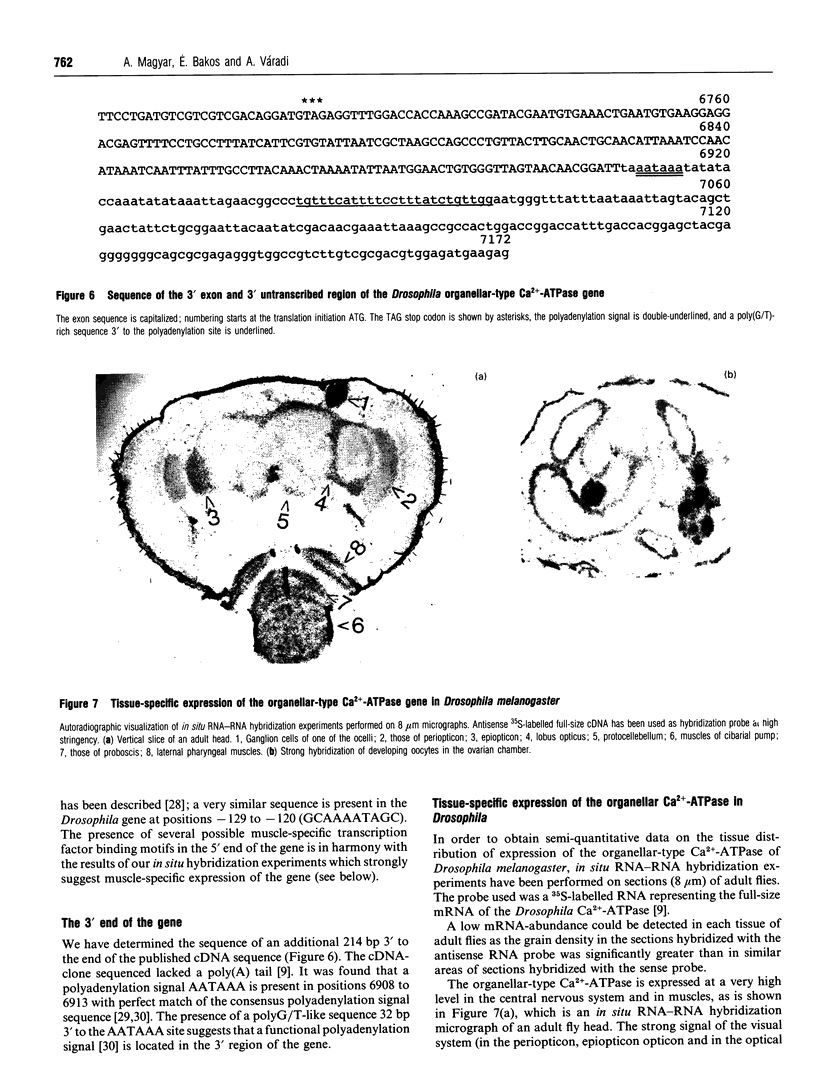
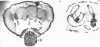Abstract
A 14 kb genomic clone covering the organellar-type Ca(2+)-ATPase gene of Drosophila melanogaster has been isolated and characterized. The sequence of a 7132 bp region extending from 1.1 kb 5' upstream of the initiation ATG codon over the polyadenylation signal at the 3' end has been determined. The gene consists of nine exons including one with an exceptional size of 2172 bp representing 72% of the protein coding region. Introns are relatively small (< 100 bp) except for the 3' intron which has a size of 2239 bp, an exceptionally large size among Drosophila introns. Five of the introns are in the same positions in Drosophila, Artemia and rabbit SERCA1 Ca(2+)-ATPase genes. There is only one organellar-type Ca(2+)-ATPase gene in the Drosophila genome, as was shown by Southern-blot analysis [Váradi, Gilmore-Hebert and Benz (1989) FEBS Lett. 258, 203-207] and by chromosomal localization [Magyar and Váradi (1990) Biochem. Biophys. Res. Commun. 173, 872-877]. Primer extension and S1-nuclease assays revealed a potential transcription initiation site 876 bp upstream of the translation initiation ATG with a TATA-box 23 bp upstream of this site. Analysis of the 5' region of the Drosophila organellar-type Ca(2+)-ATPase gene suggests the presence of potential recognition sequences of various muscle-specific transcription factors and shows a region with remarkable similarity to that in the rabbit SERCA2 gene. The tissue distribution of expression of the organellar-type Ca(2+)-ATPase gene has been studied by in situ RNA-RNA hybridization on microscopic sections. A low mRNA abundance can be detected in each tissue of adult flies, suggesting a housekeeping function for the gene. On the other hand a pronounced tissue specificity of expression has also been found as the organellar-type Ca(2+)-ATPase is expressed at a very high level in cell bodies of the central nervous system and in various muscles.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bingham P. M., Chou T. B., Mims I., Zachar Z. On/off regulation of gene expression at the level of splicing. Trends Genet. 1988 May;4(5):134–138. doi: 10.1016/0168-9525(88)90136-9. [DOI] [PubMed] [Google Scholar]
- Boorstein W. R., Craig E. A. Primer extension analysis of RNA. Methods Enzymol. 1989;180:347–369. doi: 10.1016/0076-6879(89)80111-9. [DOI] [PubMed] [Google Scholar]
- Brandl C. J., Green N. M., Korczak B., MacLennan D. H. Two Ca2+ ATPase genes: homologies and mechanistic implications of deduced amino acid sequences. Cell. 1986 Feb 28;44(4):597–607. doi: 10.1016/0092-8674(86)90269-2. [DOI] [PubMed] [Google Scholar]
- Brandl C. J., deLeon S., Martin D. R., MacLennan D. H. Adult forms of the Ca2+ATPase of sarcoplasmic reticulum. Expression in developing skeletal muscle. J Biol Chem. 1987 Mar 15;262(8):3768–3774. [PubMed] [Google Scholar]
