Abstract
We examined whether mixtures of urinary concentrations of bisphenol A (BPA), parabens and phthalate metabolites were associated with serum lipid levels among 175 pregnant women who enrolled in the Environment and Reproductive Health (EARTH) Study (2005–2017), including triglycerides, total cholesterol, high-density lipoprotein (HDL), non-HDL, and low-density lipoprotein (LDL). We applied Bayesian Kernel Machine Regression (BKMR) and quantile g-computation while adjusting for confounders. In the BKMR models, we found no associations between chemical mixture and lipid levels, e.g., total cholesterol [mean difference (95% CRI, credible interval) = 0.02 (−0.31, 0.34)] and LDL [mean difference (95% CRI) = 0.10 (−0.22, 0.43)], when comparing concentrations at the 75th to the 25th percentile. When stratified by BMI, we found suggestive positive relationships between urinary propylparaben and total cholesterol and LDL among women with high BMI [mean difference (95% CRI) = 0.25 (−0.26, 0.75) and 0.35 (−0.25, 0.95)], but not with low BMI [mean difference (95% CRI) = 0.00 (−0.06, 0.07) and 0.00 (−0.07, 0.07)]. No association was found by quantile g-computation. This exploratory study suggests mixtures of phenol and phthalate metabolites were not associated with serum lipid levels during pregnancy, while there were some suggestive associations for certain BMI subgroups. Larger longitudinal studies with multiple assessments of both exposure and outcome are needed to corroborate these novel findings.
Keywords: mixture, endocrine disruptors, EDC, lipid, pregnancy
1. Introduction
Endocrine-disrupting chemicals (EDCs) are exogenous chemicals that can interfere with hormone action, and some are widely used in household products [1]. Specifically, di(2-ethylhexyl) phthalate (DEHP) is widely used in plasticizers, making plastics softer and more flexible, and can be found in many household items and medical devices, such as wall and floor coverings, food packaging, and medical tubing. DEHP is further metabolized into mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), and mono(2-ethyl-5-carboxypentyl) phthalate (MECPP) in humans. Other low-molecular-weight phthalate metabolites such as monoethyl phthalate (MEP), mono-n-butyl phthalate (MBP) and monobenzyl phthalate (MBzP) are mainly found in people after exposure to personal care products (such as lotions and deodorants) and other consumer products [2,3]. Bisphenol A (BPA) is commonly used in polycarbonate plastics, epoxy resin in canned food liners, certain dental sealants and thermal receipts [4]. Parabens are used as preservatives in cosmetics, personal care products, pharmaceuticals, and food [5]. These EDCs can be detected and quantified in urine samples collected from almost all individuals in the industrialized world [6,7].
The adverse effects of maternal EDC exposure during pregnancy on the offspring are well-studied [8,9,10,11,12,13]. Pregnancy is a potentially sensitive period in relation to EDC exposure, with increased susceptibility to dyslipidemia and cardiovascular disease (CVD) [14]. During pregnancy, the body undergoes rapid cardiovascular and metabolic changes to adapt to the energy needs of the mother and the fetus [15]. Dyslipidemia is a well-known CVD risk factor characterized by high levels of triglycerides, total cholesterol and low-density lipoprotein (LDL), as well as lower levels of high-density lipoprotein (HDL), in the circulation [16]. Dyslipidemia during pregnancy has been associated with adverse fetal development [17,18], short- and long-term CVD risk [19,20,21] and higher BMI in the offspring [22]. It was reported in mice that high cholesterol levels during pregnancy impaired long-term vascular function in the mother [23], which supports the hypothesis that gestational dyslipidemia contributes to cardiovascular disorders later in life. Therefore, it is important to study the impact of EDCs during pregnancy, and the biomarkers and predictors of pregnancy-related and long-term cardiovascular health.
Some phthalates, parabens and BPA have been identified as metabolic disruptors [24,25], which contribute to metabolic disorders such as type 2 diabetes, fatty liver disease and metabolic syndrome. Some have been considered obesogens [26], which are defined as xenobiotic chemicals that can disrupt adipogenesis and energy balance [27]. Previously, in single-chemical analyses, we have found associations between lipid profiles during pregnancy and urinary phthalate metabolites [28], as well as parabens and phenols [29]. In real life, we are exposed to a mixture of EDCs, which may be associated with cholesterol and increase susceptibility to CVD. Interactions between these EDCs are biologically plausible, because previous experimental studies have demonstrated that exposure to parabens, phthalates and BPA can affect adipogenesis, as these chemicals can bind to peroxisome proliferator-activated receptors (PPARs) [30], which are present in adipose tissue and are key regulators of lipid metabolism [31,32]. When studying exposure to EDCs during pregnancy and its health effects, modern statistical tools are now available to evaluate their effects as a mixture [33].
Based on the metabolic-disrupting and obesogenic effects of phenols and phthalates, and our previous single-pollutant results, we hypothesized that among these pregnant women, mixtures of phenols and phthalate metabolite biomarkers would be related to serum lipid levels reflecting dyslipidemia. We also hypothesized that the associations would be stronger among women with overweight or obesity based on the presence of PPAR-γ in adipose tissues. Given the limited evidence related to EDC mixture and lipid profiles in pregnant women [34], we examined whether pregnancy mixtures of urinary concentrations of BPA, parabens and phthalate metabolites were associated with serum cholesterol levels among women who participated in the Environment and Reproductive Health (EARTH) Study. We further analyzed two subpopulations stratified by body mass index (BMI) to examine these associations.
2. Materials and Methods
2.1. Study Population
This study evaluated a subset of women enrolled in the Environment and Reproductive Health (EARTH) Study, a prospective cohort at the Massachusetts General Hospital (MGH) Fertility Center established to assess environmental and dietary determinants of fertility [35]. Women using their own gametes for fertility treatment between 18 and 45 years old were eligible to participate and approximately 60% of those contacted by the research staff were enrolled. This exploratory study includes 175 women enrolled from 2005 to 2017 who have data on both urinary concentrations of phenol and phthalate metabolite biomarkers as well as serum lipid biomarker measurements during pregnancy. The urine samples and blood samples were collected on the same day. Participants’ date of birth was collected at enrollment and their weight and height were measured by experienced study staff. Sociodemographic, lifestyle, and medical history questionnaires were administered to participants at the same entry visit. Study participants completed a comprehensive questionnaire on medical, family and reproductive history, use of consumer products, physical activity and smoking history. Infertility diagnosis under the Society of Assisted Reproductive Technology definitions (SART) was assigned by physicians [36]. Pregnancy-related covariates were abstracted from electronic medical records by trained study staff. The study obtained approvals from the Human Subject Committees of the Harvard T.H. Chan School of Public Health, MGH, and the Centers for Disease Control and Prevention (CDC). Informed consent was signed by participants after the study procedures had been explained, and all questions were answered in detail by trained staff.
