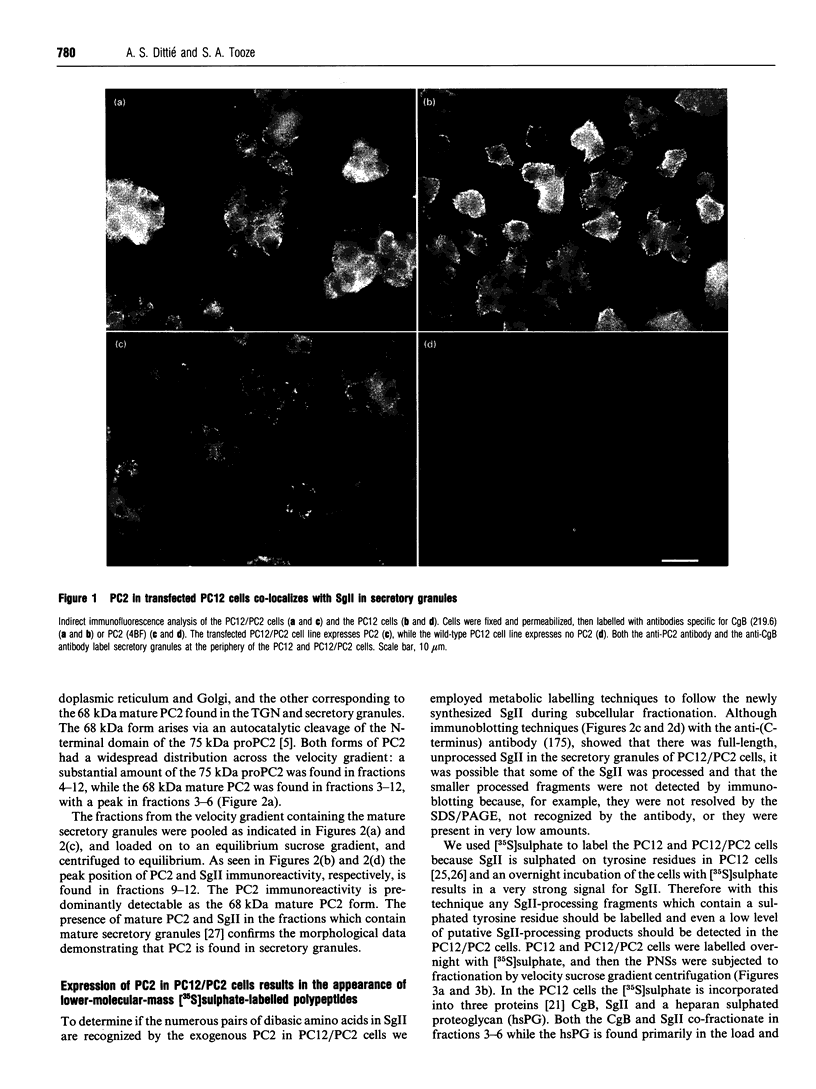
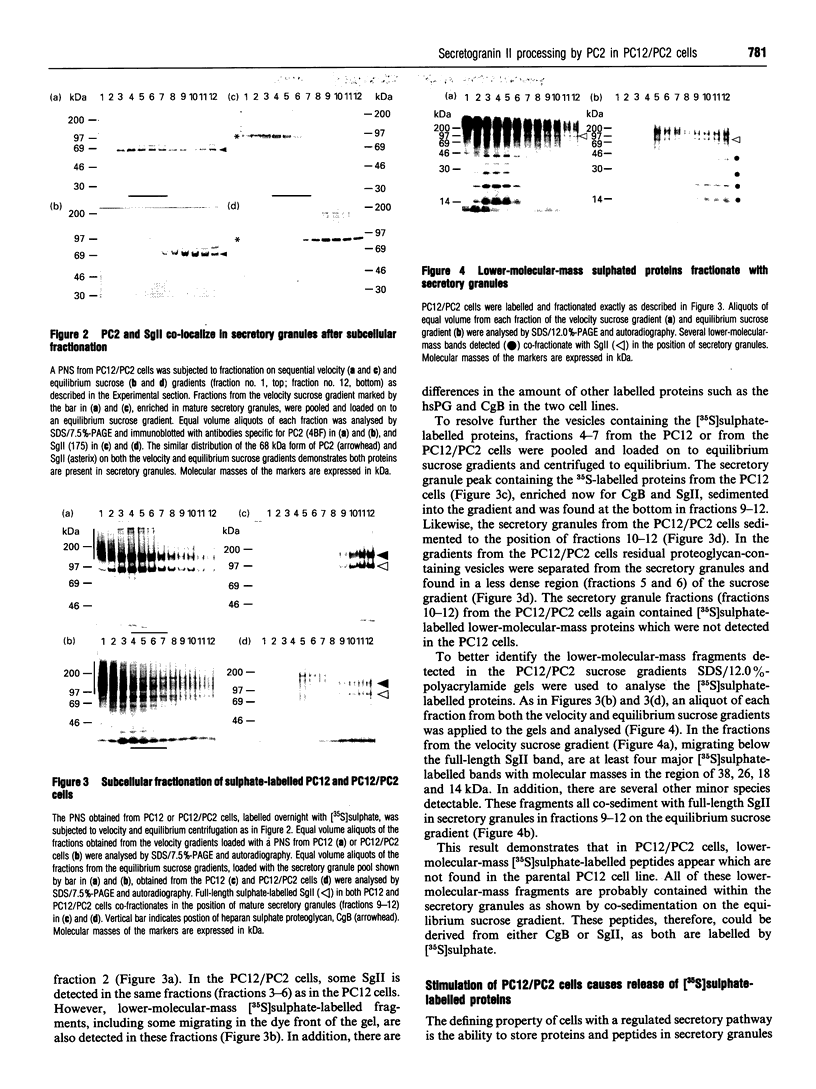
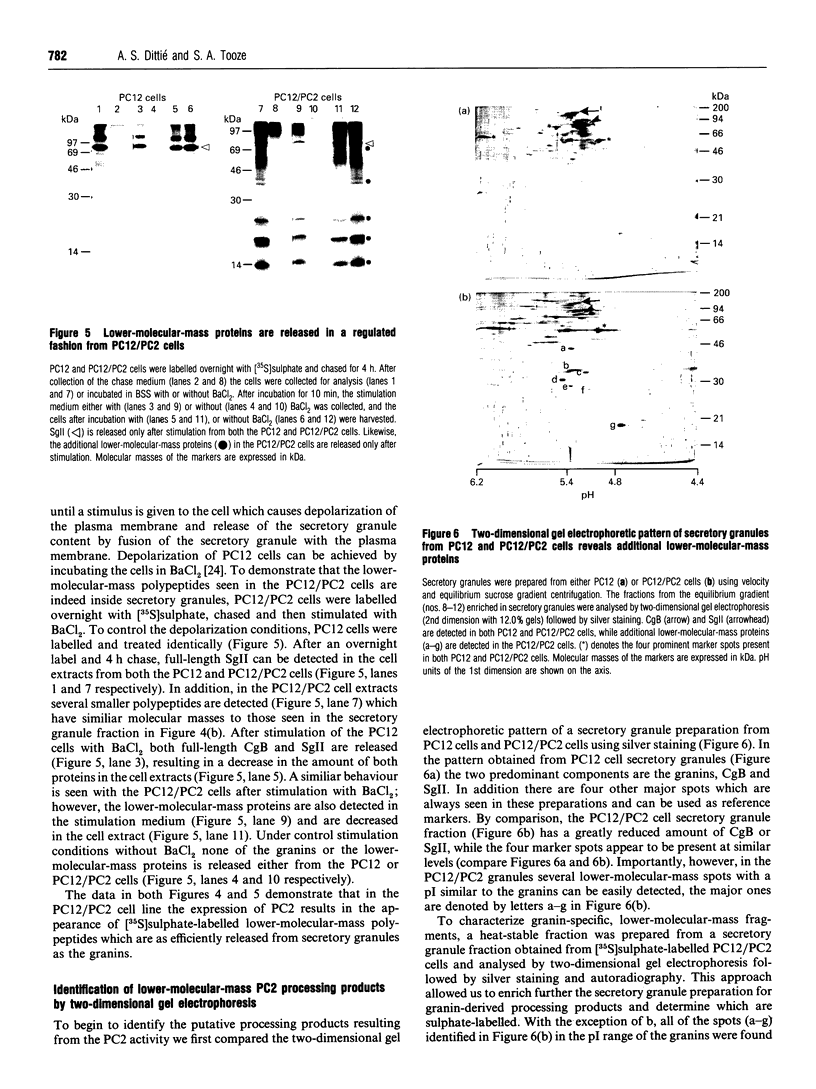
Abstract
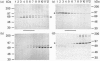
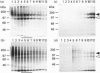
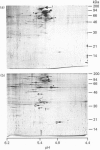
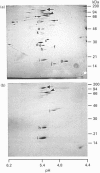
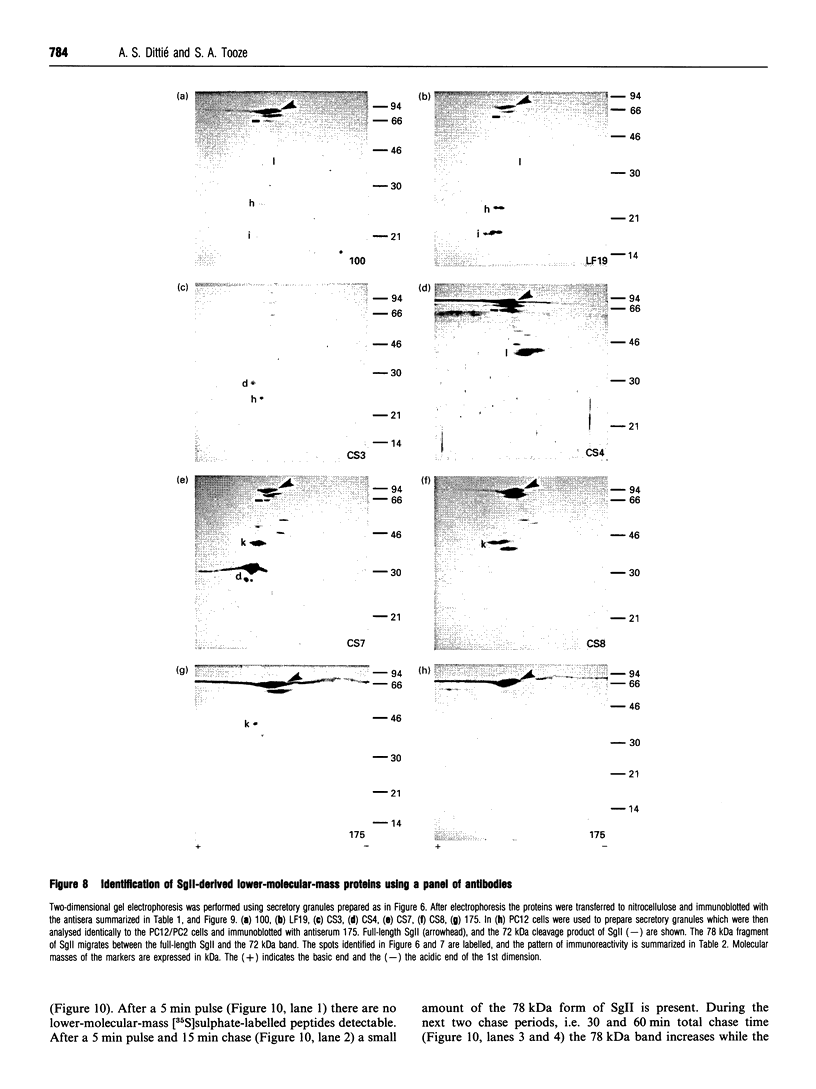
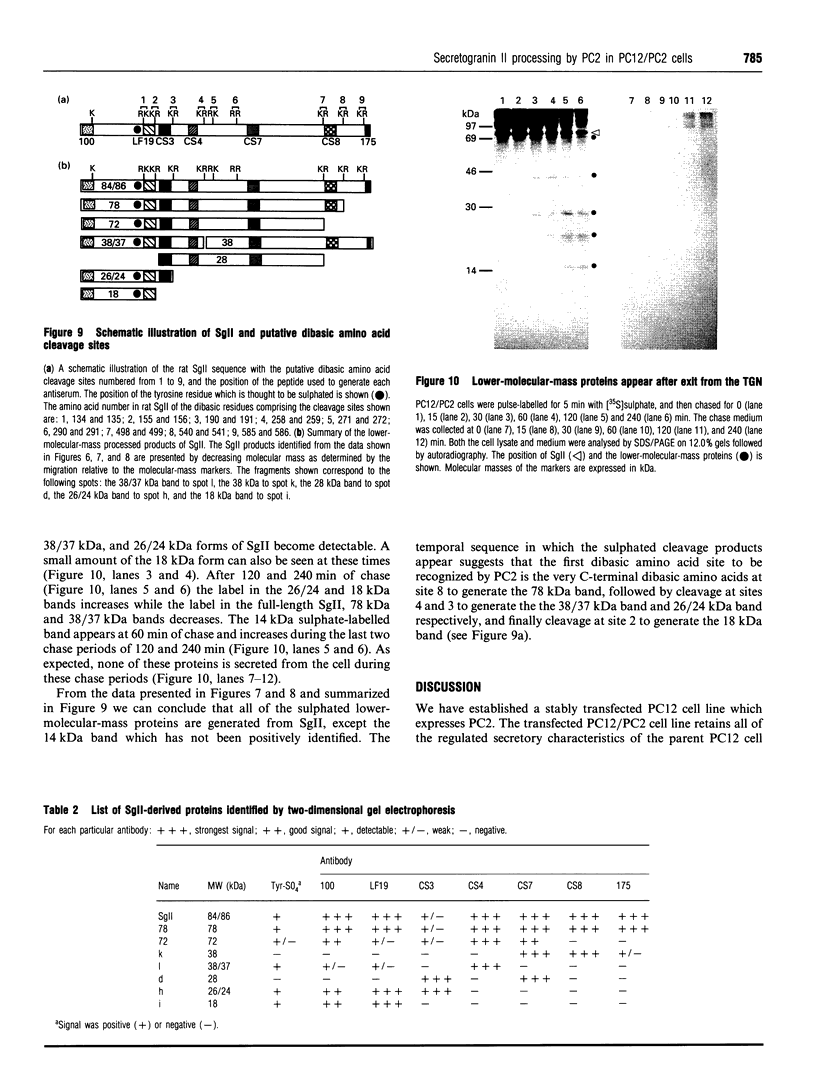
To study the processing of secretogranin II (SgII) by the prohormone convertase PC2 we have generated a stable PC12 cell line which expresses mouse PC2. We here present the characteristics of the PC12/PC2 cell line and demonstrate that the exogenous PC2 is sorted and stored in secretory granules in the PC12/PC2 cell line as efficiently as the endogenous granins. By indirect immunofluorescence with antibodies specific for chromogranin B (CgB) and PC2 we were able to establish that the PC2 is stored in secretory granules in the PC12/PC2 cell line. After subcellular fractionation, followed by immunoblotting, the mature 68 kDa form of PC2 was found co-sedimented with SgII in fractions containing secretory granules. Two-dimensional gel electrophoresis was used to characterize a secretory granule fraction obtained from the PC12/PC2 cells, and a comparison was done of the electrophoretic pattern obtained from the PC12/PC2 cells with the parent cell line PC12. The products derived from the processing of SgII by PC2 were identified by immunoblotting with a panel of antibodies directed against SgII. Using [35S]sulphate to label the newly synthesized SgII, we performed a time course to monitor the appearance of the lower-molecular-mass fragments of SgII: beginning 15 min after a 5 min pulse of [35S]sulphate we were able to detect the first proteolytic fragment of SgII. Our results demonstrate that SgII is proteolytically processed by PC2 in the immature secretory granule into several lower-molecular-mass proteins, the major ones being an 18 kDa sulphated fragment and a 28 kDa fragment.