partial sequence analysis of pp185, the major cellular substrate of the insulin receptor tyrosine kinase. J Biol Chem. 1991 May 5;266(13):8302–8311. [PubMed] [Google Scholar]
- Saad M. J., Araki E., Miralpeix M., Rothenberg P. L., White M. F., Kahn C. R. Regulation of insulin receptor substrate-1 in liver and muscle of animal models of insulin resistance. J Clin Invest. 1992 Nov;90(5):1839–1849. doi: 10.1172/JCI116060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad M. J., Folli F., Kahn J. A., Kahn C. R. Modulation of insulin receptor, insulin receptor substrate-1, and phosphatidylinositol 3-kinase in liver and muscle of dexamethasone-treated rats. J Clin Invest. 1993 Oct;92(4):2065–2072. doi: 10.1172/JCI116803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorb W., Peeler T. C., Madigan N. N., Conrad K. M., Baker K. M. Angiotensin II-induced protein tyrosine phosphorylation in neonatal rat cardiac fibroblasts. J Biol Chem. 1994 Jul 29;269(30):19626–19632. [PubMed] [Google Scholar]
- Souza S. C., Frick G. P., Yip R., Lobo R. B., Tai L. R., Goodman H. M. Growth hormone stimulates tyrosine phosphorylation of insulin receptor substrate-1. J Biol Chem. 1994 Dec 2;269(48):30085–30088. [PubMed] [Google Scholar]
- Sun X. J., Rothenberg P., Kahn C. R., Backer J. M., Araki E., Wilden P. A., Cahill D. A., Goldstein B. J., White M. F. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991 Jul 4;352(6330):73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- Taubman M. B., Berk B. C., Izumo S., Tsuda T., Alexander R. W., Nadal-Ginard B. Angiotensin II induces c-fos mRNA in aortic smooth muscle. Role of Ca2+ mobilization and protein kinase C activation. J Biol Chem. 1989 Jan 5;264(1):526–530. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend R. R., DiPette D. J. Pressor doses of angiotensin II increase insulin-mediated glucose uptake in normotensive men. Am J Physiol. 1993 Sep;265(3 Pt 1):E362–E366. doi: 10.1152/ajpendo.1993.265.3.E362. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Riedel H., Yarden Y., Coussens L., Gray A., Dull T., Schlessinger J., Waterfield M. D., Parker P. J. Protein kinases in cellular signal transduction: tyrosine kinase growth factor receptors and protein kinase C. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 2):713–724. doi: 10.1101/sqb.1986.051.01.084. [DOI] [PubMed] [Google Scholar]
- Wang L. M., Keegan A. D., Li W., Lienhard G. E., Pacini S., Gutkind J. S., Myers M. G., Jr, Sun X. J., White M. F., Aaronson S. A. Common elements in interleukin 4 and insulin signaling pathways in factor-dependent hematopoietic cells. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4032–4036. doi: 10.1073/pnas.90.9.4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. M., Myers M. G., Jr, Sun X. J., Aaronson S. A., White M., Pierce J. H. IRS-1: essential for insulin- and IL-4-stimulated mitogenesis in hematopoietic cells. Science. 1993 Sep 17;261(5128):1591–1594. doi: 10.1126/science.8372354. [DOI] [PubMed] [Google Scholar]
- White M. F., Kahn C. R. The insulin signaling system. J Biol Chem. 1994 Jan 7;269(1):1–4. [PubMed] [Google Scholar]
- Williams L. T. Signal transduction by the platelet-derived growth factor receptor. Science. 1989 Mar 24;243(4898):1564–1570. doi: 10.1126/science.2538922. [DOI] [PubMed] [Google Scholar]
- Wong P. C., Price W. A., Jr, Chiu A. T., Carini D. J., Duncia J. V., Johnson A. L., Wexler R. R., Timmermans P. B. Nonpeptide angiotensin II receptor antagonists. Studies with EXP9270 and DuP 753. Hypertension. 1990 Jun;15(6 Pt 2):823–834. doi: 10.1161/01.hyp.15.6.823. [DOI] [PubMed] [Google Scholar]
- Yonezawa K., Ueda H., Hara K., Nishida K., Ando A., Chavanieu A., Matsuba H., Shii K., Yokono K., Fukui Y. Insulin-dependent formation of a complex containing an 85-kDa subunit of phosphatidylinositol 3-kinase and tyrosine-phosphorylated insulin receptor substrate 1. J Biol Chem. 1992 Dec 25;267(36):25958–25965. [PubMed] [Google Scholar]