2.2. Exposure Assessment
Enrolled women provided urine samples at the clinic during pregnancy. For this study, we included the urine sample that was collected on the same day the blood sample was collected at the clinic visit during pregnancy. The specific gravity of the urine was measured by a handheld refractometer (National Instrument Company, Inc., Baltimore, MD, USA) at room temperature, calibrated with deionized water before each measurement. We included specific gravity as a covariate in the statistical models as previously described [37,38]. Urine samples were stored at −80 °C after collection and then shipped frozen to the CDC for analysis overnight. As previously described [39,40], we applied strict quality controls and used online solid-phase extraction along with isotope dilution–high-performance liquid chromatography–tandem mass spectrometry to quantify the concentrations of phenol and phthalate biomarkers in the urine samples collected. The measured chemicals included four phenols (BPA, methylparaben, propylparaben and butylparaben) four phthalate metabolites [mono-n-butyl phthalate (MBP), mono-isobutyl phthalate (MiBP), monoethyl phthalate (MEP) and monobenzyl phthalate (MBzP)] and four DEHP phthalate metabolites [mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP) and mono(2-ethyl-5-carboxypentyl) phthalate (MECPP)]. The above twelve biomarkers have been examined as a mixture in this study. Limits of detection (LODs) ranged from 0.2 to 1.2 µg/L, depending on the chemical biomarkers (Supplemental Table S1). Quality control was performed as previously described using statistical probability rules [41].
2.3. Outcome Assessment
We included one non-fasting blood sample per participant collected on the same day as the urine sample. If the participant provided several samples from different timepoints during pregnancy, we randomly selected one sample at one timepoint. Blood samples were prepared as previously described [29] and transferred to the Clinical and Epidemiologic Laboratory (CERLab) at Boston Children’s Hospital (Boston, MA, USA), which was certified by the Centers for Disease Control and Prevention/National Heart, Lung, and Blood Institute Lipid Standardization Program. Total cholesterol, triglycerides and HDL cholesterol levels (mg/dL) were measured in the serum sample with the Roche Cobas 6000 system, and reagents and calibrators were provided by Roche Diagnostics (Indianapolis, IN, USA), which were approved by the Food and Drug Administration (FDA) for clinical use.
Triglycerides were measured enzymatically with correction for endogenous glycerol, as previously described [42]. Cholesterol levels were also measured enzymatically [43]. The concentrations of HDL-C were measured by a direct enzymatic colorimetric assay, which meets the rigid requirements established by the Lipid Standardization Program [44]. Triglyceride concentrations were determined with an intra- and inter- day-to-day reproducibility of 1.8% and 1.7%, respectively, and the corresponding figures for HDL-C levels were 3.3% and 1.7%. The coefficients of variation (CVs) for total cholesterol concentrations were 1.7% and 1.6%. Non-HDL levels were calculated as the difference between total and HDL cholesterol concentrations. LDL cholesterol was estimated using the Friedewald formula [45].
2.4. Statistical Analysis
The demographic, reproductive characteristics of the study population and serum lipid biomarker levels were described as median ± inter-quartile ranges (IQRs) for continuous variables and count (percentage) for discrete variables. The serum lipid biomarker levels were not log-transformed because they appeared to be normally distributed [28] based on Kolmogorov–Smirnov tests for normality. Distributions of urinary concentrations of chemical biomarkers were reported using percentiles, geometric mean and mean ± standard deviations (SDs). Detection frequencies of the urinary chemical biomarkers were reported. Given that the samples were analyzed in multiple batches over the years, some biomarkers had different LODs at various time points; for those biomarkers, we reported the maximum LOD (Supplemental Table S1). Concentrations below the LODs were imputed using R package multiLODmice (version 0.1.0), which extended the multiple imputation method to allow different LODs for different observations [46]. Correlations between urinary chemical biomarkers were estimated using Spearman correlation coefficients. All urinary chemical biomarkers were log-transformed by a natural logarithm before including them in the statistical models.
Covariates were selected based on prior knowledge regarding their impact on exposures and outcomes. Primary models were adjusted for urine specific gravity, age (years), pre-pregnancy BMI (kg/m2) at sample collection, race (white/Caucasian and other, combined given the relatively low proportion of women of color included in this study), education level (graduate degree attainment), infertility diagnosis by physician (female factor; male factor; unexplained cause), mode of conception [without treatment; use of in vitro fertilization (IVF)/intrauterine insemination (IUI)], multiple gestations (singleton; twins/triplets) and trimester at sample collection (1st; 2nd; 3rd). In stratified models, the pre-pregnancy BMI was not adjusted. Mixture effect analyses were performed using two modern approaches: Bayesian Kernel Machine Regression (BKMR) [47] and quantile g-computation [48]. The BKMR method aims to model the complex relationship between a number of variables and the outcome (dependent variable) using the flexible non-linear or additive function . The general modeling framework considered is:
where is the monotonic link function, , is the flexible function of predictors (exposure variables ), is a vector of covariates considered to have linear relationships with the outcome, and is the corresponding coefficients for each sample in a total of samples [47].
In the BKMR analysis, to account for the collinearity of biomarker concentrations, hierarchical variable selection was applied, which means biomarker concentrations were categorized into non-overlapping groups, and variable selection was performed first at the group level and then within groups [47]. We grouped the chemical biomarkers based on prior knowledge of their sources, their correlations, and previous findings in the same cohort: (1) BPA and DEHP metabolites (MEHP, MEHHP, MEOHP and MECPP); (2) parabens (methylparaben, propylparaben, butylparaben); (3) other phthalate metabolites (MBP, MiBP, MEP, MBzP). In each analysis, the number of iterations was 20,000. We reported group-specific posterior inclusion probabilities (PIPs) and conditional posterior inclusion probabilities, which represent the group contribution and the chemical biomarker’s individual contribution to the non-null association between exposure and the outcome.
In secondary analyses, we also evaluated stratification by BMI (above and below 25 kg/m2), which is the threshold for overweight and obesity [49], and studied chemicals individually and as the molar sums of the chemicals within each group (only when applying BKMR). We illustrated graphically for each chemical: (1) exposure–response relationships, while holding all other biomarkers at median concentrations, and (2) the mean difference between the 75th and 25th percentiles (estimates and 95% credible intervals) when concentrations of all other biomarker were held at the 25th, 50th, and 75th.
In the quantile g-computation models, we reported the mixture effect by the mean differences and 95% confidence intervals for each outcome. All analyses were performed in R environment (version 4.0.5). BKMR analyses were conducted using the R package bkmr (version 0.2.2) and quantile g-computation analyses were conducted using the R package qgcomp (version 2.15.2).
3. Results
The 175 women included in this exploratory study had a median (IQR) age of 35 (32, 38) years at sample collection during pregnancy and a pre-pregnancy BMI of 22.9 (21.2, 25.6) kg/m2 (Table 1). Thirty percent of the participants (N = 53) had overweight or obesity. The majority of the cohort were white (88%) and highly educated (60% possessed a graduate degree), and few were current or past smokers (29%). Of the women included in this study, 83% became pregnant after medical intervention, with 57% using IVF and 26% using IUI. Most women had singleton pregnancies (82%). Compared to women with no exposure and outcome assessment, the included women were more likely to undergo IVF treatments and diagnosed female-factor-caused infertility [22,23]. The median (IQR) serum concentrations of total triglycerides, total cholesterol, HDL, non-HDL and LDL cholesterol were 181 (112, 251), 229 (190, 279), 68 (58, 79), 161 (122, 204) and 120 (92, 158) mg/dL, respectively.