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benjannet S., Reudelhuber T., Mercure C., Rondeau N., Chrétien M., Seidah N. G. Proprotein conversion is determined by a multiplicity of factors including convertase processing, substrate specificity, and intracellular environment. Cell type-specific processing of human prorenin by the convertase PC1. J Biol Chem. 1992 Jun 5;267(16):11417–11423. [PubMed] [Google Scholar]
- Benjannet S., Rondeau N., Paquet L., Boudreault A., Lazure C., Chrétien M., Seidah N. G. Comparative biosynthesis, covalent post-translational modifications and efficiency of prosegment cleavage of the prohormone convertases PC1 and PC2: glycosylation, sulphation and identification of the intracellular site of prosegment cleavage of PC1 and PC2. Biochem J. 1993 Sep 15;294(Pt 3):735–743. doi: 10.1042/bj2940735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D. L., Bailyes E. M., Nielsen E., Guest P. C., Rutherford N. G., Arden S. D., Hutton J. C. Identification of the type 2 proinsulin processing endopeptidase as PC2, a member of the eukaryote subtilisin family. J Biol Chem. 1992 Jul 25;267(21):15229–15236. [PubMed] [Google Scholar]
- Braks J. A., Martens G. J. 7B2 is a neuroendocrine chaperone that transiently interacts with prohormone convertase PC2 in the secretory pathway. Cell. 1994 Jul 29;78(2):263–273. doi: 10.1016/0092-8674(94)90296-8. [DOI] [PubMed] [Google Scholar]
