Abstract
Background
Vascular Ehlers‐Danlos syndrome has a high mortality rate due to hemorrhagic complications.
Case Presentation
We report a case of vascular‐type Ehlers‐Danlos syndrome diagnosed due to rupture of multiple celiac aneurysms. The patient was a 25‐year‐old Japanese man with a history of a sigmoid perforation. He was admitted to a nearby hospital because of abdominal pain. On day 9 of hospitalization, the patient experienced shock. Enhanced abdominal computed tomography revealed a hepatic aneurysm and intra‐abdominal bleeding, and the patient was transferred to our hospital. Emergency abdominal angiography revealed multiple aneurysms in the celiac, common, and right hepatic arteries. The right hepatic artery was considered responsible and was embolized. The patient had characteristic physical findings of the syndrome, aiding in confirming the genetic analysis of COL3A1 gene abnormality.
Conclusion
Juvenile‐onset colonic perforation and rupture of the celiac arteries are key findings in the suspicion of vascular‐type Ehlers‐Danlos syndrome.
Keywords: celiac artery aneurysm, connective tissue disorder, Ehlers‐Danlos syndrome, interventional radiology, trans‐arterial embolization
The patient was a young Japanese man with a history of sigmoid perforation. He went into a state of shock owing to acute intra‐abdominal hemorrhage caused by rupture of multiple aneurysms at the celiac, common hepatic, and right hepatic arteries. Genetic analysis revealed COL3A1 gene abnormality.
INTRODUCTION
Ehlers‐Danlos syndrome (EDS) is a rare genetic disorder resulting from defective formation of type 3 collagen, leading to impaired connective tissue synthesis and abnormalities of luminal organs and blood vessels. 1 Vascular EDS (previously known as Type IV) can be fatal due to rupture of aneurysms and perforation of the intestinal tract. 2 Here, we report a case of intra‐abdominal hemorrhage due to a ruptured aneurysm of a region of the celiac artery, which was diagnosed as vascular EDS because of a history of sigmoid colon perforation.
CASE
A 25‐year‐old Japanese man was admitted to a nearby hospital for acute abdominal pain of unknown cause. He experienced repeated relief and aggravation of symptoms after the analgesic administration. On day 9 of hospitalization, the patient went into hemorrhagic shock. Enhanced abdominal computed tomography (CT) revealed severe intraabdominal bleeding. The patient was transferred to an emergency medical center.
On admission, he was conscious; however, blood pressure was 100/72 mmHg; heart rate, 124 beats/min; and respiratory rate, 30 breaths/min, indicating shock. Tenderness was observed throughout the abdomen with guarding. The patient underwent a laparotomy for sigmoid colon perforation 12 years ago.
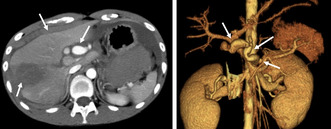
Contrast‐enhanced CT of the abdomen on admission to the ER (Figure 1) revealed hematogenous ascites on the liver surface, a swollen aneurysm of the celiac artery, and a wedge‐shaped, low‐density area in the lateral area of the liver. Intra‐abdominal hemorrhage was diagnosed because of rupture of a hepatic artery aneurysm, which required massive transfusion and emergency angioembolization within 1 h of the ER visit.
FIGURE 1.
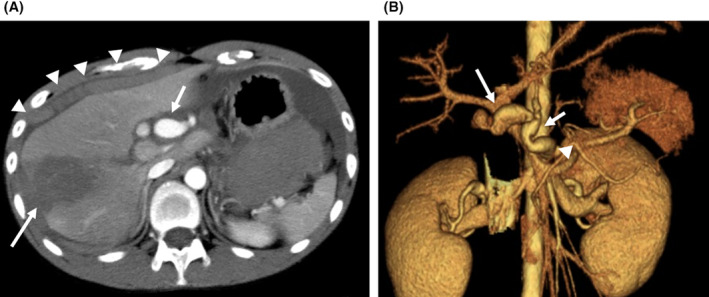
The finding of enhanced abdominal computed tomography (CT) on ER admission. (A) Enhanced abdominal CT showing swelling of the celiac artery trunk (short arrow) and a low‐density area (long arrow) in the lateral segment of the liver with blood ascites around the liver (arrowhead). (B) Abdominal CT angiography with 3D imaging demonstrating irregular aneurysms in the celiac artery (arrowhead), common hepatic artery (short arrow), and right hepatic artery (long arrow).