- Campbell A. M., Kessler P. D., Sagara Y., Inesi G., Fambrough D. M. Nucleotide sequences of avian cardiac and brain SR/ER Ca(2+)-ATPases and functional comparisons with fast twitch Ca(2+)-ATPase. Calcium affinities and inhibitor effects. J Biol Chem. 1991 Aug 25;266(24):16050–16055. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cruzen M. E., Arfin S. M. Nucleotide and deduced amino acid sequence of human threonyl-tRNA synthetase reveals extensive homology to the Escherichia coli and yeast enzymes. J Biol Chem. 1991 May 25;266(15):9919–9923. [PubMed] [Google Scholar]
- Eggermont J. A., Wuytack F., Casteels R. Characterization of the mRNAs encoding the gene 2 sarcoplasmic/endoplasmic-reticulum Ca2+ pump in pig smooth muscle. Biochem J. 1990 Mar 15;266(3):901–907. [PMC free article] [PubMed] [Google Scholar]
- Escalante R., Sastre L. Similar alternative splicing events generate two sarcoplasmic or endoplasmic reticulum Ca-ATPase isoforms in the crustacean Artemia franciscana and in vertebrates. J Biol Chem. 1993 Jul 5;268(19):14090–14095. [PubMed] [Google Scholar]
- Escalante R., Sastre L. Structure of Artemia franciscana sarco/endoplasmic reticulum Ca-ATPase gene. J Biol Chem. 1994 Apr 29;269(17):13005–13012. [PubMed] [Google Scholar]
- Garcia-Bellido A., Moscoso del Pradio J. Genetic analysis of maternal information in Drosophila. Nature. 1979 Mar 22;278(5702):346–348. doi: 10.1038/278346a0. [DOI] [PubMed] [Google Scholar]
- Gunteski-Hamblin A. M., Greeb J., Shull G. E. A novel Ca2+ pump expressed in brain, kidney, and stomach is encoded by an alternative transcript of the slow-twitch muscle sarcoplasmic reticulum Ca-ATPase gene. Identification of cDNAs encoding Ca2+ and other cation-transporting ATPases using an oligonucleotide probe derived from the ATP-binding site. J Biol Chem. 1988 Oct 15;263(29):15032–15040. [PubMed] [Google Scholar]
- Hultman T., Bergh S., Moks T., Uhlén M. Bidirectional solid-phase sequencing of in vitro-amplified plasmid DNA. Biotechniques. 1991 Jan;10(1):84–93. [PubMed] [Google Scholar]
- Korczak B., Zarain-Herzberg A., Brandl C. J., Ingles C. J., Green N. M., MacLennan D. H. Structure of the rabbit fast-twitch skeletal muscle Ca2+-ATPase gene. J Biol Chem. 1988 Apr 5;263(10):4813–4819. [PubMed] [Google Scholar]
- Lytton J., MacLennan D. H. Molecular cloning of cDNAs from human kidney coding for two alternatively spliced products of the cardiac Ca2+-ATPase gene. J Biol Chem. 1988 Oct 15;263(29):15024–15031. [PubMed] [Google Scholar]
- Lytton J., MacLennan D. H. Molecular cloning of cDNAs from human kidney coding for two alternatively spliced products of the cardiac Ca2+-ATPase gene. J Biol Chem. 1988 Oct 15;263(29):15024–15031. [PubMed] [Google Scholar]
- MacLennan D. H., Brandl C. J., Korczak B., Green N. M. Amino-acid sequence of a Ca2+ + Mg2+-dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature. 1985 Aug 22;316(6030):696–700. doi: 10.1038/316696a0. [DOI] [PubMed] [Google Scholar]