- Chanat E., Huttner W. B. Milieu-induced, selective aggregation of regulated secretory proteins in the trans-Golgi network. J Cell Biol. 1991 Dec;115(6):1505–1519. doi: 10.1083/jcb.115.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool D. R., Loh Y. P. Identification of a sorting signal for the regulated secretory pathway at the N-terminus of pro-opiomelanocortin. Biochimie. 1994;76(3-4):265–270. doi: 10.1016/0300-9084(94)90156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson H. W., Rhodes C. J., Hutton J. C. Intraorganellar calcium and pH control proinsulin cleavage in the pancreatic beta cell via two distinct site-specific endopeptidases. Nature. 1988 May 5;333(6168):93–96. doi: 10.1038/333093a0. [DOI] [PubMed] [Google Scholar]
- Gerdes H. H., Rosa P., Phillips E., Baeuerle P. A., Frank R., Argos P., Huttner W. B. The primary structure of human secretogranin II, a widespread tyrosine-sulfated secretory granule protein that exhibits low pH- and calcium-induced aggregation. J Biol Chem. 1989 Jul 15;264(20):12009–12015. [PubMed] [Google Scholar]
- Huttner W. B., Gerdes H. H., Rosa P. The granin (chromogranin/secretogranin) family. Trends Biochem Sci. 1991 Jan;16(1):27–30. doi: 10.1016/0968-0004(91)90012-k. [DOI] [PubMed] [Google Scholar]
- Kirchmair R., Hogue-Angeletti R., Gutierrez J., Fischer-Colbrie R., Winkler H. Secretoneurin--a neuropeptide generated in brain, adrenal medulla and other endocrine tissues by proteolytic processing of secretogranin II (chromogranin C). Neuroscience. 1993 Mar;53(2):359–365. doi: 10.1016/0306-4522(93)90200-y. [DOI] [PubMed] [Google Scholar]
- Lazure C., Benjannet S., Seidah N. G., Chrétien M. Processed forms of neuroendocrine proteins 7B2 and secretogranin II are found in porcine pituitary extracts. Int J Pept Protein Res. 1991 Oct;38(4):392–400. doi: 10.1111/j.1399-3011.1991.tb01519.x. [DOI] [PubMed] [Google Scholar]
