Abstract
The utilization of full-fat high-oleic soybean meal in layer diets could lead to value-added poultry products. To test this idea, 336 hens were randomly assigned to 4 isonitrogenous (18.5% CP) and isocaloric (2,927 kcal/kg) formulated diets and fed the following diets for eight weeks: conventional control solvent-extracted defatted soybean meal (CON); extruded-expelled defatted soybean meal (EENO); full fat normal-oleic soybean meal (FFNO); or full fat high-oleic soybean meal (FFHO). Body weights (BW) were collected at week 0 and week 8. Eggs were collected daily, and the totals counted each week. Feed consumption was measured weekly, and egg quality was measured bi-weekly. Eggs were collected at wk 0 and wk 8 for fatty acid analysis. There were no significant treatment differences in any of the production parameters measured, BW, feed consumption, feed conversion ratio or egg production (P > 0.05). Eggshell strength was significantly greater in eggs produced from the EENO group as compared to the control (P < 0.01), while egg yolk color was significantly darker in eggs of the control and EENO treatment groups relative to the FFNO and FFHO treatments (P < 0.0001). Eggs produced by hens fed the FFHO diet had a 52% increase in monounsaturated n-9 oleic acid content (P < 0.0001) and reduced palmitic (P < 0.01) and stearic (P < 0.0001) saturated fatty acid levels as compared to the conventional controls. These results validate the utilization of FFHO as a value-added poultry feed ingredient to enrich the eggs and/or poultry meat produced.
Key words: layer, high-oleic soybean, oilseed, alternative poultry feed ingredient, egg
INTRODUCTION
The U.S. poultry and swine industry utilizes approximately 60% of U.S. produced commercial defatted soybean meal annually (United Soybean Board, 2020). Poultry feeding trials have demonstrated the effective use of protease supplemented diets containing full-fat soybean meal prepared from whole normal-oleic soybeans (Park et al. 2017; Erdaw et al. 2017a, 2018; Karimi et al. 2022). However, few studies have examined the use of full-fat high-oleic soybean meal in the diets of poultry. Weld et al. (2018) demonstrated that dairy cows fed whole high-oleic soybeans had an increase in milk fat secretion compared to whole normal-oleic soybeans. Moreover, layer and broiler feeding trials conducted within the Food Science & Market Quality and Handling Research Unit, Agricultural Research Service, U.S. Department of Agriculture (Raleigh, NC) demonstrated an approximate 2-fold increase in yolk color, β-carotene and oleic fatty acid content in eggs, with a reduction in saturated and trans-fatty acids in eggs (Toomer et al., 2019) and chicken breast (Toomer et al., 2021) produced from birds fed whole high-oleic peanuts.
Traditionally, defatted soybean meal and supplemental dietary vegetable oil are commonly utilized in poultry and livestock feed within the U.S. However, full-fat soybean meal could potentially be utilized in animal food production as a replacement to these feed ingredients.
Conventional soybean oil has a fatty acid profile of 11 % palmitic acid (16:0), 4 % stearic acid (18:0), 25 % oleic acid (18:1), 52 % linoleic acid (18:2), and 7 % linolenic acid (18:3) (Fehr 2007). These relatively high levels of linoleic and linolenic acid in conventional soybean oil cause low oxidative stability and rapid rancidity (Warner and Fehr, 2008). As a result, industry began the process of hydrogenation, in which hydrogen atoms are added to some of the double bonds of the unsaturated fatty acids (Shurtleff and Aoyagi, 2007) in the oil to reduce the low oxidative stability and rapid rancidity of conventional soybean oil for end use industrial food applications. Nonetheless, the hydrogenation process introduced artificial trans fats in conventional soy oil, which has been shown to increase the risk of obesity and heart disease (Mozaffarian et al. 2009) and in 2018 the US. Food and Drug Administration banned partially hydrogenated oils containing artificial trans-fats in food products (US. Food and Drug Administration, 2024). As a result, soybean oil with a high-oleic acid and low linolenic acid profile has become more desirable due to improved oxidative stability, increased shelf-life and reduced artificial trans-fats.
High-oleic soybeans are resultant of molecular genetic mutations of microsomal Delta 12 fatty acid desaturase enzymes FAD2-1A (Glyma10g42470) and FAD2-1B (Glyma20g24530) which are responsible for the step conversion of the precursor oleic acid (18:1) to linoleic (18:2) in the soybean seed lipid biosynthesis pathway (Schlueter et al., 2007; Pham et al., 2010). Hence, these 2 key genes have been the target of soybean breeding programs aimed at altering the oleic acid content in soybeans (Tang et al., 2005; Pham et al., 2010). High-oleic soybeans launched commercially in 2012 and currently grown in 13 states throughout the U.S with more than 800,000 acres of high-oleic soybeans grown in 2022, with varieties such as Plenish from Corteva, Vistive Gold from Bayer and SOYLEIC (United Soybean Board, 2024).
Currently, the U.S. food industry is the top end user of more than 70% of high-oleic soybean oil in food manufacturing. Therefore, we aim to conduct applied research studies to increase the utilization of high-oleic oilseeds beyond edible human foods and to identify new uses within animal food production markets. In this study we aim to determine the value-added utilization of high-oleic soybeans to produce FFHO for use as a feed ingredient to enhance the performance of food production animals and to enrich the nutritional value of the products produced for human consumption. To achieve this, we aimed to determine the effects of feeding full-fat high-oleic soybean meal to layers on performance, egg quality and fatty acid composition in an 8-week feeding trial.
High-oleic soybean oil containing approximately 75% oleic acid, 13% linoleic acid and 4% linolenic acid (U.S. Soy, 2021) has been used in the diets of swine to extend the shelf product life and fry life of pork due to its increased oxidative stability as a dietary fat source compared to conventional normal-oleic soybean oil with 51% linoleic acid and 23% oleic acid (Knowlton, 2022; Gaffield et al. 2022a). Interestingly, swine feed supplemented with high-oleic soybean oil had increased visual marbling in loin chops and increased red color (a*) in loin chops compared to loin chops from pigs fed a diet containing dried distiller's grains with solubles (Gaffield et al., 2022a). Saturated fatty acid level was greatest in meat produced from pigs fed the DDGS diets as compared to the meat produced from pigs fed diets containing high-oleic soybean oil (Gaffield et al., 2022a).
Studies conducted in layers fed diets containing high-oleic soybean oil (40 g/kg diet) and co-supplemented with flaxseed oil (40 g/kg diet), reported elevated oleic acid levels and reduced omega 3 fatty acid levels in egg yolks as compared to the egg yolks produced by hens fed a flaxseed oil supplemented diet alone (Elkin et al., 2018). These results parallel high-oleic peanut layer feeding trials demonstrating that eggs produced from hens fed a 20% high-oleic peanut diet had reduced omega 3 fatty acid content and elevated oleic acid content as compared to eggs produced from hens fed a conventional layer diet (Toomer et al., 2019). Interestingly, the stearic acid, linoleic acid, and total omega 6 fatty acid levels were similar in eggs produced by layers fed a high-oleic peanut diet (20%) and an oleic acid oil supplemented diet (3%) in an 8-week layer feeding trial. In contrast the palmitic acid, oleic acid, linolenic acid, and omega 3 fatty acids levels were significantly different between these 2 treatment groups (Toomer et al., 2021), suggesting that dietary supplementation with oil extracted from high-oleic oilseeds may have differing effects on egg enrichment as compared to feeding whole high-oleic oilseeds or full-fat high-oleic meals.
While a number of animal feeding trials have investigated the dietary supplementation of oil extracted from high-oleic oilseeds in the diets of production animals (Martínez-Marín et al., 2012; Toomer et al., 2021; Elkin et al., 2018; Gaffield et al.,2022a, 2022b), there are few published poultry or animal feeding trials investigating the effects of full-fat high-oleic soybean meal (FFHO) on performance, meat or egg quality and nutritional content of the meat or eggs produced and intended for human consumption.
MATERIALS AND METHODS
Experimental Design, Animal Husbandry and Dietary Treatments
All methods and procedures used for animal research in this feeding trial were approved by the North Carolina State University Institutional Animal Care and Use Committee (19-761-07-A) following an accredited internal research animal protocol review set forth by the Association for Assessment and Accreditation of Laboratory Animal Care Institution accreditation program.
Soybean Analysis, Processing, Meal Preparation and Experimental Diets
Near isogenic lines of normal oleic soybean (<25% oleic acid, >7% linolenic –USDA NC-Roy) and high-oleic soybean (>80% oleic acid, <2% linolenic-USDA N16-1286 BC4 NIL) cultivars were bred and harvested by the U.S. Department of Agriculture, Agriculture Research Service, Soybean and Nitrogen Fixation Research Unit, ARS (SNFRU, Raleigh, NC). Upon harvesting, all foreign material was removed using an Eclipse 324 seed and grain cleaner (Seedburo, Equipment Company, Des Plaines, IL) and all whole soybeans were dried to approximately 10% moisture using ambient temperature and natural air drying.