Table 1.
Demographics, reproductive characteristics and serum lipid profiles among 175 pregnant women enrolled in the Environment and Reproductive Health (EARTH) Study.
| Demographic Characteristics | |
|---|---|
| Age at pregnancy, years, median (IQR) | 35 (32, 38) |
| Race, N (%) | |
| White | 154 (88) |
| Black | 5 (2) |
| Asian | 8 (5) |
| Other | 8 (5) |
| Pre-pregnancy body mass index, kg/m2 | 22.9 (21.2, 25.6) |
| 122 (70) | |
| 53 (30) | |
| Ever smoked, N (%) | 50 (29) |
| Graduate degree attainment, N (%) | 105 (60) |
| Primary infertility diagnosis, N (%) | |
| Male factor | 58 (33) |
| Female factor | 59 (33) |
| Unexplained | 58 (33) |
| Mode of conception, N (%) | |
| IUI | 45 (26) |
| IVF | 101 (57) |
| Natural | 29 (17) |
| Number of babies, N (%) | |
| Singleton | 144 (82) |
| Twins and triplets | 31 (18) |
| Trimester of sample collection, N (%) | |
| 1st | 61 (35) |
| 2nd | 47 (27) |
| 3rd | 67 (38) |
| Serum lipid level, mg/dL, median (IQR) | |
| Total triglycerides | 181 (112, 251) |
| Total cholesterol | 229 (190, 279) |
| HDL cholesterol | 68.0 (58.0, 79.0) |
| Non-HDL cholesterol | 161 (122, 204) |
| LDL cholesterol | 120 (92.0, 158) |
N: Number of participants. IUI: intrauterine insemination. IVF: in vitro fertilization. HDL: high-density lipoprotein. LDL: low-density lipoprotein.
The detection frequencies for BPA (87%), butylparaben (58%) and MEHP (69%) were lower than for other chemical biomarkers (93%) (Supplemental Table S1). Compared to adult females participating in the National Health and Nutrition Examination Survey (NHANES) [6], women in this study had similar urinary chemical biomarker concentrations, except for lower concentrations of MBzP and higher concentrations of propylparaben. Urinary concentrations of the four DEHP metabolites (MEOHP, MEHHP, MECPP, MEHP) were highly correlated (Spearman r range from 0.76 to 0.98, Supplemental Figure S1). DEHP metabolites were moderately correlated with BPA (Spearman r range from 0.37 to 0.47). In addition, concentrations of methylparaben and propylparaben were highly correlated (Spearman r = 0.86). Butylparaben was weakly correlated with other chemicals (Spearman r ≤ 0.31).
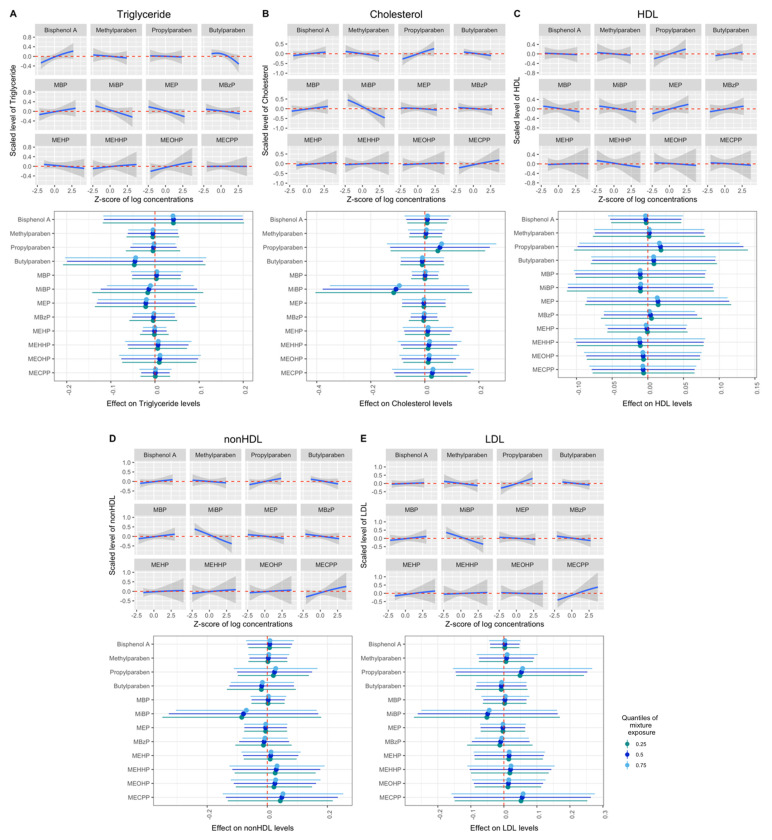
The primary BKMR models showed no significant overall mixture or single-pollutant effects when comparing mixed-exposure biomarker concentrations at the 75th to the 25th percentile on total triglycerides (mean difference = −0.03, 95% CRI = −0.29, 0.23), total cholesterol (mean difference = 0.02, 95% CRI = −0.31, 0.34), HDL (mean difference = 0.00, 95% CRI = −0.27, 0.26), non-HDL (mean difference = 0.04, 95% CRI = −0.27, 0.35), or LDL (mean difference = 0.10, 95% CRI = −0.22, 0.43) cholesterol (Figure 1), although PIPs were greater than 50% in some models (Supplemental Table S2). In addition, no significant interactions between chemical biomarkers were observed (Figure 1). In addition, no associations were found between a quartile increase in the chemical biomarker mixture and total triglycerides using quantile g-computation (mean difference = −0.11, 95% CRI = −0.32, 0.09), total cholesterol (mean difference = 0.04, 95% CRI = −0.17, 0.26), HDL (mean difference = 0.05, 95% CRI = −0.24, 0.34), non-HDL (mean difference = 0.03, 95% CRI = −0.18, 0.25) or LDL (mean difference = 0.09, 95% CRI = −0.17, 0.34) (Supplemental Table S3).
Figure 1.
Bayesian Kernel Machine Regression (BKMR) mixture associations of phenol and phthalate metabolites and lipid biomarkers. (A–E) Mixture associations with total triglycerides, total cholesterol, HDL, non-HDL and LDL cholesterol in BKMR. Upper half: exposure–response relationships for each biomarker while holding all other biomarkers at their medians. Lower half: mean difference between the 75th and 25th percentiles of exposure (estimates and 95% credible intervals) when other biomarker concentrations were fixed at the 25th, 50th, and 75th percentiles. BKMR models were adjusted for age, education level, race, infertility diagnosis, mode of conception, number of fetuses, trimester and specific gravity. HDL: high-density lipoprotein. LDL: low-density lipoprotein. MEHP: mono(2-ethylhexyl) phthalate. MEHHP: mono(2-ethyl-5-hydroxyhexyl) phthalate. MEOHP: mono(2-ethyl-5-oxohexyl) phthalate. MECPP: mono(2-ethyl-5-carboxypentyl). MBP: mono-n-butyl phthalate. MiBP: mono-isobutyl phthalate. MEP: monoethyl phthalate. MBzP: monobenzyl phthalate.