- Lee R. W., Huttner W. B. Tyrosine-O-sulfated proteins of PC12 pheochromocytoma cells and their sulfation by a tyrosylprotein sulfotransferase. J Biol Chem. 1983 Sep 25;258(18):11326–11334. [PubMed] [Google Scholar]
- Matthews G., Shennan K. I., Seal A. J., Taylor N. A., Colman A., Docherty K. Autocatalytic maturation of the prohormone convertase PC2. J Biol Chem. 1994 Jan 7;269(1):588–592. [PubMed] [Google Scholar]
- Natori S., Huttner W. B. Peptides derived from the granins (chromogranins/secretogranins). Biochimie. 1994;76(3-4):277–282. doi: 10.1016/0300-9084(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Amherdt M., Madsen O., Vassalli J. D., Perrelet A. Direct identification of prohormone conversion site in insulin-secreting cells. Cell. 1985 Sep;42(2):671–681. doi: 10.1016/0092-8674(85)90124-2. [DOI] [PubMed] [Google Scholar]
- Rosa P., Hille A., Lee R. W., Zanini A., De Camilli P., Huttner W. B. Secretogranins I and II: two tyrosine-sulfated secretory proteins common to a variety of cells secreting peptides by the regulated pathway. J Cell Biol. 1985 Nov;101(5 Pt 1):1999–2011. doi: 10.1083/jcb.101.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saria A., Troger J., Kirchmair R., Fischer-Colbrie R., Hogue-Angeletti R., Winkler H. Secretoneurin releases dopamine from rat striatal slices: a biological effect of a peptide derived from secretogranin II (chromogranin C). Neuroscience. 1993 May;54(1):1–4. doi: 10.1016/0306-4522(93)90377-r. [DOI] [PubMed] [Google Scholar]