Soybean sub-samples were analyzed for mycotoxins using standard methodologies (vomitoxin, aflatoxin, fumonisin, ochratoxin, T-2 toxin, zearalenone) and fatty acid composition at a commercial laboratory (ATC Scientific, Little Rock, AR) using AOAC-approved methods. Vomitoxin analysis was conducted using high-performance liquid chromatography (Schweighardt et al., 1980) with DONtest reference column purchased from Vicam (Watertown, MA). AOAC official method 991.31 (2002) was utilized for the determination of aflatoxins B1, B2, G1, and G2 using immunoaffinity column cleanup with liquid chromatography. AOAC official method 2001.04 (2001) was utilized for the determination of fumonisin using high-performance liquid chromatography (HPLC) methods. Ochratoxin, T-2 toxin, and zearalenone levels were determined by modified HPLC- Mass Spectrometry (MS)/MS methods (Zhang et al., 2023) using respective HPLC reference columns purchased from Vicam (Watertown, MA). Levels of mycotoxins in the soybean sub-samples were below the detection thresholds (Vomitoxin <0.10 ppm, Aflatoxin <2.0 ppb, Fumonisin <100.0 ppb, Ochratoxin < 1.0 ppb, T-2 Toxin <25.0 ppb, Zearalenone <100.0 ppb) and the proximate composition and fatty acid analysis were within the expected parameters. The nutrient and energy values of the soybeans were obtained by the NC State University Feed Mill (Raleigh, NC) using near-infrared spectroscopy (Pérez-Marın et al., 2004) and AOAC-approved methods (Crude fat-AOAC 954.02 (2023) acid hydrolysis, fatty acid-AOCS Ce 2-66 / Ce 1e 91 and AOAC 996.06 (2008)) at a commercial laboratory (ATC Scientific, Little Rock, AR). Gross energy was also performed by ATC Scientific using an adiabatic oxygen bomb calorimeter with standard methods. A total of 5 replicates per soybean source were analyzed to obtain near-infrared spectroscopy (NIRS) values, and the average value was used in the diet formulation. Levels of mycotoxins in the soybean sub-samples were below the detection thresholds for each analysis, the proximate composition, and fatty acid analysis were within the parameters expected (Table 1).
Table 1.
Proximate composition from normal-oleic and high-oleic raw soybean cultivars.
| Parameters | Normal-oleic1 | High-oleic1 |
|---|---|---|
| Crude protein (%) | 36.6 | 38.15 |
| Crude fat (%) | 17.94 | 16.38 |
| Gross energy(kcal/kg) | 5238 | 5236 |
| Palmitic acid (%) | 10.5 | 6.92 |
| Stearic acid (%) | 2.89 | 0.58 |
| Oleic acid (%) | 19.5 | 81.53 |
| Linoleic acid (%) | 51.56 | 4.76 |
High-oleic: high-oleic and low linoleic soybean cultivar N16-1286 BC4 NIL; Normal oleic=NC-Roy variety normal oleic and normal linolenic. A total of 5 replicates per soybean source was analyzed to obtain all values. Near-infrared spectroscopy (NIRS) was utilized to determine crude protein, and standard AOAC methods at a commercial laboratory (ATC Scientific (Little Rock, AR, USA) were used to determine crude fat, gross energy, and fatty acids. The average values were used in the diet formulation.
Crude Protein content = g crude fat/g total sample weight * 100; Crude Fat content = g crude protein/g total sample weight * 100. Fatty acid content (palmitic acid, stearic acid, oleic acid, linoleic acid) = g of fatty acid/g total lipid content * 100.
Solvent extracted defatted soybean meal for use in the control diets was purchased commercially from Perdue Agribusiness (Cofield, NC). The production of the 3 different soybean meals was done at a local commercial feed mill, Mule City Feeds (Benson, NC). Whole soybeans having a moisture content of 10±0.5% were coarsely ground using a hammer mill to break the soybeans roughly into 4 pieces. The broken soybeans were fed through a hopper to a single screw dry extruder (InstaPro 2000 R, Grimes, IA). The soybeans exited the extruder through a die which had a temperature of 155°C to produce the full-fat normal oleic, full-fat high-oleic soybean meal. Further, a portion of the full-fat normal oleic soybean meal was diverted to a mechanical expeller using an inclined cleated conveyor belt to extract the oil to produce extruded-expelled normal oleic soybean. Sub-samples of each of the 4 soybean meals (Control-solvent extracted defatted soybean meal, EENO-extruder expelled soybean meal, FFNO-full-fat normal-oleic soybean meal, FFHO-full-fat high-oleic soybean meal) were analyzed using 3 replicates for particle size (PS) using the ANSI/ASAE S319.4 “Method of determining and expressing fineness of feed materials by sieving” standard using 13 sieves and an electric sieve shaker (ANSI). The lowest particle size was 950 µm for SENO, therefore the particle size of all other experimental SBMs produced were reduced to approximately the same PS, using a 2-pair roller mill (Model C128889, RMS, Sea, SD) at 2 different roller settings. A roller mill was used instead of the commonly used hammer milling to prevent clogging of the sieves of the hammer mill due to its inherent oil content. The 50:50 setting was used for FFNO and FFHO; while the particle size reduction of EENO was achieved by setting 50:25. Both settings had a roller pair spacing that ranged from 0.03 inch (0.76 mm) at setting 0 to 0.09 inch (228.6 mm) at setting 50. After grinding, the soybean meal sub-samples were analyzed for chemical composition (protein, lipid, anti-nutritional factors, amino acid, and fatty acid content) and is shown in Table 2.
Table 2.
Chemical composition of experimental soybean meals postproduction.
| Type of soybean meal |
||||
|---|---|---|---|---|
| Parameters | SE-SBM | EENO | FFNO | FFHO |
| 1Crude protein (%) | 45.7 | 43.8 | 39.6 | 39.9 |
| 1Crude fat (%) | 4.75 | 7.12 | 17.3 | 15.5 |
| 1Crude fiber (%) | 5.50 | 7.20 | 6.90 | 7.90 |
| 1Crude ash (%) | 6.16 | 6.02 | 5.39 | 5.10 |
| 1Moisture content (%) | 10.0 | 5.58 | 5.43 | 7.83 |
| UAI (ΔpH) | 0.07 | 0.06 | 0.29 | 0.27 |
| Trypsin inhibitor (mg/g) | 2.40 | 7.64 | 7.99 | 6.92 |
| Gross energy (kcal/kg) | 4105 | 4598 | 4863 | 4890 |
| Palmitic acid (%) | 14.1 | 11.3 | 11.1 | 7.74 |
| Stearic acid (%) | 3.67 | 3.68 | 3.55 | 3.11 |
| Oleic acid (%) | 14.3 | 19.7 | 18.0 | 71.7 |
| Linoleic acid (%) | 55.9 | 53.2 | 55.2 | 11.0 |
SE-SBM: solvent extracted defatted soybean meal commercially purchased from Perdue Agribusiness (Cofield, NC).
EENO: extruder expelled normal oleic soybean meal.
FFNO: full-fat normal-oleic soybean meal.
FFHO: full-fat high-oleic soybean meal.
Solvent extracted defatted soybean meal was purchased commercially from Perdue Agribusiness (Cofield, NC, USA). Soybean meals (EENO, FFNO and FFHO) were manufactured at Mule City Feeds (Benson, NC, USA). The proximate composition and fatty acid analysis of the various meals was analyzed by a commercial lab (ATC Scientific, Little Rock, AR, USA) using standard AOAC-approved methods. UAI: Urease Activity Index-measured as the change in pH.
Fatty acid content (palmitic acid, stearic acid, oleic acid, linoleic acid) = g of fatty acid/g total lipid content * 100.
1Parameters measured as g/g total sample weight * 100
Subsequently, 4 experimental diets (control diet with solvent extracted defatted soybean meal, extruded-expelled soybean meal diet, full-fat normal oleic soybean meal diet, full-fat high-oleic soybean meal) were formulated to be isocaloric (2927 kcal/kg) and isonitrogenous (18.5% crude protein) using Concept 5 (level 2, version 10.0) software to meet and/or exceed the nutrient requirements for layers (Table 3). Three hundred and thirty-six (6 replicates per treatment, 14 birds per replicate) White Shaver hens at 36 wk of lay were randomly assigned to one of 4 experimental diets 1) control (solvent extracted defatted soybean meal/corn diet), 2) extruded-expelled normal-oleic defatted soybean meal/corn diet (EENO), 3) full-fat normal-oleic soybean meal/corn diet (FFNO), 4) full-fat high-oleic soybean meal/corn diet (FFHO). Hens were housed individually in cages 12 inches wide × 18 inches deep × 23.5 inches tall with 14:10 L:D and provided water and feed ad libitum for 8 wk in House 8 at the Chicken Education Unit, NC State University (Raleigh, NC). Body weights were individually recorded for each hen at wk 0 and 8, with feed weights recorded bi-weekly. Shell eggs were collected daily, labeled, and stored at 4°C in a walk-in cooler. At the end of each week the total number of collected eggs was counted for each replicate and treatment. Feed intake was calculated as the 8-week daily average feed consumed per bird (56 d). Feed Conversion Ratio (FCR) was calculated as the average 8-wk feed consumed (grams)/8-wk average egg weight (grams) for each treatment. At wk 0 and wk 8, 6 pooled egg samples were collected from each treatment (24 pooled egg samples total at each time point) with each pool consisting of 12 homogenously combined whole eggs (2 eggs from each replicate). Crude fat (AOAC 954.02 acid hydrolysis), total cholesterol (AOAC 976.26, 2023), and fatty acid (AOCS Ce 2-66 / Ce 1e 91 (AOAC 996.06, 2008) analysis were conducted by ATC Scientific (Little Rock, AR) using AOAC-approved methods.
Table 3.
Composition of formulated experimental laying hen diets.1,*
| Feed ingredient, % | Control | EENO | FFNO | FFHO |
|---|---|---|---|---|
| Yellow corn | 57.9 | 58.1 | 57.9 | 58.4 |
| Soybean meal | 20.6 | 21.4 | 24.6 | 23.9 |
| Calcium carbonate | 9.69 | 9.72 | 9.69 | 9.69 |
| Dicalcium phosphate | 1.64 | 1.62 | 1.59 | 1.59 |
| Corn gluten meal | 3.75 | 3.75 | 3.22 | 3.46 |
| Sodium bicarbonate | 0.15 | 0.15 | 0.20 | 0.20 |
| Sodium chloride | 0.25 | 0.25 | 0.23 | 0.25 |
| DL-methionine | 0.10 | 0.12 | 0.12 | 0.12 |
| Pro Fam 974 (soy protein) | 2.00 | 2.00 | 1.97 | 2.00 |
| Soybean oil | 3.51 | 2.48 | 0.00 | 0.00 |
| Santoquin2 | 0.05 | 0.05 | 0.05 | 0.05 |
| Choline chloride | 0.07 | 0.06 | 0.06 | 0.07 |
| Trace mineral premix3 | 0.20 | 0.20 | 0.20 | 0.20 |
| Vitamin premix4 | 0.05 | 0. 05 | 0.05 | 0.05 |
| Selenium premix5 | 0.05 | 0.05 | 0.05 | 0.05 |
| Metabolizable energy (kcal/kg) | 2927 | 2927 | 2927 | 2927 |
Four experimental isonitrogenous (18.5% crude protein) diets were formulated: Control=conventional diet containing solvent extracted defatted soybean meal and corn; EENO=diet containing extruded-expelled defatted normal-oleic soybean meal and corn; FFNO = diet containing full fat normal-oleic soybean meal and corn; FFHO = diet containing full fat high-oleic soybean meal and corn
Santoquin®= Feed antioxidant and preservative to prevent fat oxidation in stored feed (Novus International, St. Charles, MO).