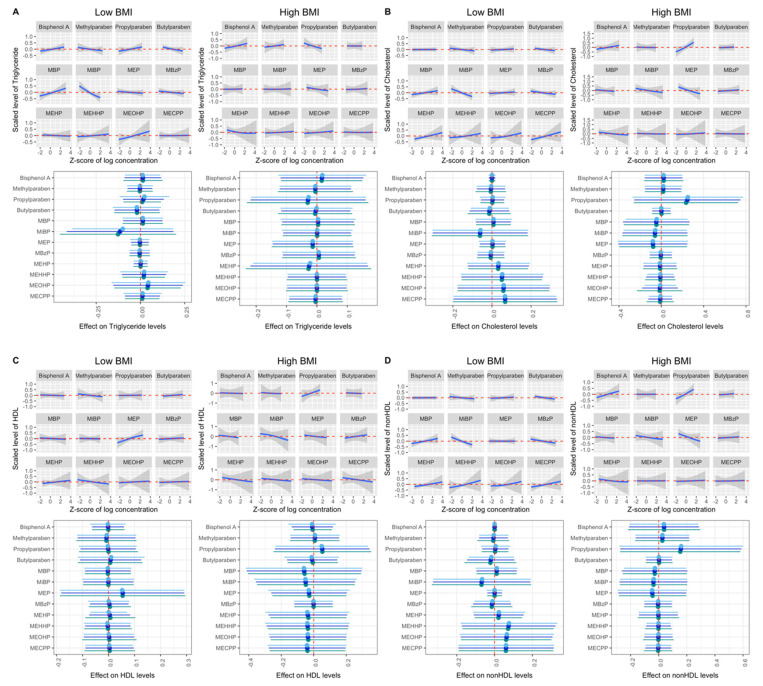
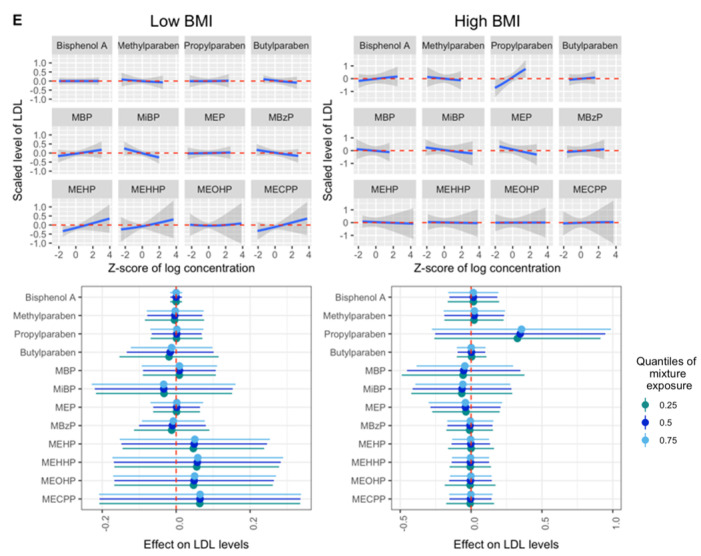
We then examined the associations between chemical biomarker mixtures and lipid profiles, stratifying by BMI. We observed suggestive positive relationships of urinary propylparaben with total cholesterol and LDL among women with high BMI [mean difference (95% CRI) = 0.25 (−0.26, 0.75) and 0.35 (−0.25, 0.95), conditional PIPs = 79.0% and 83.2%, Figure 2 and Supplemental Table S4] when comparing concentrations at the 75th to the 25th percentile, fixing other chemicals at their medians. The corresponding figures of urinary propylparaben with total cholesterol and LDL were 0.00 (−0.06, 0.06) and 0.00 (−0.07, 0.07), with conditional PIPs of 24.8% and 25.5% among women with low BMI. While we observed different estimates for women with high and low BMI, the differences were not statistically significant. The associations between mixtures of these EDCs and lipid profiles did not differ between high- and low-BMI groups when applying quantile g-computation (Supplemental Table S5).
Figure 2.
Bayesian Kernel Machine Regression (BKMR) mixture associations of phenol and phthalate metabolites and lipid biomarkers when stratified by BMI. (A–E) Mixture associations with total triglycerides, total cholesterol, HDL, non-HDL and LDL cholesterol in BKMR. Upper half: exposure–response relationships for each biomarker while holding all other biomarkers at their median. Lower half: mean difference between the 75th and 25th percentile of exposure (estimates and 95% credible intervals) when other biomarker concentrations were fixed at the 25th, 50th, and 75th percentiles. BKMR models were adjusted for age, education level, race, infertility diagnosis, mode of conception, number of fetuses, trimester and specific gravity. HDL: high-density lipoprotein. LDL: low-density lipoprotein. MEHP: mono(2-ethylhexyl) phthalate. MEHHP: mono(2-ethyl-5-hydroxyhexyl) phthalate. MEOHP: mono(2-ethyl-5-oxohexyl) phthalate. MECPP: mono(2-ethyl-5-carboxypentyl). MBP: mono-n-butyl phthalate. MiBP: mono-isobutyl phthalate. MEP: monoethyl phthalate. MBzP: monobenzyl phthalate.
4. Discussion
In this exploratory study, we examined whether mixtures of urinary concentrations of four phenol and eight phthalate metabolites were associated with serum lipid levels during pregnancy, including total triglycerides, total cholesterol, HDL, non-HDL and LDL cholesterol, among pregnant women in the EARTH Study cohort. The mixture models were evaluated using BKMR modeling and quantile g-computation. We observed no overall associations of urinary phenol and phthalate metabolite biomarker mixtures with lipid biomarkers in both models. Nevertheless, when stratified by BMI, we found suggestive positive relationships between propylparaben, total cholesterol and LDL among women with high BMI. It is important to note that these relationships were not statistically significant, possibly due to the moderate sample size included in the study, which limited our study power. No difference in mixture effect was observed using quantile q-computation models among women with high and low BMI.