- Magyar A., Váradi A. Molecular cloning and chromosomal localization of a sarco/endoplasmic reticulum-type Ca2(+)-ATPase of Drosophila melanogaster. Biochem Biophys Res Commun. 1990 Dec 31;173(3):872–877. doi: 10.1016/s0006-291x(05)80867-8. [DOI] [PubMed] [Google Scholar]
- Mar J. H., Ordahl C. P. M-CAT binding factor, a novel trans-acting factor governing muscle-specific transcription. Mol Cell Biol. 1990 Aug;10(8):4271–4283. doi: 10.1128/mcb.10.8.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty A., Kedes L. Upstream regions of the human cardiac actin gene that modulate its transcription in muscle cells: presence of an evolutionarily conserved repeated motif. Mol Cell Biol. 1986 Jun;6(6):2125–2136. doi: 10.1128/mcb.6.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M., Burks C., Hertz G., Stormo G. D., White O., Fields C. Splicing signals in Drosophila: intron size, information content, and consensus sequences. Nucleic Acids Res. 1992 Aug 25;20(16):4255–4262. doi: 10.1093/nar/20.16.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K., Tanabe K., Takada S. Structure of a Plasmodium yoelii gene-encoded protein homologous to the Ca(2+)-ATPase of rabbit skeletal muscle sarcoplasmic reticulum. J Cell Sci. 1990 Nov;97(Pt 3):487–495. doi: 10.1242/jcs.97.3.487. [DOI] [PubMed] [Google Scholar]
- Palmero I., Sastre L. Complementary DNA cloning of a protein highly homologous to mammalian sarcoplasmic reticulum Ca-ATPase from the crustacean Artemia. J Mol Biol. 1989 Dec 20;210(4):737–748. doi: 10.1016/0022-2836(89)90106-x. [DOI] [PubMed] [Google Scholar]
- Piette J., Bessereau J. L., Huchet M., Changeux J. P. Two adjacent MyoD1-binding sites regulate expression of the acetylcholine receptor alpha-subunit gene. Nature. 1990 May 24;345(6273):353–355. doi: 10.1038/345353a0. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Váradi A., Gilmore-Heber M., Benz E. J., Jr Amplification of the phosphorylation site-ATP-binding site cDNA fragment of the Na+,K(+)-ATPase and the Ca2(+)-ATPase of Drosophila melanogaster by polymerase chain reaction. FEBS Lett. 1989 Dec 4;258(2):203–207. doi: 10.1016/0014-5793(89)81653-9. [DOI] [PubMed] [Google Scholar]
- Wahle E., Keller W. The biochemistry of 3'-end cleavage and polyadenylation of messenger RNA precursors. Annu Rev Biochem. 1992;61:419–440. doi: 10.1146/annurev.bi.61.070192.002223. [DOI] [PubMed] [Google Scholar]
- Wimmers L. E., Ewing N. N., Bennett A. B. Higher plant Ca(2+)-ATPase: primary structure and regulation of mRNA abundance by salt. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9205–9209. doi: 10.1073/pnas.89.19.9205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarain-Herzberg A., MacLennan D. H., Periasamy M. Characterization of rabbit cardiac sarco(endo)plasmic reticulum Ca2(+)-ATPase gene. J Biol Chem. 1990 Mar 15;265(8):4670–4677. [PubMed] [Google Scholar]