Figure 2 shows the angiographic findings. Stenosis was observed at the celiac trunk origin, and aneurysms were observed in the celiac, common hepatic, and right hepatic arteries. However, the periphery of the right hepatic artery could not be visualized. A right hepatic artery aneurysm was considered responsible for the vascular irregularities and aneurysmal dilatation, while no extravasation was detected. The portal vein was markedly stenotic, possibly caused by pressure from the hematoma. Coil packing was performed using 21 microcoils for the right hepatic artery aneurysm. The portal vein was narrowed; the right lobe of the liver had already been infarcted. We decided not to embolize the celiac or common hepatic artery aneurysm because further embolization might have aggravated the infarction. Angiography of other major abdominal arteries revealed bilateral renal artery aneurysms. The surgery was completed within 90 min. The total required transfusion was 18 units of concentrated red blood cells and 16 units of fresh frozen plasma.
FIGURE 2.
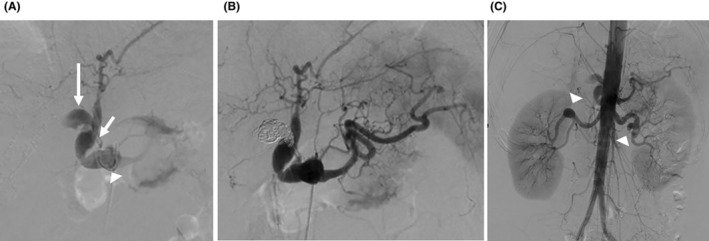
The findings and treatment of angiography. (A) Digitally subtracted image showing stenosis at the origin of the celiac trunk artery and aneurysms in the celiac artery (arrowhead), common hepatic artery (short arrow), and right hepatic artery (long arrow). However, the periphery of the right hepatic artery could not be visualized. (B) Postembolization of the right hepatic artery by multiple coils. (C) Digitally subtracted image showing multiple aneurysms (arrowheads) in both renal arteries.
The serum liver enzyme (ALT) of the patient rose up to about 1300 IU/L on day 3, but did peak out. Slight high serum CRP level (about 10 mg/dL) without fever and abdominal pain owing to remaining abdominal hematoma persisted by day 12 while serum leukocytes of the patient were normalized in day 5 (File S1). He became ambulant on day 20 and was discharged from the hospital on day 26 after rehabilitation. I followed him as an outpatient and advised him to avoid strenuous exercise.
He had the characteristic physical findings 2 of vascular EDS (Figure 3), including increased vascular permeability of the skin, a small jaw, keloids on the surgical scar from the past 12‐years, and joint hypermobility. Brain magnetic resonance angiography revealed a left internal carotid artery aneurysm.
FIGURE 3.