To the best of our knowledge, only one cohort study examined the mixture effect of EDCs on circulating lipid levels in pregnant women [34]. The authors evaluated BPA, phthalates, polybrominated diphenyl ethers (PBDEs), and per- and polyfluoroalkyl substances (PFAS), but not parabens. No overall mixture associations with the examined lipids were reported, either. However, they identified urinary MBzP, measured at 16 weeks of pregnancy, as an important contributor to triglycerides levels. Other individual phthalate biomarkers and BPA were weak contributors to the association with total lipid, cholesterol and triglycerides levels, which is consistent with our results. In our study, we found no associations between the urinary concentration of MBzP and triglyceride levels. This may be partially explained by the fact that the pregnant women in our study had considerably lower urinary MBzP concentrations compared to women in the HOME Study (75th quantile: 5.75 μg/L vs. 24.5 μg/L). Another reason may be related to the strategy used when analyzing chemical biomarker exposure mixtures, as our primary analyses applied hierarchical variable selection in BKMR and included the exposure biomarkers as groups based on their correlation and previous publications in EARTH. Given the scarce literature on the topic in relation to pregnant women, additional studies are warranted.
Previously, in single-pollutant analyses among the same women in the EARTH Study, it was found that urinary propylparaben concentrations were positively associated with total cholesterol, as well as non-HDL and LDL cholesterol, levels when comparing concentrations in the highest tertile to concentrations in the lowest [29]. They also found several associations between urinary MBP, BPA and several DEHP metabolites and lipid levels when comparing the concentrations in the highest group versus the lowest group [28]. In single-biomarker analyses, urinary propylparaben was associated with total, non-HDL and LDL cholesterol. In the previous study, urinary concentrations of pollutants were evaluated as categorical variables, and statistical testing was performed against the lowest quantiles, whereas in this mixture manuscript, urinary biomarker concentrations were examined as continuous variables within a determined group. Some of the previously observed positive associations may also be neutralized by the negative effects of other EDC biomarkers. For example, there is a decreasing trend in the association between MiBP and total cholesterol, non-HDL and LDL (Figure 1). The moderate sample size included in the study also limited our study’s ability to find significant associations by BKMR. This may explain the discrepancy between single and mixture model results for the other examined biomarkers.
We observed suggestive positive relationships of propylparaben with total cholesterol and LDL among women with high BMI scores. Some parabens were identified as obesogens [26]. Wen and colleagues found positive associations between urinary paraben concentrations, including propylparaben and mixtures of parabens, and gestational weight gain among pregnant women in Wuhan, China [50]. They also observed that these associations were stronger among women with overweight or obesity compared to women with normal- and underweight.
Exposure to parabens might result in elevated circulating lipid levels by activating the peroxisome proliferator-activated receptor (PPAR)-γ [30], which is presented mainly in adipose tissue and key regulators in the lipid metabolism [31,51]. In addition, parabens were found in adipose tissue [52]. Thus, women with overweight and obesity might be more sensitive to paraben exposure than others. Particularly, urinary propylparaben showed a suggestive positive relationship with total cholesterol and LDL among women with high BMI, and was the main contributor. Women in our study had higher concentrations of parabens compared to the Wuhan cohort, and higher concentrations of propylparaben compared to adult females participating in NHANES, while they had similar concentrations of other parabens [6]. This fact may explain why we observed a stronger relationship with propylparaben in single-pollutant analyses in BKMR, but not with the other parabens among women with higher BMI. However, additional studies on both animal models and humans are required to further understand the relationship between BMI, parabens and circulating lipid profile.
There are some limitations to this study that should be noted. First, the generalizability of the results may be limited as this study includes subfertile women seeking treatment in fertility clinics. However, these women are also at higher risk of CVD compared to women in the general population [53]. Second, we cannot confirm that all serum samples were collected after fasting, and this may affect the results. Third, lipid levels physiologically rise during pregnancy, and adjusting for trimester may not fully account for this change. Fourth, the sample sizes after stratification by high (N = 53) and low BMI (N = 122) were relatively low, which may limit our ability to detect significant associations. Fifth, exposure misclassification is possible considering the biomarkers’ short biological half-lives [3] and the episodic nature of exposures [54], especially since we used one urine sample per study participant. However, in the context of EARTH, we have previously reported the moderate to high correlations of chemical biomarkers measured across multiple urine samples in women demonstrating low variability [55]. Lastly, as a cross-sectional analysis, reverse causation is also possible. Residual confounding by other exposures, lifestyle and nutritional factors is possible.
Despite the above limitations, our study has several strengths. Our study is one of the first studies to evaluate mixtures of BPA, parabens and phthalate metabolites simultaneously in pregnant women. A major strength of this study is that we applied two sophisticated statistical methods to estimate associations of phenol and phthalate biomarkers with outcomes of interest as a mixture: BKMR and quantile g-computation. Quantile g-computation assumes linearity and additivity on the quantile scale of the chemical biomarkers’ concentrations, and estimates the association between a quantile increase in all exposed chemical biomarkers simultaneously and the outcome [48]. The study is interpretable and efficient, in line with the conventional mode of comparison among quantiles. BKMR, on the other hand, allows for non-additive interactions between chemical biomarkers and complex non-linear exposure–response relationships [47]. This modeling method is flexible and powerful, yet not that interpretable or efficient compared to quantile g-computation. Furthermore, hierarchical variable selection was applied when taking highly correlated pollutants and pollutants with the same sources, such as DEHP metabolites, into account. This grouping method utilized existing knowledge of co-exposure and observed correlations, enabling the more precise evaluation of individual relevance. Previously, approaches modeling EDC exposures as a mixture have been successfully applied in reproductive epidemiology [56,57,58,59], and provided valuable insights for their joint effects on glucose metabolism during pregnancy, ovarian reserves, lipid profiles in adult women, and risk of infertility. Applying such sophisticated statistical methods will help us better understand the effects of mixtures of EDCs on human health. Other strengths include the comprehensive adjustments for other reproductive and demographical confounders, as well as the evaluation of effects on lipid profiles in a well-established sub-fertile cohort with higher CVD risk, who may be particularly vulnerable to the impacts of EDCs as metabolic disruptors.
5. Conclusions
In summary, we observed overall no association between a mixture of BPA, parabens and phthalate metabolites and circulating lipid levels, including total triglycerides, total cholesterol, and HDL, non-HDL and LDL cholesterol, among 175 pregnant women. However, we found suggestive positive associations between urinary propylparaben, total cholesterol and LDL among women with high BMI. This study suggests there is no association between the mixture of phenols and phthalate metabolites and serum lipid levels among pregnant women, while there are some suggestive associations for certain BMI subgroups. Larger longitudinal studies with multiple assessments of both exposure and outcome are needed to corroborate these novel findings. These results, if confirmed, add to our knowledge of the effects of exposure to multiple EDCs and pregnancy health.
Note: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification purposes only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Services.