- Schimmel A., Bräunling O., Rüther U., Huttner W. B., Gerdes H. H. The organisation of the mouse secretogranin II gene. FEBS Lett. 1992 Dec 21;314(3):375–380. doi: 10.1016/0014-5793(92)81509-k. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Chrétien M., Day R. The family of subtilisin/kexin like pro-protein and pro-hormone convertases: divergent or shared functions. Biochimie. 1994;76(3-4):197–209. doi: 10.1016/0300-9084(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Hendy G. N., Hamelin J., Paquin J., Lazure C., Metters K. M., Rossier J., Chrétien M. Chromogranin A can act as a reversible processing enzyme inhibitor. Evidence from the inhibition of the IRCM-serine protease 1 cleavage of pro-enkephalin and ACTH at pairs of basic amino acids. FEBS Lett. 1987 Jan 26;211(2):144–150. doi: 10.1016/0014-5793(87)81425-4. [DOI] [PubMed] [Google Scholar]
- Shen F. S., Seidah N. G., Lindberg I. Biosynthesis of the prohormone convertase PC2 in Chinese hamster ovary cells and in rat insulinoma cells. J Biol Chem. 1993 Nov 25;268(33):24910–24915. [PubMed] [Google Scholar]
- Shennan K. I., Smeekens S. P., Steiner D. F., Docherty K. Characterization of PC2, a mammalian Kex2 homologue, following expression of the cDNA in microinjected Xenopus oocytes. FEBS Lett. 1991 Jun 24;284(2):277–280. doi: 10.1016/0014-5793(91)80703-6. [DOI] [PubMed] [Google Scholar]
- Shennan K. I., Taylor N. A., Jermany J. L., Matthews G., Docherty K. Differences in pH optima and calcium requirements for maturation of the prohormone convertases PC2 and PC3 indicates different intracellular locations for these events. J Biol Chem. 1995 Jan 20;270(3):1402–1407. doi: 10.1074/jbc.270.3.1402. [DOI] [PubMed] [Google Scholar]