Mineral premix provides per kg of diet: manganese, 120 mg; zinc, 120 mg; iron, 80 mg; copper, 10 mg; iodine, 2.5 mg; and cobalt 500ppm.
Vitamin premix provides per kg of diet: 13,200 IU vitamin A, 4000 IU vitamin D3, 33 IU vitamin E, 0.02 mg vitamin B12, 0.13 mg biotin, 2 mg menadione (K3), 2 mg thiamine, 6.6 mg riboflavin, 11 mg d-pantothenic acid, 4 mg vitamin B6, 55 mg niacin, and 1.1 mg folic acid.
Selenium premix=1 mg Selenium premix provides 0.2 mg Se (as Na2SeO3) per kg of diet.
ME = metabolizable energy (kcal/kg).
Egg Quality
Egg quality was assessed at wk 0, 2, 4, and 6 using a 120 sub-sample of eggs randomly selected from each treatment (6 eggs/replicate) in the Egg Quality Lab, Prestage Department Poultry Science, NC State University (Raleigh, NC, USA). Egg quality parameters measured included shell strength, vitelline membrane elasticity (VME), egg weight, albumen height, Haugh unit (HU), yolk color, shell color, and shell thickness. Eggshell strength was determined using the TA-HDplus texture analyzer (Stable Micro Systems, Surrey, UK) with a 250 kg load cell measuring in grams of force. The TA-HDplus has a trigger force of 0.02 kg and a testing speed of 1 mm/s. Vitelline membrane elasticity (VME) was measured using methods described in the manufacturers’ instructions for the TA.XTplus Texture Analyzer (Stable Micro Systems, Surrey, UK) and modified methods described by (Anderson et al., 2011) with a 1mm blunt probe and 500-gram load cell. The trigger force was 0.0001 kg with a 3.2 mm/s testing speed. HU was calculated using the following calculation: HU = 100*Log(h - 1.7w + 7.6), with h = egg albumen height (mm) and w=weight of egg (g). Values range from 0 to 130, and HU scores below 60 indicate un-fresh eggs (Nematinia et al. 2018). Yolk color was also determined using the TSS QCD System yolk color scan. The yolk color scan was calibrated using the DSM Yolk Color Fan that determines color density from lightest to darkest with a scale of 1 to 15 (Vuilleumier, 1969). Shell color was determined using refractometry of black, blue, and red wavelengths combined to score 83.3% (white) to 0% (black).
Statistical Analysis
Linear mixed-effects models were fit to the data to assess the effect of diet treatment on each response variable. Experimental diet treatment was included in the model as a fixed effect. For response variables that were measured on each individual at week 8 only or summed across the entire measurement period (body weight, absolute liver weight, liver weight relative to body weight, and total number of eggs produced), we included a random intercept for each row and for each replicate nested within the row. For response variables that were measured for each replicate at week 8 only or summed across the entire measurement period (total feed intake, feed conversion ratio, and all individual and total fatty acid concentrations), we included only a random intercept for each row. Variables on a ratio scale (relative liver weight and feed conversion ratio) were logarithmically transformed before model fitting. Individual fatty acid concentrations were transformed with an ln(x+0.01) transformation. Logarithmic transformations were done because multiplicative changes in ratios or concentrations are more biologically meaningful than additive changes. Egg quality variables that were measured bi-weekly (shell strength, elasticity, vitelline membrane elasticity, shell color, egg weight, albumen height, and Haugh units) were also analyzed with linear mixed-effects models with the fixed effect of treatment and random intercepts for row and replicate nested within row. Roche yolk color score, which is an ordered categorical variable ranging from 1 to 8, was analyzed with a cumulative link mixed model with treatment as a fixed effect and random intercepts for row and replicate nested within row.
In all cases, we conducted an analysis of variance (ANOVA) using Type III sum of squares and Satterthwaite's method to estimate denominator degrees of freedom for the F distribution. For the Roche yolk color model, we conducted a chi-squared likelihood ratio test comparing the model that includes treatment as a fixed effect with a null model including only the random effects of row and replicate. For all response variables, we estimated marginal means for each diet treatment and did pairwise t-tests comparing each pair of treatments, using the Kenward-Roger approximation of the degrees of freedom. The Sidak adjustment was used to correct the p-values and 95% confidence intervals to account for multiple comparisons (4 treatments resulting in 6 pairwise comparisons). For the case of Roche yolk color, we estimated the marginal means of each treatment by taking an average per treatment of the scores on the color scale weighted by the marginal mean probability of each score, then compared the means between treatments with z-tests and adjusted for multiple comparisons with the Tukey adjustment. Principal component analysis was done on the scaled fatty acid concentration variables to determine whether the bulk of the variation in overall fatty acid profile between the 4 diet groups could be explained with fewer dimensions. A 95% confidence ellipse around the centroid of each treatment was calculated for plotting purposes. Analyses were conducted using R software version 4.1.2 (R Core Team, 2021), including the lme4 (Bates et al., 2015), lmerTest (Kuznetsova et al., 2017), emmeans (Lenth, 2022), multcomp (Hothorn et al., 2008), and ordinal (Christensen, 2019) packages.
RESULTS AND DISCUSSION
Crude protein (normal-oleic-37%, high-oleic-38%), crude fat (normal-oleic-18%, high-oleic-16%), and gross energy (normal-oleic-5238 kcal/kg, high-oleic-5236 kcal/kg) levels were similar between the normal-oleic and high-oleic whole unprocessed soybeans used in preparation of the various soybean meals (Table 1). However, levels of palmitic acid (normal-oleic-11%, high-oleic-6.9%), stearic acid (normal-oleic-2.9%, high-oleic-0.58%), oleic acid (normal-oleic-20%, high-oleic-82%) and linoleic acid (normal-oleic-52%, high-oleic-4.8%) were dissimilar between the 2 soybean cultivars used. Hence, while normal-oleic and high-oleic soybeans have similar levels of crude fat, the fatty acid composition is modified between the 2 cultivars.
In parallel, FFHO soybean meal prepared from high-oleic soybeans had lower levels of palmitic acid, and linoleic acid with higher levels of oleic acid relative to the EENO and FFNO soybean meals prepared from the normal-oleic soybeans (Table 2). Crude protein, crude fiber, crude ash, and gross energy levels were relatively similar between the 4 types of soybean meals. All 4 soybean meal varieties had low urease activity (0.3 or below) and within conventional quality control standards (University of Georgia-Athens Extension, 2023). Trypsin inhibitor levels were highest in the EENO, FFNO, and FFHO soybean meals (range 7.0 to 8.0%) relative to the control soybean meal (solvent extracted defatted soybean meal) and were above the recommended level of 4mg/g (Erdaw et al., 2017b).
All experimental diets were formulated to be isonitrogenous (18.5% crude protein) and isocaloric (metabolizable energy of 2927 kcal/kg). All 4 experimental diets contained yellow corn (58% inclusion), soybean meal source (21–25% inclusion), 60% crude protein corn gluten meal (3.22–3.75% inclusion) and soy protein (2.0%). Supplemental soybean oil was included in the control (3.5% inclusion) and EENO (2.48% inclusion) experimental diets to balance energy (Table 3). Chemical analysis revealed that the 4 experimental diets ranged in crude fat content between 5% and 6%, crude protein content between 17% and 19%, with each experimental diet providing approximately 4,000 kcal/kg gross energy (Table 4). In parallel to the nutrient analysis of the FFHO soybean meal (Table 2), the FFHO experimental diet had higher levels of oleic acid (Table 4) relative to the other treatment groups.
Table 4.
Chemical analysis and absolute fatty acid content of experimental layer diets.1
| Analyzed values | Control | EENO | FFNO | FFHO |
|---|---|---|---|---|
| Crude fat2, % | 5.28 | 5.76 | 4.63 | 6.09 |
| Crude protein2, % | 19.1 | 18.2 | 17.2 | 17.3 |
| Gross energy, kcal/kg | 3966 | 3976 | 4079 | 4007 |
| AMEn, kcal/kg | 2578 | 2518 | 2524 | 2622 |
| *Palmitic (C16:0), g/100g | 0.59 | 0.63 | 0.52 | 0.47 |
| *Stearic (C18:0), g/100g | 0.19 | 0.21 | 0.16 | 0.18 |
| *Saturated fat, g/100g | 0.84 | 0.91 | 0.73 | 0.73 |
| *Omega 3 fatty acids, g/100g | 0.39 | 0.44 | 0.36 | 0.16 |
| *Omega 6 fatty acids, g/100g | 2.91 | 3.16 | 2.56 | 0.70 |
| *Omega 9 fatty acids, g/100g | 1.11 | 1.22 | 0.96 | 4.46 |
| *Trans fats, g/100g | 0.001 | 0.001 | 0.002 | 0.001 |
| *Oleic acid, C 18:1, g/100g | 1.08 | 1.19 | 0.94 | 4.44 |
| *Linoleic (C18:2), g/100g | 2.89 | 3.16 | 2.55 | 0.69 |
All data represent the mean of the chemical analysis of 3 individual samples from the finished feed of the 4 experimental diets. Four dietary treatments were chemically analyzed by an AOAC-certified lab, (ATC Scientific, Little Rock, AR, USA) using standard AOAC-approved methods.
Dietary treatments: Control=conventional diet containing solvent extracted defatted soybean meal and corn; EENO=diet containing extruded-expelled defatted normal-oleic soybean meal and corn; FFNO=diet containing full fat normal-oleic soybean meal and corn; FFHO=diet containing full fat high-oleic soybean meal and corn.