Acknowledgments
The authors gratefully acknowledge all members of the EARTH study team, specifically the Harvard T. H. Chan School of Public Health research staff Myra Keller, Ramace Dadd and Alex Azevedo, physicians and staff at Massachusetts General Hospital Fertility Center as well as CDC lab personnel. A special thank you is given to all of the study participants.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/toxics12080574/s1, Table S1: Distribution of urinary concentration (μg/L) of phenol and phthalate metabolites among pregnant women in Environment and Reproductive Health (EARTH) Study (2005–2017); Table S2: Bayesian Kernel Machine Regression (BKMR) posterior inclusion probabilities (PIPs) (%) of phenol and phthalate metabolites in relation to serum lipid biomarkers; Table S3: Quantile g-computation measurement of the association between a quartile increase in mixture chemical biomarker concentrations and lipid biomarkers; Table S4: Bayesian Kernel Machine Regression (BKMR) posterior inclusion probabilities (PIPs) (%) for chemicals in relation to lipid profiles stratified by high vs. low BMI; Table S5: Quantile g-computation measurement of the association between a quartile increase in mixture chemical biomarker concentrations and lipid biomarkers stratified by high vs. low BMI; Figure S1: Spearman correlation between pairs of phenol and phthalate metabolite concentrations.
Author Contributions
Conceptualization, L.M.-A., R.H. and J.E.C.; methodology, M.G.-W., X.S. and P.L.W.; software, M.G.-W., X.S. and P.L.W.; formal analysis, X.S.; investigation, J.B.F.; data curation, A.M.C., J.B.F. and the EARTH study team; writing—original draft preparation, X.S. and L.M.-A.; writing—review and editing, L.M.-A., R.H., J.E.C., D.Z., M.G.-W., A.M.C., P.L.W., K.M.R., T.J.-T. and X.S.; visualization, X.S.; supervision, L.M.-A.; project administration, J.B.F. and the EARTH Study Team; funding acquisition, D.Z., L.M.-A. and R.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Human Subject Committees of the Harvard T.H. Chan School of Public Health (MGH), and the Centers for Disease Control and Prevention (CDC) (protocol code: 1999P008167 and date of approval: 29 January 2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are not publicly available due to privacy and confidentiality reasons.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
The project was funded by grants R01ES022955, R01ES009718, R01ES034700, R01ES033651 and P30ES000002, from the National Institute of Environmental Health Sciences (NIEHS). The research was also supported by Zhejiang University Global Partnership Fund.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Gore A.C., Chappell V.A., Fenton S.E., Flaws J.A., Nadal A., Prins G.S., Toppari J., Zoeller R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015;36:E1–E150. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braun J.M., Just A.C., Williams P.L., Smith K.W., Calafat A.M., Hauser R. Personal Care Product Use and Urinary Phthalate Metabolite and Paraben Concentrations during Pregnancy among Women from a Fertility Clinic. J. Expo. Sci. Environ. Epidemiol. 2014;24:459–466. doi: 10.1038/jes.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hauser R., Calafat A. Phthalates and Human Health. Occup. Environ. Med. 2005;62:806–818. doi: 10.1136/oem.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mínguez-Alarcón L., Hauser R., Gaskins A.J. Effects of Bisphenol A on Male and Couple Reproductive Health: A Review. Fertil. Steril. 2016;106:864–870. doi: 10.1016/j.fertnstert.2016.07.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen F.A. Final Amended Report on the Safety Assessment of Methylparaben, Ethylparaben, Propylparaben, Isopropylparaben, Butylparaben, Isobutylparaben, and Benzylparaben as Used in Cosmetic Products. Int. J. Toxicol. 2008;27:1–82. doi: 10.1080/10915810802548359. [DOI] [PubMed] [Google Scholar]
- 6.CDC Centers for Disease Control and Prevention . National Report on Human Exposure to Environmental Chemicals, Updated Tables. CDC Centers for Disease Control and Prevention; Atlanta, GA, USA: 2022. [DOI] [Google Scholar]
- 7.United Nations Environment Programme . Overview Report II: An Overview of Current Scientific Knowledge on the Life Cycles, Environmental Exposures, and Environmental Effects of Select Endocrine Disrupting Chemicals (EDCs) and Potential EDCs. UNEP; Nairobi, Kenya: 2017. [Google Scholar]
- 8.Moya J., Phillips L., Sanford J., Wooton M., Gregg A., Schuda L. A Review of Physiological and Behavioral Changes during Pregnancy and Lactation: Potential Exposure Factors and Data Gaps. J. Expo. Sci. Environ. Epidemiol. 2014;24:449–458. doi: 10.1038/jes.2013.92. [DOI] [PubMed] [Google Scholar]
- 9.Soma-Pillay P., Nelson-Piercy C., Tolppanen H., Mebazaa A. Physiological Changes in Pregnancy. Cardiovasc. J. Afr. 2016;27:89–94. doi: 10.5830/CVJA-2016-021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alonso-Magdalena P., Quesada I., Nadal A. Endocrine Disruptors in the Etiology of Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2011;7:346–353. doi: 10.1038/nrendo.2011.56. [DOI] [PubMed] [Google Scholar]
- 11.Braun J.M. Early-Life Exposure to EDCs: Role in Childhood Obesity and Neurodevelopment. Nat. Rev. Endocrinol. 2017;13:161–173. doi: 10.1038/nrendo.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caporale N., Leemans M., Birgersson L., Germain P.-L., Cheroni C., Borbély G., Engdahl E., Lindh C., Bressan R.B., Cavallo F., et al. From Cohorts to Molecules: Adverse Impacts of Endocrine Disrupting Mixtures. Science. 2022;375:eabe8244. doi: 10.1126/science.abe8244. [DOI] [PubMed] [Google Scholar]
- 13.Liu J., Shi J., Hernandez R., Li X., Konchadi P., Miyake Y., Chen Q., Zhou T., Zhou C. Paternal Phthalate Exposure-Elicited Offspring Metabolic Disorders Are Associated with Altered Sperm Small RNAs in Mice. Environ. Int. 2023;172:107769. doi: 10.1016/j.envint.2023.107769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mustieles V., Rolland M., Pin I., Thomsen C., Sakhi A.K., Sabaredzovic A., Muckle G., Guichardet K., Slama R., Philippat C. Early-Life Exposure to a Mixture of Phenols and Phthalates in Relation to Child Social Behavior: Applying an Evidence-Based Prioritization to a Cohort with Improved Exposure Assessment. Environ. Health Perspect. 2023;131:87006. doi: 10.1289/EHP11798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salazar P., Villaseca P., Cisternas P., Inestrosa N.C. Neurodevelopmental Impact of the Offspring by Thyroid Hormone System-Disrupting Environmental Chemicals during Pregnancy. Environ. Res. 2021;200:111345. doi: 10.1016/j.envres.2021.111345. [DOI] [PubMed] [Google Scholar]