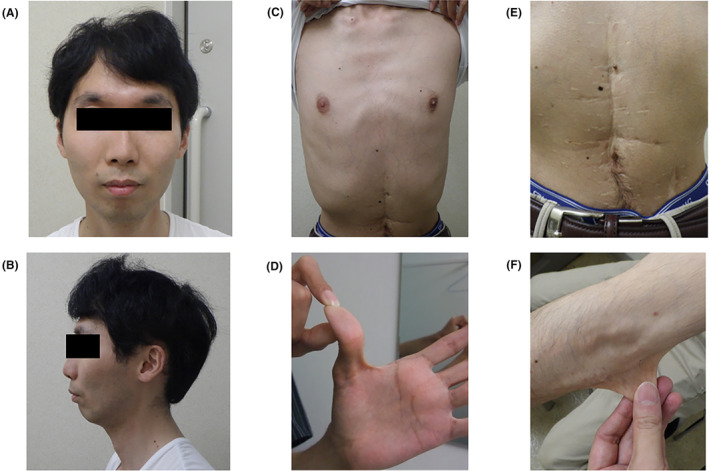
The physical characteristics of the case. ※Permission for exposure of body parts was obtained from the patient. (A, B) Front and side faces featuring thin lips, small chin, and large eyes (eyes are sealed to ensure anonymity). (C) Chest wall featuring thin translucent skin. (D) Thumb featuring hypermobility of finger joints. (E) Abdominal skin featuring keroid formation of surgical scar. (F) Elbow featuring hyperextensibility of skin.
On day 60, genetic analysis revealed a COL3A1 abnormality, which confirmed the diagnosis. The patient is currently being treated with celiprolol, and the aneurysms have remained stable under outpatient observation.
DISCUSSION
EDS is an inherited connective tissue disease with various systemic symptoms, characterized by fragile and hyperextensible skin, hypermobility of joints, aneurysms, and perforation of the gastrointestinal tract. 2 EDS is divided into several subtypes, with the vascular type having a prevalence of approximately 1/50,000 individuals or 3% of all EDS. The prognosis is extremely poor and most patients die in their 40s. 2
The patient discussed herein had multiple physical features indicative of EDS including increased skin permeability, excessive mobility of small joints, and characteristic facial features such as thin lips. 1 Vessel and bowel walls in vascular type EDS have reduced total collagen content and thin walls with irregular fibrils. 2 , 3 These abnormalities predispose patients to aneurysms and bowel perforation at a relatively young age. 2 This case was suspected to be vascular EDS due to intra‐abdominal hemorrhage caused by the rupture of the celiac artery at a young age, history of surgery for colon perforation, and archetypal physical characteristics. A definitive diagnosis was obtained by genetic analysis.
This patient had sigmoid colon perforation during his teenage years. Colon perforation is common in vascular EDS; one study reported that 50/51 (97%) emergency surgeries for peritonitis in patients with vascular EDS were for sigmoid colon perforation. 4 In our patient's case, the abdominal surgical wound from previous surgery had a well‐defined keloid scar, suggesting poor skin fusion. Bowel perforation is the cause of death in 3% of all patients with vascular EDS, most of whom die of the vascular lesion complications described below. 2
Herein, the abdominal arterial lesion was characteristic; most of the vascular lesions in the vascular EDS were in the branch arteries of the abdominal aorta rather than in the aortic lesions. In one report, 54 cases (80%) of arterial lesions were in the branches of the abdominal aorta 5 likely because peripheral branch arteries are more susceptible to injury from hypertension than the aorta and are comparatively rich in elastic fibers.
Aneurysmal lesions tend to develop in multiple locations after surgical or endovascular treatment, also known as vascular catastrophe. 6 Herein, the arterial lesions may have been aggravated by increased collagenase hyperactivity during surgery at a young age. During the follow‐up period, approximately 15% of patients with vascular EDS with intestinal complications had vascular lesions. 2 In a recent case report, a new aneurysm appeared in a branch artery of the celiac artery a few days after surgery for colon perforation. 7
IVR is risky but must be performed urgently in cases of ruptured aneurysms. A higher mortality rate exists for open surgical repair of arterial complications (24%) than for endovascular surgery (10%) 8 ; therefore, IVR was considered appropriate in this case. Stenting would have risked stretching the artery; therefore, transarterial catheter embolization was the only option. Transarterial embolization of the subclavian artery without a stent has also been reported. 9 In this case, we believe that coil embolization was appropriate. However, transcatheter therapy and angiography have been associated with high rates of complications, morbidity, and death in these patients, 8 highlighting the need for careful approach.