Crude Fat content = g crude fat/g total sample weight * 100; Crude Protein content = g crude protein/g total sample weight * 100
Absolute fatty acid content (g/100g diet) = (g of fatty acid/g total lipid content) * % crude fat.
There were no significant differences in the production parameters measured, average body weights (at wk 8), feed intake, feed conversion ratio (FCR), or egg production between the treatment groups (Table 5, P ≥ 0.05). Moreover, there were no significant treatment differences in the relative liver weights at termination (P ≥ 0.05). These results parallel other layer feeding trials demonstrating that feeding full-fat soybean meal in layer diets does not adversely affect hen body weights, feed conversion ratio or egg production (Maharjan et al. 2023). Similarly, Elkin et al. (2018) demonstrated that supplementing the diets of layers with high-oleic soybean oil did not adversely impact production performance parameters (egg production, egg weights, feed efficiency). Also, feeding high-oleic peanuts to layers did not adversely affect hen's 8-wk body weights (Toomer et al. 2021), 8-wk feed conversion ratio (Redhead et al. 2021; Toomer et al. 2021) or hen egg production (Redhead et al. 2021).
Table 5.
Comparative body weights of hens fed a full-fat high-oleic soybean meal diet.1
| Treatments | BW2 (kg) | Relative Liver Weight3 | Feed intake4 (gm) |
FCR5 (gm feed/gm egg mass) |
Ave hen house egg production6 (%) |
|---|---|---|---|---|---|
| Control | 1.64 | 0.02 | 117 | 1.90 | 99.8 |
| EENO | 1.62 | 0.02 | 111 | 1.82 | 98.3 |
| FFNO | 1.62 | 0.02 | 115 | 1.96 | 98.5 |
| FFHO | 1.61 | 0.02 | 111 | 1.84 | 98.4 |
| SEM | 0.02 | 0.001 | 2.74 | 0.05 | 0.8 |
| P-value | 0.76 | 0.10 | 0.15 | 0.06 | 0.56 |
Three hundred and thirty-six hens were fed 4 diets (18.5% crude protein, 2927kcal/kg), 6 replicates/treatment for 8 weeks. Diets: Trmt1-Control=conventional diet containing solvent extracted defatted soybean meal and corn; Trmt2-EENO=diet containing extruded-expelled defatted normal-oleic soybean meal and corn; Trmt3-FFNO = diet containing full fat normal-oleic soybean meal and corn; Trmt4-FFHO = diet containing full fat high-oleic soybean meal and corn.
Eight-week average body weights.
Relative Liver Weight Ratio= weight of fresh liver (g)/final layer carcass body weight (g).
Average daily 8-week feed consumed per bird (56 d).
Feed Conversion Ratio (FCR)= average 8-week feed consumed (grams)/average 8 average egg weight (grams).
Eight-week average House Egg Production= total number of eggs produced per bird over number of days fed each dietary treatment.
*P-value= statistically significant treatment effect (P < 0.05) from analysis of variance.
a,b,cMeans within the same column lacking a common superscript differ significantly (P < 0.05) after adjusting for multiple comparisons.
The 8-wk average of each egg quality parameter (egg weight, vitelline membrane elasticity, shell color, shell strength, albumen height, Haugh Unit, yolk color) measured at wk 2, 4, 6, and 8, is represented in Table 6. There were no significant (P > 0.05) treatment differences in the following egg quality parameters: shell color, vitelline membrane elasticity, albumen height and Haugh Unit. Haugh Unit is the most widely used measurement of egg white quality (Stadelman, 1995), with higher Haugh Unit values indicating fresher higher quality eggs. This suggests that all eggs produced in this study had similar freshness and egg white quality. Vitelline membrane mechanical properties are an important measure for egg freshness with vitelline membrane elasticity as an important measured parameter during egg breaking operations for intact separation of the egg white from the yolk (Alig et al. 2023). Also, there were no significant treatment differences in the egg vitelline membrane elasticity (Control 0.280 mm, EENO 0.270 mm, FFNO 0.283 mm, FFHO 0.275 mm, P = 0.75). There was significant variation in egg weights between the treatment groups (P < 0.05), with the average egg mass of the conventional controls larger compared to the egg mass of the FFNO treatment group. Average egg masses were similar between the conventional controls, EENO, and FFHO treatment groups.
Table 6.
Comparative egg quality of eggs produced by hens fed a full fat high-oleic soybean meal diet.1
| Treatments | Egg Wt3 (g) | Haugh Unit (HU) | Albumen Ht (mm) | Shell Color2 (%) | Vitelline membrane elasticity (mm) | Shell strength (g force) |
Yolk color |
|---|---|---|---|---|---|---|---|
| Control | 61.8a | 90.5 | 8.40 | 80.9 | 0.28 | 4603a | 5.03b |
| EENO | 61.1ab | 90.7 | 8.41 | 80.3 | 0.27 | 4888b | 5.26b |
| FFNO | 59.7b | 90.9 | 8.35 | 80.8 | 0.28 | 4660ab | 4.73a |
| FFHO | 59.9ab | 91.1 | 8.42 | 80.7 | 0.28 | 4762aab | 4.50a |
| SEM | 0.56 | 0.65 | 0.11 | 0.36 | 0.01 | 62 | 0.06 |
| P-value | 0.04 | 0.92 | 0.97 | 0.73 | 0.75 | 0.01 | < 0.0001 |
All data represent the 8-week average of each (2, 4, 6 and 8 week) egg quality parameter. Egg quality analysis was conducted at weeks 0, 2, 4, 6 and 8 using a 120 sub-sample of eggs randomly selected from each treatment (6 eggs/replicate) using Technical Services and Supplies QCD system, with calibration with the DSM Color Fan for yolk color. Egg wt=egg weight, HU=Haugh Unit, Albumen Ht.=albumen height, yolk color=index 1–15 (lightest to darkest color intensity).
Three hundred and thirty-six hens were fed 4 diets (18.5% crude protein, 2,927 kcal/kg), 6 rep/trmt for 8 wk. Diets: Trmt1-Control=conventional diet containing solvent extracted defatted soybean meal and corn; Trmt2-EENO=diet containing extruded-expelled defatted normal-oleic soybean meal and corn; Trmt3-FFNO = diet containing full fat normal-oleic soybean meal and corn; Trmt4-FFHO = diet containing full fat high-oleic soybean meal and corn.
Shell color is based on the reflectance of the shell surface; White shell = 83.3% reflectance; Black = 0% reflectance.
Egg weight p-value from the F-test was P < 0.05, however, t-test means separation did not indicate any 2 of the means were significantly different from each other.
*P-value= statistically significant treatment effect (P < 0.05) from analysis of variance.
Means within the same column lacking a common superscript differ significantly (P < 0.05) after adjusting for multiple comparisons.
Hens fed the EENO produced eggs with significantly greater shell strength compared to conventional control eggs (P < 0.01). Eggshell strength is an important egg quality parameter for layer production and serves to prevent the entry of bacteria and to prevent eggshell breakage, which enhances durability during transport and storage. Thus, many commercial egg producers aim to develop feeding regimens that support increased eggshell strength to prevent economic losses. Hence, a number of studies have been conducted to determine dietary strategies to enhance eggshell strength (Kim et al. 2013a,b; 2022). Several studies have reported that dietary supplementation of magnesium enhances eggshell strength (Seo et al. 2010; Kim et al. 2022). More recently, a study by Heo et al. (2023) demonstrated that reductions in dietary crude protein levels did not affect eggshell qualities including eggshell thickness, and eggshell strength of the eggs produced. In this study the EENO and control diets had similar nutrient composition (19% crude protein control diet, 18% crude protein EENO) with similar dietary levels of all feed ingredients, especially in the calcium carbonate, dicalcium phosphate, trace mineral, vitamin, and selenium premixes, with the slight exception of the quantities of soybean oil included in the 2 diets (Table 3). Therefore, additional layer feeding trials are warranted to better assess the dietary effects of full-fat soybean meal on shell egg quality and to explain the mechanisms of action.
Egg yolk color was significantly different between the treatment groups (P < 0.0001), with darker yolk color in the conventional control and EENO-fed treatment groups and a lighter color in the FFNO and FFHO treatment groups. These results parallel results published by Maharjan et al. (2023) demonstrating that eggs produced by layers fed a full-fat normal-oleic or high-oleic soybean meal diet had lower yolk color scores relative to layers fed diets containing defatted soybean meal. Egg yolk color is one of the most important egg quality parameters that affect consumer perception and choices, which is typically associated with geographical location and culture, with most consumers around the world preferring deeply hued yolks (Beardswort and Hernandes, 2004; Czarnowska-Kujawska et al. 2021). Egg yolk color is strongly determined by the levels of carotenoids present in the diet, which transfer from the diet to the eggs especially when the feeds contain high levels of dietary fat and is also dependent upon the health status of the bird and layer production management practices (Maguregui, 2020). It has been demonstrated that plant-derived compounds in layer diets readily transfer to the yolk of the eggs produced (Olson et al. 2008). Additionally, egg yolk color has been shown to be enhanced with dietary lipids and/or the lipid profile found within the feed (Suksombat et al. 2006). The pigmented carotenoids in yellow corn and corn gluten meal are often contributors to egg yolk color. However, in this layer feeding trial, there was a very small difference (<1%) in the inclusion of yellow corn between the treatment groups (57.87% yellow corn Control diet, 58.06% yellow corn EENO diet, 57.93% yellow corn FFNO and 58.35% yellow corn FFHO), and mostly likely too small of a dietary difference in between the treatment groups to attribute to yolk color variation. Also, there were very small difference (<1%) in the inclusion of corn gluten meal between the treatment groups (3.75% corn gluten meal Control diet, 3.75% corn gluten meal EENO diet, 3.22% corn gluten meal FFNO diet, and 3.46% corn gluten meal FFHO diet) and most likely too small of a dietary difference in this feed ingredient between the treatment groups to attribute to yolk color variations. Hence, we conjecture that yolk color may have been enhanced in the control and EENO diets containing 3.51% and 2.48% soybean oil, respectively as compared to the FFNO and FFHO diets containing no inclusion of soybean oil in the diets. The dietary inclusion of soybean oil in the control and EENO diets may have facilitated improved nutrient absorption and uptake of the carotenoid pigments found in the diet and transfer to the yolk of the eggs produced. Also, studies by Heo et al. (2023) demonstrated that decreasing dietary crude protein levels in layer diets reduces yolk color in the eggs produced. Therefore, this may be a plausible cause of reduced egg yolk color in the FFNO and FFHO (control-19% crude protein, EENO-18% crude protein, FFNO-17% crude protein, FFHO-17% crude protein) treatment groups.