- 16.Virani S.S., Alonso A., Aparicio H.J., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Cheng S., Delling F.N., et al. Heart Disease and Stroke Statistics-2021 Update: A Report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 17.Chen K.-Y., Lin S.-Y., Lee C.-N., Wu H.-T., Kuo C.-H., Kuo H.-C., Chuang C.-C., Kuo C.-H., Chen S.-C., Fan K.-C., et al. Maternal Plasma Lipids During Pregnancy, Insulin-like Growth Factor-1, and Excess Fetal Growth. J. Clin. Endocrinol. Metab. 2021;106:e3461–e3472. doi: 10.1210/clinem/dgab364. [DOI] [PubMed] [Google Scholar]
- 18.Wang H., Dang Q., Zhu H., Liang N., Le Z., Huang D., Xiao R., Yu H. Associations between Maternal Serum HDL-c Concentrations during Pregnancy and Neonatal Birth Weight: A Population-Based Cohort Study. Lipids Health Dis. 2020;19:93. doi: 10.1186/s12944-020-01264-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palinski W. Effect of Maternal Cardiovascular Conditions and Risk Factors on Offspring Cardiovascular Disease. Circulation. 2014;129:2066–2077. doi: 10.1161/CIRCULATIONAHA.113.001805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Napoli C., Glass C.K., Witztum J.L., Deutsch R., D’Armiento F.P., Palinski W. Influence of Maternal Hypercholesterolaemia during Pregnancy on Progression of Early Atherosclerotic Lesions in Childhood: Fate of Early Lesions in Children (FELIC) Study. Lancet. 1999;354:1234–1241. doi: 10.1016/S0140-6736(99)02131-5. [DOI] [PubMed] [Google Scholar]
- 21.Gaillard R., Jaddoe V.W.V. Maternal Cardiovascular Disorders before and during Pregnancy and Offspring Cardiovascular Risk across the Life Course. Nat. Rev. Cardiol. 2023;20:617–630. doi: 10.1038/s41569-023-00869-z. [DOI] [PubMed] [Google Scholar]
- 22.Cacciatore F., Bruzzese G., Abete P., Russo G., Palinski W., Napoli C. Maternal Hypercholesterolaemia during Pregnancy Affects Severity of Myocardial Infarction in Young Adults. Eur. J. Prev. Cardiol. 2022;29:758–765. doi: 10.1093/eurjpc/zwab152. [DOI] [PubMed] [Google Scholar]
- 23.Sáez T., Pageé A., Kirschenman R., Quon A., Spaans F., Davidge S.T. A High Cholesterol Diet during Late Pregnancy Impairs Long-Term Maternal Vascular Function in Mice. Arter. Thromb. Vasc. Biol. 2023;43:120–132. doi: 10.1161/ATVBAHA.122.318421. [DOI] [PubMed] [Google Scholar]
- 24.Casals-Casas C., Desvergne B. Endocrine Disruptors: From Endocrine to Metabolic Disruption. Annu. Rev. Physiol. 2011;73:135–162. doi: 10.1146/annurev-physiol-012110-142200. [DOI] [PubMed] [Google Scholar]
- 25.Heindel J.J., Blumberg B., Cave M., Machtinger R., Mantovani A., Mendez M.A., Nadal A., Palanza P., Panzica G., Sargis R., et al. Metabolism Disrupting Chemicals and Metabolic Disorders. Reprod. Toxicol. 2017;68:3–33. doi: 10.1016/j.reprotox.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heindel J.J., Blumberg B. Environmental Obesogens: Mechanisms and Controversies. Annu. Rev. Pharmacol. Toxicol. 2019;59:89–106. doi: 10.1146/annurev-pharmtox-010818-021304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grün F., Blumberg B. Environmental Obesogens: Organotins and Endocrine Disruption via Nuclear Receptor Signaling. Endocrinology. 2006;147:S50–S55. doi: 10.1210/en.2005-1129. [DOI] [PubMed] [Google Scholar]
- 28.Mínguez-Alarcón L., Williams P.L., James-Todd T., Souter I., Ford J.B., Rexrode K.M., Calafat A.M., Hauser R., Chavarro J.E. Association of Urinary Phthalate and Phthalate Replacement Metabolite Concentrations with Serum Lipid Biomarker Levels among Pregnant Women Attending a Fertility Center. Toxics. 2022;10:292. doi: 10.3390/toxics10060292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mínguez-Alarcón L., Frueh L., Williams P.L., James-Todd T., Souter I., Ford J.B., Rexrode K.M., Calafat A.M., Hauser R., Chavarro J.E. Pregnancy Urinary Concentrations of Bisphenol A, Parabens and Other Phenols in Relation to Serum Levels of Lipid Biomarkers: Results from the EARTH Study. Sci. Total Environ. 2022;833:155191. doi: 10.1016/j.scitotenv.2022.155191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu L., Liu W., Wang X., Ye Z., Tan Q., Qiu W., Nie X., Li M., Wang B., Chen W. A Review of Practical Statistical Methods Used in Epidemiological Studies to Estimate the Health Effects of Multi-Pollutant Mixture. Environ. Pollut. 2022;306:119356. doi: 10.1016/j.envpol.2022.119356. [DOI] [PubMed] [Google Scholar]
- 31.Lazarevic N., Barnett A.G., Sly P.D., Knibbs L.D. Statistical Methodology in Studies of Prenatal Exposure to Mixtures of Endocrine-Disrupting Chemicals: A Review of Existing Approaches and New Alternatives. Environ. Health Perspect. 2019;127:026001. doi: 10.1289/EHP2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L., Asimakopoulos A.G., Kannan K. Accumulation of 19 Environmental Phenolic and Xenobiotic Heterocyclic Aromatic Compounds in Human Adipose Tissue. Environ. Int. 2015;78:45–50. doi: 10.1016/j.envint.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 33.Fajas L., Auboeuf D., Raspé E., Schoonjans K., Lefebvre A.M., Saladin R., Najib J., Laville M., Fruchart J.C., Deeb S., et al. The Organization, Promoter Analysis, and Expression of the Human PPARgamma Gene. J. Biol. Chem. 1997;272:18779–18789. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- 34.Pereira-Fernandes A., Demaegdt H., Vandermeiren K., Hectors T.L.M., Jorens P.G., Blust R., Vanparys C. Evaluation of a Screening System for Obesogenic Compounds: Screening of Endocrine Disrupting Compounds and Evaluation of the PPAR Dependency of the Effect. PLoS ONE. 2013;8:e77481. doi: 10.1371/journal.pone.0077481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vuong A.M., Braun J.M., Sjödin A., Calafat A.M., Yolton K., Lanphear B.P., Chen A. Exposure to Endocrine Disrupting Chemicals (EDCs) and Cardiometabolic Indices during Pregnancy: The HOME Study. Environ. Int. 2021;156:106747. doi: 10.1016/j.envint.2021.106747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mínguez-Alarcón L., Gaskins A.J., Chiu Y.-H., Souter I., Williams P.L., Calafat A.M., Hauser R., Chavarro J.E. Dietary Folate Intake and Modification of the Association of Urinary Bisphenol A Concentrations with In Vitro Fertilization Outcomes among Women from a Fertility Clinic. Reprod. Toxicol. 