- Soszynski D., Metz-Boutigue M. H., Aunis D., Bader M. F. Secretogranin II: regulation of synthesis and post-translational proteolysis in bovine adrenal chromaffin cells. J Neuroendocrinol. 1993 Dec;5(6):655–662. doi: 10.1111/j.1365-2826.1993.tb00536.x. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Smeekens S. P., Ohagi S., Chan S. J. The new enzymology of precursor processing endoproteases. J Biol Chem. 1992 Nov 25;267(33):23435–23438. [PubMed] [Google Scholar]
- Thorne B. A., Viveros O. H., Thomas G. Expression and processing of mouse proopiomelanocortin in bovine adrenal chromaffin cells. A model system to study tissue-specific prohormone processing. J Biol Chem. 1991 Jul 25;266(21):13607–13615. [PubMed] [Google Scholar]
- Tooze S. A., Hollinshead M., Dittié A. S. Antibodies to secretogranin II reveal potential processing sites. Biochimie. 1994;76(3-4):271–276. doi: 10.1016/0300-9084(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Tooze S. A., Huttner W. B. Cell-free formation of immature secretory granules and constitutive secretory vesicles from trans-Golgi network. Methods Enzymol. 1992;219:81–93. doi: 10.1016/0076-6879(92)19012-u. [DOI] [PubMed] [Google Scholar]
- Tooze S. A., Huttner W. B. Cell-free protein sorting to the regulated and constitutive secretory pathways. Cell. 1990 Mar 9;60(5):837–847. doi: 10.1016/0092-8674(90)90097-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry H., Conlon J. M. Identification of a peptide arising from the specific post-translation processing of secretogranin II. FEBS Lett. 1991 Jun 17;284(1):31–33. doi: 10.1016/0014-5793(91)80754-q. [DOI] [PubMed] [Google Scholar]
- Winkler H., Fischer-Colbrie R. The chromogranins A and B: the first 25 years and future perspectives. Neuroscience. 1992 Aug;49(3):497–528. doi: 10.1016/0306-4522(92)90222-N. [DOI] [PMC free article] [PubMed] [Google Scholar]