At week 8, egg samples were analyzed to determine the crude fat, total cholesterol, and fatty acid composition. Fatty acids values >0.01 were reported in Table 7. The following fatty acids had values <0.01: myristoleic acid (14:1), pentadecyclic acid (15:0) and pentadecenoic acid (15:1). Crude fat was measured by acid hydrolysis and reported as gram individual fatty acid/gram crude fat *100 (Table 7). There were no significant differences in crude fat or total cholesterol content between egg samples of the 4 treatment groups (P > 0.05). Individual fatty acids were reported in Table 7 as Absolute fatty acid content (g/100g diet) = (g of fatty acid/g total lipid content) * % crude fat. The were no significant differences in the saturated fatty acid (SFA) content of eggs produced by hens fed the 4 dietary treatment groups at 8 weeks (P > 0.05). While the absolute stearic acid content was lowest in eggs produced from hens fed the FFHO dietary treatment (0.547), there was no significant statistical (P > 0.05) the absolute stearic acid levels between the 4 dietary treatments (Control=0.742, EENO=0.695, FFNO=0.740) . Moreover, there were no significant differences in the absolute content of palmitoleic acid, margaroleic acid and n9 t elaidic acid monounsaturated fatty acid content between the 4 dietary treatments (P > 0.05). Previous layer feeding trials have demonstrated that eggs produced by hens fed a diet containing 20% whole unprocessed high-oleic peanuts had significantly lower levels of palmitoleic acid (P < 0.001) as compared to eggs produced by hens fed a control conventional layer diet (Toomer et al. 2019). Elkin et al. (2018) demonstrated a dose dependent reduction in palmitoleic acid levels with decreasing dietary supplementation of HOSO (0, 10, 20, 40 g HOSO/kg diet).
Table 7.
Comparative fatty acid profile and total cholesterol of eggs produced by hens fed full fat high-oleic soybean meal diet.1
| Dietary treatments1 |
|||||||
|---|---|---|---|---|---|---|---|
| Classification | Fatty acid⁎⁎ (Carbon:# double bonds) |
Control | EENO | FFNO | FFHO | SEM | P-value |
| *Crude Fat | 7.69 | 6.72 | 7.00 | 7.22 | 0.65 | 0.71 | |
| ⁎⁎SFA | myristic acid (C14:0) | 0.027 | 0.025 | 0.023 | 0.022 | 0.003 | 0.51 |
| palmitic acid (C16:0) | 1.88 | 1.64 | 1.69 | 1.69 | 0.24 | 0.76 | |
| margaric acid (C17:0) | 0.020 | 0.017 | 0.020 | 0.015 | 0.004 | 0.48 | |
| stearic acid (C18:0) | 0.742 | 0.695 | 0.740 | 0.547 | 0.10 | 0.19 | |
| arachidic acid (C20:0) | 0.001 | 0.003 | 0.003 | 0.002 | 0.002 | 0.86 | |
| heneicosanic acid (C21:0) | 0.015 | 0.017 | 0.022 | 0.010 | 0.004 | 0.10 | |
| lignoceric acid (C24:0) | 0.023 | 0.020 | 0.015 | 0.017 | 0.009 | 0.77 | |
| ⁎⁎MUFA | palmitoleic acid (C16:1) | 0.033 | 0.019 | 0.019 | 0.030 | 0.019 | 0.10 |
| margaroleic acid (C17:1) | 0.015 | 0.015 | 0.017 | 0.012 | 0.004 | 0.70 | |
| oleic acid (C18:1) | 2.64b | 2.29b | 2.21b | 3.74a | 0.78 | 0.0008 | |
| n9 t elaidic acid (C18:1) | 0.007 | 0.007 | 0.003 | 0.007 | 0.003 | 0.61 | |
| gondoic acid (C20:1) | 0.022ab | 0.018b | 0.017b | 0.028a | 0.003 | 0.007 | |
| ⁎⁎PUFA | linoleic acid (C18:2) | 1.73a | 1.49a | 1.74a | 0.71b | 0.56 | < 0.0001 |
| α-linolenic acid (C18:3) | 0.09a | 0.08a | 0.10a | 0.03b | 0.01 | < 0.0001 | |
| ɣ-linolenic acid (C18:3) | 0.02 | 0.01 | 0.01 | 0.01 | 0.003 | 0.25 | |
| eicosadienoic acid (C20:2) | 0.03a | 0.02 ab | 0.03a | 0.01b | 0.006 | 0.04 | |
| arachidonic acid (C20:4)2 | 0.15 | 0.13 | 0.14 | 0.14 | 0.02 | 0.71 | |
| docosahexaenoic acid(C22:6) | 0.08 | 0.08 | 0.09 | 0.07 | 0.01 | 0.26 | |
| Total Cholesterol3 | (mg/100 g) | 350a | 297a | 323a | 306a | 28.7 | 0.44 |
Three hundred and thirty-six hens were fed 4 diets (18.5% crude protein, 2927 kcal/kg), 6 replicates/treatment for 8 weeks. Diets: Trmt1-Control=conventional diet containing solvent extracted defatted soybean meal and corn; Trmt2-EENO=diet containing extruded-expelled defatted normal-oleic soybean meal and corn; Trmt3-FFNO=diet containing full fat normal-oleic soybean meal and corn; Trmt4-FFHO=diet containing full fat high-oleic soybean meal and corn.
Each value represents the average of pooled egg samples (n=6). Each pool contained 12 homogenously combined whole eggs (2 eggs from each replicate) collected at week 8. Each fatty acid level is represented as a percentage of the total lipids (total lipids=total cholesterol + crude fat + fatty acids). analysis conducted by a commercial lab, ATC Scientific (Little Rock, AR), using AOAC-approved methods.
Arachidonic acid P-value from the F-test was P < 0.05, however, t-test means separation did not indicate any 2 of the means were significantly different from each other.
Total cholesterol is presented as mg/100g of whole egg minus the shell as per USDA FoodData Central
Means within the same column lacking a common superscript differ significantly (P < 0.05) after adjusting for multiple comparisons.
Crude fat was measured by acid hydrolysis and reported as gram crude fat /total gram of the sample *100.
Fatty acid content presented as Absolute fatty acid content (g/100g diet) = (g of fatty acid/g total lipid content) * % crude fat; SFA-saturated fatty acid, MUFA-monounsaturated fatty acid, PUFA-polyunsaturated fatty acid.
Palmitoleic acid is produced in the liver through the conversion of palmitic acid (16:0) via desaturation by the enzyme stearoyl-CoA desaturase in vivo (Carta et al. 2017). Palmitoleic acid is an n-7 monounsaturated fatty acid found in plants (Yang and Kallio, 2001) and marine sources (Ozogul et al. 2008), such as macadamia and codfish liver oil. Epidemiology studies have reported that dietary intake of palmitoleic acid has been associated with reduced risk of type 2 diabetes in humans (Guillocheau et al. 2020), with benefits for insulin sensitivity, cholesterol metabolism, and glucose hemostasis (Morgan and Dhayal, 2010).
The absolute oleic acid (18:1) content was significantly higher in eggs produced by hens fed the FFHO dietary treatment as compared to eggs produced by hens fed the other dietary treatments (P < 0.001). Absolute oleic acid levels were similar in eggs produced by hens fed the control, EENO and FFNO dietary treatments. Similarly, chemical analysis of the experimental diets (Table 4) demonstrated that the oleic acid content was highest in FFHO dietary treatment as compared to the other 3 experimental diets. Likewise, other studies have demonstrated that dietary supplementation of layer diets with high-oleic oilseeds (Toomer et al. 2019, 2021, Elkin et al. 2018) or the oil extracted from high-oleic oilseeds enriches the eggs produced with oleic acid. Oleic acid (cis‐18:1 n−9) is a nonessential monounsaturated n-9 fatty acid that is found in various animal and vegetable dietary sources. Fatty acid analysis of various olive oils has reported a fatty acid profile of 64 to 75% oleic acid, 7 to 15% linoleic acid, < 1.0% linolenic acid, < 2.0% palmitoleic acid, 11 to 16% palmitic acid, and < 3.0% stearic acid (Di Serio et al. 2016; Revelou et al. 2021). Hence, the high levels of oleic acid in olive oil have been attributed to multiple health benefits associated with the dietary consumption of olive oil. Increased dietary intake of oleic acid has been shown to reduce low-density lipoprotein serum cholesterol levels in humans (Kwon and Choi, 2015; Nogoy et al. 2020) and to increase beneficial high‐density lipoprotein serum cholesterol levels (Gilmore et al. 2011; Nogoy et al. 2020).
Gondoic acid (20:1) levels were significantly higher in eggs produced by hens fed the FFHO dietary treatment as compared to eggs produced by hens fed the EENO and FFNO diets (P < 0.01), with similar levels to eggs produced by hens fed the control diet. Gondoic acid is an n-9 monounsaturated long-chain fatty acid generated as an elongation product of oleic acid. Gondoic acid is found in various botanical oils, such as camelina seed oil. While a few studies have examined the use of gondoic acid in transdermal application (Morimoto et al. 1996), there is a paucity of published data reporting the health benefits or risks associated with dietary consumption of gondoic acid.