2016;65:104–112. doi: 10.1016/j.reprotox.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mok-Lin E., Ehrlich S., Williams P.L., Petrozza J., Wright D.L., Calafat A.M., Ye X., Hauser R. Urinary Bisphenol A Concentrations and Ovarian Response among Women Undergoing IVF. Int. J. Androl. 2010;33:385–393. doi: 10.1111/j.1365-2605.2009.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barr D.B., Wilder L.C., Caudill S.P., Gonzalez A.J., Needham L.L., Pirkle J.L. Urinary Creatinine Concentrations in the U.S. Population: Implications for Urinary Biologic Monitoring Measurements. Environ. Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schisterman E.F., Whitcomb B.W., Buck L.G.M., Louis T.A. Lipid Adjustment in the Analysis of Environmental Contaminants and Human Health Risks. Environ. Health Perspect. 2005;113:853–857. doi: 10.1289/ehp.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silva M.J., Samandar E., Preau J.L., Reidy J.A., Needham L.L., Calafat A.M. Quantification of 22 Phthalate Metabolites in Human Urine. J. Chromatogr. B. 2007;860:106–112. doi: 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 41.Zhou X., Kramer J.P., Calafat A.M., Ye X. Automated On-Line Column-Switching High Performance Liquid Chromatography Isotope Dilution Tandem Mass Spectrometry Method for the Quantification of Bisphenol A, Bisphenol F, Bisphenol S, and 11 Other Phenols in Urine. J. Chromatogr. B. 2014;944:152–156. doi: 10.1016/j.jchromb.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Caudill S.P., Schleicher R.L., Pirkle J.L. Multi-Rule Quality Control for the Age-Related Eye Disease Study. Stat. Med. 2008;27:4094–4106. doi: 10.1002/sim.3222. [DOI] [PubMed] [Google Scholar]
- 43.Stinshoff K., Weisshaar D., Staehler F., Hesse D., Gruber W., Steier E. Relation between Concentrations of Free Glycerol and Triglycerides in Human Sera. Clin. Chem. 1977;23:1029–1032. doi: 10.1093/clinchem/23.6.1029. [DOI] [PubMed] [Google Scholar]
- 44.Allain C.C., Poon L.S., Chan C.S.G., Richmond W., Fu P.C. Enzymatic Determination of Total Serum Cholesterol. Clin. Chem. 1974;20:470–475. doi: 10.1093/clinchem/20.4.470. [DOI] [PubMed] [Google Scholar]
- 45.Rifai N., Cole T.G., Iannotti E., Law T., Macke M., Miller R., Dowd D., Wiebe D.A. Assessment of Interlaboratory Performance in External Proficiency Testing Programs with a Direct HDL-Cholesterol Assay. Clin. Chem. 1998;44:1452–1458. doi: 10.1093/clinchem/44.7.1452. [DOI] [PubMed] [Google Scholar]
- 46.Roberts W.C. The Friedewald-Levy-Fredrickson Formula for Calculating Low-Density Lipoprotein Cholesterol, the Basis for Lipid-Lowering Therapy. Am. J. Cardiol. 1988;62:345–346. doi: 10.1016/0002-9149(88)90248-2. [DOI] [PubMed] [Google Scholar]
- 47.Lapidus N., Chevret S., Resche-Rigon M. Assessing Assay Agreement Estimation for Multiple Left-censored Data: A Multiple Imputation Approach. Stat. Med. 2014;33:5298–5309. doi: 10.1002/sim.6319. [DOI] [PubMed] [Google Scholar]
- 48.Bobb J.F., Valeri L., Claus Henn B., Christiani D.C., Wright R.O., Mazumdar M., Godleski J.J., Coull B.A. Bayesian Kernel Machine Regression for Estimating the Health Effects of Multi-Pollutant Mixtures. Biostatistics. 2015;16:493–508. doi: 10.1093/biostatistics/kxu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keil A.P., Buckley J.P., O’Brien K.M., Ferguson K.K., Zhao S., White A.J. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environ. Health Perspect. 2020;128:047004. doi: 10.1289/EHP5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.World Health Organization Obesity and Overweight. [(accessed on 24 October 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- 51.Wen Q., Zhou Y., Wang Y., Li J., Zhao H., Liao J., Liu H., Li Y., Cai Z., Xia W. Association between Urinary Paraben Concentrations and Gestational Weight Gain during Pregnancy. J. Expo. Sci. Environ. Epidemiol. 2020;30:845–855. doi: 10.1038/s41370-020-0205-7. [DOI] [PubMed] [Google Scholar]
- 52.Kodani S.D., Overby H.B., Morisseau C., Chen J., Zhao L., Hammock B.D. Parabens Inhibit Fatty Acid Amide Hydrolase: A Potential Role in Paraben-Enhanced 3T3-L1 Adipocyte Differentiation. Toxicol. Lett. 2016;262:92–99. doi: 10.1016/j.toxlet.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parikh N.I., Cnattingius S., Mittleman M.A., Ludvigsson J.F., Ingelsson E. Subfertility and Risk of Later Life Maternal Cardiovascular Disease. Hum. Reprod. 2012;27:568–575. doi: 10.1093/humrep/der400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Braun J.M., Smith K.W., Williams P.L., Calafat A.M., Berry K., Ehrlich S., Hauser R. Variability of Urinary Phthalate Metabolite and Bisphenol A Concentrations before and during Pregnancy. Environ. Health Perspect. 2012;120:739–745. doi: 10.1289/ehp.1104139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bai J., Ma Y., Zhao Y., Yang D., Mubarik S., Yu C. Mixed Exposure to Phenol, Parabens, Pesticides, and Phthalates and Insulin Resistance in NHANES: A Mixture Approach. Sci. Total Environ. 2022;851:158218. doi: 10.1016/j.scitotenv.2022.158218. [DOI] [PubMed] [Google Scholar]
- 56.Bellavia A., Chiu Y.-H., Brown F.M., Mínguez-Alarcón L., Ford J.B., Keller M., Petrozza J., Williams P.L., Ye X., Calafat A.M., et al. Urinary Concentrations of Parabens Mixture and Pregnancy Glucose Levels among Women from a Fertility Clinic. Environ. Res. 2019;168:389–396. doi: 10.1016/j.envres.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Génard-Walton M., McGee G., Williams P.L., Souter I., Ford J.B., Chavarro J.E., Calafat A.M., Hauser R., Mínguez-Alarcón L. Mixtures of Urinary Concentrations of Phenols and Phthalate Biomarkers in Relation to the Ovarian Reserve among Women Attending a Fertility Clinic. Sci. Total Environ. 2023;898:165536. doi: 10.1016/j.scitotenv.2023.165536. [DOI] [PubMed] [Google Scholar]
- 58.Nguyen H.D., Oh H., Kim M.-S. The Effects of Chemical Mixtures on Lipid Profiles in the Korean Adult Population: Threshold and Molecular Mechanisms for Dyslipidemia Involved. Environ. Sci. Pollut. Res. 2022;29:39182–39208. doi: 10.1007/s11356-022-18871-2. [DOI] [PubMed] [Google Scholar]
- 59.Zhan W., Yang H., Zhang J., Chen Q. Association between Co-Exposure to Phenols and Phthalates Mixture and Infertility Risk in Women. Environ. Res. 2022;215:114244. doi: 10.1016/j.envres.2022.114244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are not publicly available due to privacy and confidentiality reasons.