Celiprolol prevents aneurysm development. 10 Our patient had been under no medication for over 10 years since laparotomy for colon perforation. Earlier prescriptions may have prevented the progression of the aneurysms.
CONCLUSION
Juvenile‐onset colonic perforation and rupture of the celiac artery are important findings in the diagnosis of vascular‐type EDS.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Approval of the research protocol: N/A.
Informed consent: N/A.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Supporting information
File S1.
ACKNOWLEDGMENTS
We acknowledge proofreading and editing by Benjamin Phillis at the Clinical Study Support Center at Wakayama Medical University and Editage (www.editage.jp) for English language editing.
Tanaka M, Ueda K, Yonemitsu T, Tamura S, Ikoma A, Sonomura T, et al. Vascular type Ehlers‐Danlos syndrome with intra‐abdominal hemorrhage due to ruptured hepatic aneurysm: A case report. Acute Med Surg. 2024;11:e984. 10.1002/ams2.984
DATA AVAILABILITY STATEMENT
Approval of the research protocol: N/A.
Informed consent: N/A.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
REFERENCES
- 1. Barabas AP. Heterogeneity of the Ehlers‐Danlos syndrome: description of three clinical types and a hypothesis to explain the basic defect(s). Br Med J. 1967;2:612–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pepin M, Schwarze U, Superti‐Furga A, Byers PH. Clinical and genetic features of Ehlers‐Danlos syndrome type IV, the vascular type. N Engl J Med. 2000;342:673–680. [DOI] [PubMed] [Google Scholar]
- 3. North KN, Whiteman DAH, Pepin MG, Byers PH. Cerebrovascular complications in Ehlers‐Danlos syndrome type IV. Ann Neurol. 1995;38:960–964. [DOI] [PubMed] [Google Scholar]
- 4. Speake D, Dvorkin L, Vaizey CJ, Carlson GL. Management of colonic complications of type IV Ehlers‐Danlos syndrome: a systematic review and evidence‐based management strategy. Color Dis. 2020;22:129–135. [DOI] [PubMed] [Google Scholar]
- 5. Akutsu K, Watanabe A, Yamada T, Sahara T, Hiraoka S, Shimizu W. Vascular involvements are common in branch arteries of the abdominal aorta rather than in the aorta in vascular Ehlers‐Danlos syndrome. CJC Open. 2023;5:72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Horowitz MB, Purdy PD, Valentine RJ, Morrill K. Remote vascular catastrophes after neurovascular interventional therapy for type 4 Ehlers‐Danlos syndrome. AJNR Am J Neuroradiol. 2000;21:974–976. [PMC free article] [PubMed] [Google Scholar]
- 7. Kakinuma D, Yamada T, Kanazawa Y, Matsuno K, Sahara T, Yoshida H. A case of vascular Ehlers‐Danlos syndrome with a ruptured hepatic artery after surgical treatment of peritonitis caused by the perforation of the colon. Surg Case Rep. 2021;7:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bergqvist D, Björck M, Wanhainen A. Treatment of vascular Ehlers‐Danlos syndrome: a systematic review. Ann Surg. 2013;258:257–261. [DOI] [PubMed] [Google Scholar]
- 9. Iida Y, Obitsu Y, Komai H, Shigematsu H. Successful coil embolization for rupture of the subclavian artery associated with Ehlers‐Danlos syndrome type IV. J Vasc Surg. 2009;50:1191–1195. [DOI] [PubMed] [Google Scholar]
- 10. Ong KT, Perdu J, De Backer J, Bozec E, Collignon P, Emmerich J, et al. Effect of celiprolol on prevention of cardiovascular events in vascular Ehlers‐Danlos syndrome: a prospective randomized, open, blinded‐endpoints trial. Lancet. 2010;376:1476–1484. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1.
Data Availability Statement
Approval of the research protocol: N/A.
Informed consent: N/A.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.