Absolute linoleic acid (18:2) levels were highest in eggs produced by hens fed the control, EENO and FFNO diets as compared to the levels in eggs produced by hens fed FFHO dietary treatments (P < 0.0001), which parallels the absolute linoleic acid levels found in the 4 experimental diets, with the lowest total n-6 fatty acid levels being in the FFHO dietary treatment (Table 4). Linoleic acid is an essential n-6 fatty acid that must be supplied by the diet and is the predominant polyunsaturated fatty acid found in the Western diet. It can be found in nuts, seeds, and vegetable oils such as sunflower, safflower, soybean, corn, and canola oils. Epidemiological studies have shown that replacing that replacing 5% of the dietary energy derived from saturated fatty acids with linoleic acid reduces low-density lipoprotein serum cholesterol by up to 10%, with a significant reduction in cardiovascular risk (Mozaffarian et al. 2010; Mensink, 2016).
Alpha (α)-linolenic acid (18:3) levels were the lowest in eggs produced by hens fed the FFHO dietary treatment as compared to the other 3 dietary treatments (P < 0.0001). In parallel, the FFHO experimental diet had the lowest total n-3 fatty acid levels compared to the other treatment groups (Table 4). Alpha (α)-linolenic acid levels were similar in eggs produced by hens fed the control, EENO and FFNO dietary treatments. Elkin et al. (2018) demonstrated that the conversion efficiency of alpha (α)-linolenic acid to very long chain n-3 polyunsaturated fatty acids was significantly decreased by increasing dietary levels of HOSO with supplemental flax oil resulting in egg yolks with lower omega 3 fatty acids. Our earlier published feeding trials demonstrated that 20% inclusion of whole unblanched high-oleic peanuts (Toomer et al. 2021, 2019) or 3% high-oleic acid oil (Toomer et al. 2021) corresponded to lower egg levels of linolenic acid and linoleic acid. Alpha (α)-linolenic acid is an n-3 essential fatty acid and must be consumed in the diet, and it can be found in small amounts in almonds, hazelnuts, and cashews, moderate amounts in rapeseed oil, soybean oil, corn oil, and olive oil, and relatively high amounts in flaxseed oil, perilla oil, chia seed oil, Agastache rugosa oil, peony oil, and Sichuan pepper oil (Yuan et al. 2022). Studies have shown that increased dietary intake of n-3 fatty acids, such as alpha-linolenic acid, has been shown to improve cardiovascular health (Stanley et al., 2007) and brain function (Bourre et al., 1991).
It has been shown in a number of studies that feeding laying hens diets containing high levels of oleic acid attenuates the deposition of alpha-linolenic acid in the egg yolk and tissue (Maharjan et al. 2023) along with the reduced deposition of hepatic synthesized very long chain n-3 derivatives, such as docosahexaenoic acid (Elkin et al. 2018; Elkin et al. 2021), which parallels this study. Therefore, it has been conjectured that these effects are resultant of increased mixed micelle formation with bile salts and subsequent increased digestive absorption of oleic acid which competitively inhibits the absorption of alpha (α)-linolenic acid (Elkin et al. 2018). Thereafter, reducing the availability of alpha (α)-linolenic acid substrates for hepatic elongation and desaturation to produce very long chain n-3 polyunsaturated fatty acids and ultimately reduced egg deposition of alpha (α)-linolenic acid and docosahexaenoic acid. In contrast, our previously published feeding trial demonstrated no significant difference in docosahexaenoic acid levels between eggs produced from hens fed a 20% whole unprocessed high-oleic peanut diet, 3% oleic acid oil supplemented diet and a control conventional layer diet (Toomer et al. 2021). Consequently, we aim to conduct additional layer feeding trials with high-oleic oilseeds and extracted oils to better define the mechanisms of action responsible for these effects.
Eicosadienoic acid levels were very low in all eggs (absolute fatty acid values ≤ 0.03) from the 4 dietary treatments. Nonetheless, absolute eicosadienoic values were lowest in eggs produced from hens fed the FFHO dietary treatment as compared to the control and FFNO treatment groups (P < 0.05).
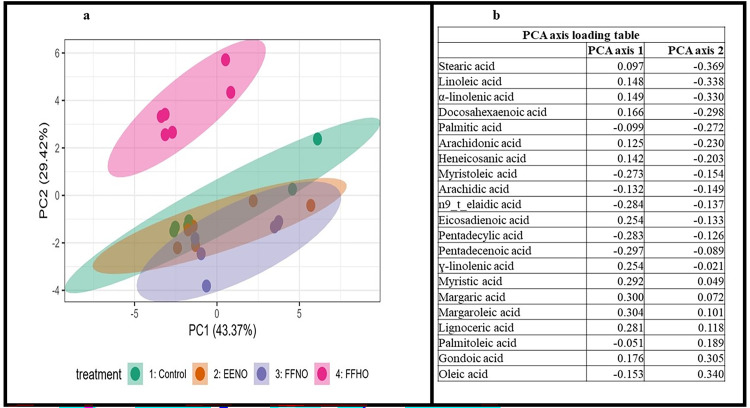
In summary, there were no significant treatment differences in the following saturated fatty acids: myristic acid, palmitic acid, margaric acid, stearic acid, arachidic acid, heneicosanic acid, lignoceric acid; monounsaturated fatty acids: palmitoleic acid, margaroleic acid, n9 t elaidic acid; and polyunsaturated fatty acids: ɣ-linolenic acid, arachidonic acid, docosahexaenoic acid in eggs collected at week 8 of the feeding trial (Table 7, P > 0.05). Additionally, a principal component analysis (PCA) was conducted to identify the overall patterns and variation in the 21 fatty acids measured in eggs produced by hens fed the 4 dietary treatments (Figure 1A). Axis 1 (PC1) explained 43.37% of the variation and axis 2 (PC2) explained 29.42% of the variation. The PCA ordination plot shows that eggs produced by hens fed the FFHO dietary treatment were clearly differentiated in fatty acid composition along axis 2 as compared to the other treatment groups. PCA axis loading table results show that axis 1 is positively associated with the following fatty acids: margaric, margaroleic, ɣ-linolenic, myristic, and lignoceric. The PCA axis loading table shows negative loadings on axis 2 for stearic, linoleic, and alpha-linolenic, and positive loadings for palmitoleic, gondoic, and oleic acids, with the FFHO dietary treatment having higher values of axis 2 than the other 3 treatments.
Figure 1.
Principal components analysis (PCA) of the fatty acid profile of eggs produced by hens fed a full-fat high-oleic soybean meal diet. The ellipse surrounding the points for each treatment represents a 95% confidence interval. Three hundred and thirty-six hens were fed 4 diets (18.5% crude protein, 2,927 kcal/kg), 6 replicates/treatment for 8 wk. Treatments: Control=conventional diet containing solvent extracted defatted soybean meal and corn; Trmt2-EENO=diet containing extruded-expelled defatted normal-oleic soybean meal and corn; Trmt3-FFNO = diet containing full fat normal-oleic soybean meal and corn; Trmt4-FFHO=diet containing full fat high-oleic soybean meal and corn. Each data point represents one replicate, with 6 replicates of pooled egg samples per treatment. Each pool contained 12 homogenously combined whole eggs (2 eggs from each replicate) collected at week 8.
This study suggest that replacement of conventional solvent extracted defatted soybean meal with full-fat high-oleic soybean meal in layer diet enriches the eggs produced with oleic acid. Nonetheless, eggs produced from hens fed full-fat high-oleic soybean meal and full-fat normal-oleic soybean meal had reduced yolk color as compared to the controls. There were very small variations in the dietary inclusion levels of yellow corn (<1%) and corn gluten meal (<1%) between the treatment groups and most likely not major contributors to differences seen in yolk color between the treatment groups. We conjecture that inclusion of conventional soybean oil at 3.6% and 2.4% in the control and EENO diets, respectively possibly enhanced the absorption carotenoids in the diet which significantly influenced yolk color. These adverse effects on egg yolk color can be potentially overcome with increased inclusion of corn gluten meal in layer diets, which contain more than 300 mg/kg xanthophylls. Studies have demonstrated that 10% dietary inclusion of corn gluten meal in layer diets significantly enhances yolk color, whereas in this study we utilized approximately 4% dietary inclusion of corn gluten meal. Overall, we documented a > 50% increase in monounsaturated n-9 oleic acid levels as compared to conventional (control) eggs with the inclusion of full-fat high-oleic soybean meal in layer diets, without adversely impacting egg quality or performance. Recently, the American Heart Association (2023) has reported that increased dietary intake of monounsaturated fats, such as oleic acid, reduces “the bad” cholesterol and lowers the risk for heart disease and stroke. Moreover, a review of several clinical trials demonstrated that increased dietary intake of monounsaturated fatty acids promotes healthy blood lipid profiles, mediates hypertension, and improves and regulates blood glucose levels (Gillingham et al. 2011). For decades, the nutrition science community has regarded plant proteins as preferable dietary choices in animal and human nutrition, providing high-quality protein, low in saturated fats, and rich in unsaturated fatty acids. Studies like this one demonstrate that the use of high-oleic oilseeds in livestock rations (broilers, layers, dairy, swine, fish) may serve as a nutritional means to produce animal proteins intended for human consumption that are rich in high-quality protein, low in saturated fat and enriched with monounsaturated fatty acids.
Acknowledgments
ACKNOWLEDGMENTS
The authors would like to acknowledge the following: The United States Soybean Board for funding for this project (1930-362-0618), Mr. Philip Lobo and the US Soybean Board-Animal Nutrition Working group for providing their guidance and leadership, Dr. Muquarrab Qureshi, Location Coordinator, ARS Raleigh Location & Research Leader, FSMQH for administrative support and guidance, the Soybean & Nitrogen Fixation Unit for the production and donation of the soybean cultivars, the Animal & Poultry Waste Processing Facility for processing of soybean cultivars, the Staff of the Prestage Department of Poultry Science, the NC State University Feed Mill, and Chicken Education Unit for assistance with animal care & husbandry and feed manufacturing, and the FSMQH-ARS for their contributions to this study. This work was also supported by appropriated funds from the Agricultural Research Service, US Department of Agriculture (CRIS 6070-43440-013-00D).
Mention of a trademark or proprietary product does not constitute a guarantee or warranty of the product by the U.S. Department of Agriculture or North Carolina Agricultural Research Service, nor does it imply approval to the exclusion of other products that may be suitable. USDA is an equal opportunity provider and employer.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- Alig B.N., Malheiros R.D., Anderson K.E. Evaluation of physical egg quality parameters of commercial brown laying hens housed in five production systems. Animals (Basel) 2023;13:716. doi: 10.3390/ani13040716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Heart Association. 2023. Monounsaturated fats.https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/fats/monounsaturated-fats#:∼:text=Monounsaturated%20fats%20can%20help%20reduce,E%2C%20an%20important%20antioxidant%20vitamin. Accessed Oct. 27, 2023.
- Anderson K.E., Lowman Z., Stomp A.M., Chang J. Duckweed as a feed ingredient in laying hen diets and its effect on egg production and composition. Int. J. Poult. Sci. 2011;10:4–7. [Google Scholar]
- AOAC Official Method 954.02, Fat (Crude) or ether extract in pet food: gravimetric method', in Dr. George W Latimer, Jr. (ed.), Official Methods of Analysis of AOAC International, 22 (New York, 2023; AOAC Publications), Accessed May 2, 2024.
- AOAC Official Method 976.26, Cholesterol in multicomponent foods: gas chromatographic method', in Dr. George W Latimer, Jr. (ed.), Official Methods of Analysis of AOAC International, 22 (New York, 2023; AOAC Publications), Accessed online May 2, 2024.
- AOAC 991.31 . Immunoaffinity Column (Aflatest) Method. AOAC Official Methods. AOAC Inc; Arlington, VA: 2002. Aflatoxins in corn, raw peanuts, and Peanut butter. [Google Scholar]
- AOAC Official Method 996.06 Fat (total, saturated, and unsaturated) in foods hydrolytic extraction gas chromatographic method first action. J AOAC INT. 2008;91:92–97. [Google Scholar]
- AOAC 2001.04 . Liquid Chromatography With Immunoaffinity Column Cleanup. AOAC Official Methods. AOAC Inc; Arlington, VA: 2001. Fumonisins B1 and B2 in corn and corn flakes. [Google Scholar]
- Bates D., Maechler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67:1–48. [Google Scholar]
- Beardswort P.M., Hernandes J.M. Yolk colour–an important egg quality attribute. Int. J. Poult. 2004;12:17–18. [Google Scholar]
- Bourre J.M., Dumont O., Piciotti M., Clément M., Chaudière J., Bonneil M., Nalbone G., Lafont H., Pascal G., Durand G. Essentiality of omega 3 fatty acids for brain structure and function. World Rev. Nutr. Diet. 1991;66:103–117. doi: 10.1159/000419283. [DOI] [PubMed] [Google Scholar]
- Carta G., Murru E., Banni S., Manca C. Palmitic acid: physiological role, metabolism and nutritional implications. Front. Physiol. 2017;8:902. doi: 10.3389/fphys.2017.00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, R.H.B. 2019. ordinal: Regression Models for ordinal data. R package version 2019.12-10. Accessed June 2024. https://CRAN.R-project.org/package=ordinal.
- Czarnowska-Kujawska M., Draszanowska A., Gujska E., Klepacka J., Kasińska M. Folate content and yolk color of hen eggs from different farming systems. Molecules. 2021;26:1034. doi: 10.3390/molecules26041034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Serio M.G., Di Loreto G., Di Giacinto L., Giansante L. Chemical and sensory characteristics of Italian virgin olive oils from Grossa di Gerace cv: Grossa di Gerace’ characterization. Eur. J. Lipid Sci. Technol. 2016;118:288–298. [Google Scholar]
- Elkin R.G., Kukorowski A.N., Ying Y., Harvatine K.J. Dietary high-oleic acid soybean oil dose dependently attenuates egg yolk content of n-3 polyunsaturated fatty acids in laying hens fed supplemental flaxseed oil. Lipids. 2018;53:235–249. doi: 10.1002/lipd.12016. [DOI] [PubMed] [Google Scholar]
- Elkin R.G., El-Zenary A.S.A., Bomberger R., Harvatine K.J. Supplemental dietary oils rich in oleic acid or linoleic acid attenuate egg yolk and tissue n-3 polyunsaturated fatty acid contents in laying hens co-fed oils enriched in either stearidonic acid or alpha-linolenic acid. Prostaglandins Leukot Essent Fatty Acids. 2021;172 doi: 10.1016/j.plefa.2021.102322. [DOI] [PubMed] [Google Scholar]
- Erdaw M.M., Perez-Maldonado R.A., Iji P.A. Apparent and standardized ileal nutrient digestibility of broiler diets containing varying levels of raw full-fat soybean and microbial protease. J. Anim. Sci. Technol. 2017;59:1. doi: 10.1186/s40781-017-0148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdaw M.M., Wu S., Iji P.A. Growth and physiological responses of broiler chickens to diets containing raw, full-fat soybean and supplemented with a high-impact microbial protease. Asian-Australas J. Anim Sci. 2017;30:1303–1313. doi: 10.5713/ajas.16.0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdaw M.M., Perez-Maldonado R.A., Iji P.A. Supplementation of broiler diets with high levels of microbial protease and phytase enables partial replacement of commercial soybean meal with raw, full-fat soybean. J. Anim. Physiol. Anim. Nutr. 2018;102:755–768. doi: 10.1111/jpn.12876. [DOI] [PubMed] [Google Scholar]
- Fehr W.R. Breeding for modified fatty acid composition in soybean. Crop Sci. 2007;47:S72–S87. [Google Scholar]
- Gaffield K.N., Boler D.D., Dilger R.N., Dilger A.C., Harsh B.N. Effects of feeding high oleic soybean oil to growing–finishing pigs on loin and belly quality. J. Anim. Sci. 2022;100:284. doi: 10.1093/jas/skac284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffield K.N., Boler D.D., Dilger R.N., Dilger A.C., Harsh B.N. Effects of feeding high oleic soybean oil to growing-finishing pigs on growth performance and carcass characteristics. J. Anim. Sci. 2022;100:071. doi: 10.1093/jas/skac071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingham L.G., Harris-Janz S., Jones P.J. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids. 2011;46:209–228. doi: 10.1007/s11745-010-3524-y. [DOI] [PubMed] [Google Scholar]
- Guillocheau E., Legrand P., Rioux V. Trans-palmitoleic acid (trans-9-C16:1, or trans-C16:1 n-7): nutritional impacts, metabolism, origin, compositional data, analytical methods and chemical synthesis-a review. Review Biochimie. 2020;169:144–160. doi: 10.1016/j.biochi.2019.12.004. [DOI] [PubMed] [Google Scholar]
- Heo Y.J., Park J., Kim Y.B., Kwon B.Y., Kim D.H., Song J.Y., Lee K.W. Effects of dietary protein levels on performance, nitrogen excretion, and odor emission of growing pullets and laying hens. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hothorn T., Bretz F., Westfall P. Simultaneous inference in general parametric models. Biom. J. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- Karimi Z., Torki M., Abdolmohammadi A. Effect of dietary roasted and autoclaved full-fat soybean on the performance of laying hens and egg quality traits. Vet Med Sci. 2022;8:1603–1610. doi: 10.1002/vms3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Choi W.J., Kwon C.H., Kil D.Y. Improvement of eggshell strength and intensity of brown eggshell color by dietary magnesium and δ-aminolevulinic acid supplementation in laying hens. Poult Sci. 2022;101 doi: 10.1016/j.psj.2021.101676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.H., Paik I.K., Kil D.Y. Effects of increasing supplementation of magnesium in diets on productive performance and eggshell quality of aged laying hens. Biol. Trace Elem. Res. 2013;151:38–42. doi: 10.1007/s12011-012-9537-z. [DOI] [PubMed] [Google Scholar]
- Kim C.H., Paik I.K., Kil D.Y., Chang M.B. Effects of dietary magnesium concentrations on performance and eggshell quality of laying hens. J. Anim. Vet. Adv. 2013;12:104–107. [Google Scholar]
- Knowlton S. Pages 53–87 in Chapter 3 - High-oleic Soybean Oil. Elsevier, Academic Press; Urbana, IL: 2022. High oleic oils-development, properties, and uses. [Google Scholar]
- Kuznetsova A., Brockhoff P.B., Christensen R.H.B. lmerTest package: tests in linear mixed effects models. J. Stat. Softw. 2017;82:1–26. [Google Scholar]
- Kwon H.N., Choi C.B. Comparison of lipid content and monounsaturated fatty acid composition of beef by country of origin and marbling score. J. Korean Soc. Food Sci. Nutr. 2015;44:1806–1812. [Google Scholar]
- Lenth, R.V. 2022. emmeans: estimated marginal means, aka least-squares means. R package version 1.7.3. Accessed June 2024. https://CRAN.R-project.org/package=emmeans.
- Maguregui E. The egg yolk color and pigments. The importance of colors in animal feed. Veterinaria Digital. 2020 https://www.veterinariadigital.com/en/articulos/the-egg-yolk-color-and-pigments/ Accessed Nov. 17, 2023. [Google Scholar]
- Maharjan P., Rahimi A., Harding K.L., Vu T.C., Malheiros R., Dean L., Anderson K.E., Toomer O.T. Effects of full-fat high-oleic and dry extruded high oleic soybean meal in layer hen diets on nutrient digestibility and egg quality parameters of a white laying hen strain. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez Marín A.L., Gómez-Cortés P., Gómez Castro G., Juárez M., Pérez Alba L., Pérez Hernández M., de la Fuente M.A. Effects of feeding increasing dietary levels of high oleic or regular sunflower or linseed oil on fatty acid profile of goat milk. J. Dairy Sci. 2012;95:1942–1955. doi: 10.3168/jds.2011-4303. [DOI] [PubMed] [Google Scholar]
- Mensink R.P. World Health Organization; Geneva: 2016. Effects of Saturated Fatty Acids on Serum Lipids and Lipoproteins: A Systematic Review and Regression Analysis.https://apps.who.int/iris/bitstream/handle/10665/246104/9789241565349-eng.pdf Accessed Nov. 17, 2023. [Google Scholar]
- Morgan N.G., Dhayal S. Unsaturated fatty acids as cytoprotective agents in the pancreatic beta-cell. Prostaglandins Leukot. Essent. Fatty Acids. 2010;82:231–236. doi: 10.1016/j.plefa.2010.02.018. [DOI] [PubMed] [Google Scholar]
- Morimoto K., Tojima H., Haruta T., Suzuki M., Kakemi M. Enhancing effects of unsaturated fatty acids with various structures on the permeation of indomethacin through rat skin. J. Pharm. Pharmacol. 1996;48:1133–1137. doi: 10.1111/j.2042-7158.1996.tb03908.x. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D., Aro A., Willett W.C. Health effects of trans-fatty acids: experimental and observational evidence. Eur. J. Clin. Nutr. 2009;63:S5–S21. doi: 10.1038/sj.ejcn.1602973. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D., Micha R., Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogoy K.M.C., Kim H.J., Lee Y., Zhang Y., Yu J., Lee D.H., Li X.Z., Smith S.B., Seong H.A., Choi S.H. High dietary oleic acid in olive oil-supplemented diet enhanced omega-3 fatty acid in blood plasma of rats. Food Sci. Nutr. 2020;8:3617–3625. doi: 10.1002/fsn3.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nematinia E., Mehdizadeh A. Assessment of egg freshness by prediction of Haugh unit and albumen ph using an artificial neural network. J. Food Meas. Charact. 2018;12:1449–1459. [Google Scholar]
- Olson J.B., Ward N.E., Koutsos E.A. Lycopene incorporation into egg yolk and effects on laying hen immune function. Poult. Sci. 2008;87:2573–2580. doi: 10.3382/ps.2008-00072. [DOI] [PubMed] [Google Scholar]
- Ozogul Y., Ozogul F., Cicek E., Polat A., Kuley E. Fat content and fatty acid compositions of 34 marine water fish species from the mediterranean Sea. Int. J. Food Sci. Nutr. 2008;29:1–12. doi: 10.1080/09637480701838175. [DOI] [PubMed] [Google Scholar]
- Park C.S., Helmbrecht A., Htoo J.K., Adeola O. Comparison of amino acid digestibility in full-fat soybean, two soybean meals, and peanut flour between broiler chickens and growing pigs. J. Anim. Sci. 2017;95:3110–3119. doi: 10.2527/jas.2017.1404. [DOI] [PubMed] [Google Scholar]
- Pérez-Marın D.C., Garrido-Varo A., Guerrero-Ginel J.E., Gómez-Cabrera A. Near-infrared reflectance spectroscopy (NIRS) for the mandatory labelling of compound feedingstuffs: chemical composition and open-declaration. Anim. Feed Sci. Technol. 2004;116:333–349. [Google Scholar]
- Pham A.T., Lee J.D., Shannon J.G., Bilyeu K.D. Mutant alleles of FAD2-1A and FAD2-1B combine to produce soybeans with the high oleic acid seed oil trait. BMC Plant Biol. 2010;10:195. doi: 10.1186/1471-2229-10-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redhead A.K., Sanders E., Vu T.C., Malheiros R.D., Anderson K.E., Toomer O.T. The effects of high-oleic peanuts as an alternate feed ingredient on performance, ileal digestibility apparent metabolizable energy, and histology of the small intestine in laying hens. Transl. Anim. Sci. 2021;5:1–11. doi: 10.1093/tas/txab015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revelou P.K., Xagoraris M., Alexandropoulou A., Kanakis C.D., Papadopoulos G.K., Pappas C.S., Tarantilis P.A. Chemometric study of fatty acid composition of virgin olive oil from four widespread Greek cultivars. Molecules. 2021;26:4151. doi: 10.3390/molecules26144151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlueter J.A., Vaslenko-Sanders I.F., Deshpande S., Yi J., Siegfried M., Roe B.A., Schlueter S.D., Scheffler B.E., Shoemaker R.C. The FAD2 gene family of soybean: insights into the structural and functional divergence of a paleoplyploid genome. Crop Sci. 2007;47:S14–S26. [Google Scholar]
- Schweighardt H., Böhm J., Abdelhamid A.M., Leibetseder J., Schuh M., Glawischnig E. Analysis of the fusariotoxins zearalenone and vomitoxin (deoxynivalenol) in human foods and animal feeds by high-performance liquid chromatography (HPLC) Chromatographia. 1980;13:447–450. [Google Scholar]
- Seo Y.M., Shin K.S., Rhee A.R., Chi Y.S., Han J., Paik I.K. Effects of dietary Fe-soy proteinate and MgO on egg production and quality of eggshell in laying hens. Asian-Australas. J. Anim. Sci. 2010;23:1043–1048. [Google Scholar]
- R Core Team, 2021. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Accessed June 2024. https://www.R-project.org/.
- Shurtleff, W., and A. Aoyagi. 2007. History of Soy Oil Hydrogenation and of research on the safety of hydrogenated vegetable oils. https://www.soyinfocenter.com/HSS/hydrogenation1.php. Accessed May 9, 2024.
- Stanley J.C., Elsom R.L., Calder P.C., Griffin B.A., Harris W.S., Jebb S.A., Lovegrove J.A., Moore C.S., Riemersma R.A., Sanders T.A. UK Food Standards Agency Workshop Report: the effects of the dietary n-6:n-3 fatty acid ratio on cardiovascular health. Br. J. Nutr. 2007;98:1305–1310. doi: 10.1017/S000711450784284X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suksombat W., Samitayotin S., Lounglawan P. Effects of conjugated linoleic acid supplementation in layer diet on fatty acid compositions of egg yolk and layer performances. Poult. Sci. 2006;85:1603–1609. doi: 10.1093/ps/85.9.1603. [DOI] [PubMed] [Google Scholar]
- Stadelman W.J. In: Egg Science and Technology. 4th edn. Stadelman W.J., Cotterill O.J., editors. Food Products Press; Binghamton, NY: 1995. Quality identification of shell eggs; pp. 51–64. (1995) [Google Scholar]
- Tang G.Q., Novitzky W.P., Griffin H.C., Huber S.C., Dewey R.E. Oleate desaturase enzymes of soybean: evidence of regulation through differential stability and phosphorylation. Plant J. 2005;44:433–446. doi: 10.1111/j.1365-313X.2005.02535.x. [DOI] [PubMed] [Google Scholar]
- Toomer O.T., Hulse-Kemp A.M., Dean L.L., Boykin D.L., Malheiros R., Anderson K.E. Feeding high-oleic peanuts to layer hens enhances egg yolk color and oleic fatty acid content in Shell Eggs. Poult. Sci. 2019;98:1732–1748. doi: 10.3382/ps/pey531. [DOI] [PubMed] [Google Scholar]
- Toomer O.T., Vu T.C., Sanders E., Redhead A.K., Malheiros R., Anderson K.E. Feeding laying hens a diet containing high-oleic peanuts or oleic acid enriches yolk color and beta-carotene while reducing the saturated fatty acid content in eggs. Agriculture. 2021;11 10.3390. [Google Scholar]
- United Soybean Board, 2020. Soybean meal: the one-stop solution for nutrition. https://www.unitedsoybean.org/hopper/soybean-meal-the-one-stop-solution-for-nutrition/. Accessed May 9, 2024.
- United Soybean Board, 2024. High oleic soybeans.https://www.unitedsoybean.org/issue-briefs/high-oleic-soybeans/#:∼:text=What%20is%20high%20oleic%3F,acres%20in%20Ohio%20and%20Indiana. Accessed May 9, 2024.
- University of Georgia-Athens Extension. 2023. Soybeans-Quality Control.https://poultry.caes.uga.edu/extension/poultry-nutrition/soybeans/quality-control.html. Accessed May 7, 2024.
- US. Food and Drug Administration, 2024. Tans-Fat. Food Ingredients & Packaging -Food Additives & Petitions.https://www.fda.gov/food/food-additives-petitions/trans-fat#:∼:text=In%202015%2C%20the%20FDA%20took,not%20add%20PHOs%20to%20foods. Accessed May 9, 2024.
- U.S. Soy, 2021. Use Soy-Benefits. United Soybean Board. https://soynewuses.org/benefits. Accessed May 9, 2024.
- Vuilleumier J.P. The ‘roche yolk colour fan‘—an instrument for measuring yolk colour. Poult. Sci. 1969;48:767–779. [Google Scholar]
- Warner K., Fehr W. Mid-Oleic/Ultra Low Linolenic Acid Soybean Oil: A Healthful New Alternative to Hydrogenated Oil for Frying. J Am Oil Chem Soc. 2008;85:945–951. [Google Scholar]
- Weld K., Armentano L. Feeding high oleic acid soybeans in place of conventional soybeans increases milk fat concentration. J. Dairy Sci. 2018;101:9768–9776. doi: 10.3168/jds.2018-14498. [DOI] [PubMed] [Google Scholar]
- Yang B., Kallio H.P. Fatty acid composition of lipids in sea buckthorn (Hippophaerhamnoides L.) berries of different origins. J. Agric. Food Chem. 2001;49:1939–1947. doi: 10.1021/jf001059s. [DOI] [PubMed] [Google Scholar]
- Yuan Q., Xie F., Huang W., Hu M., Yan Q., Chen Z., Zheng Y., Liu L. The review of alpha-linolenic acid: Sources, metabolism, and pharmacology. Phytother. Res. 2022;36:164–188. doi: 10.1002/ptr.7295. [DOI] [PubMed] [Google Scholar]
- Zhang Y.Y., Zhao M.J., Liu C.Y., Ma K., Liu T.Y., Chen F., Wu L.N., Hu D.J., Lv G.P. Comparison of two commercial methods with a UHPLC-MS/MS method for the determination of multiple mycotoxins in cereals. Food Chem. 2023;406 doi: 10.1016/j.foodchem.2022.135056. [DOI] [PubMed] [Google Scholar]