Abstract
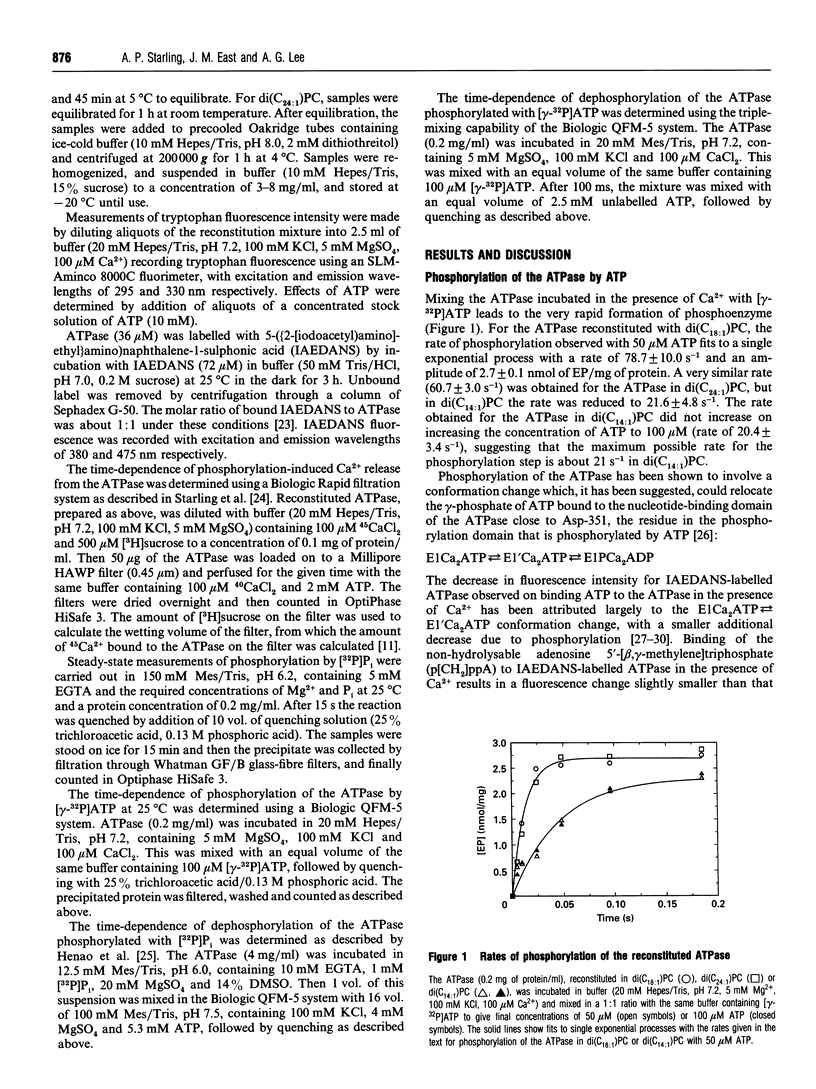
The kinetics of the Ca(2+)-ATPase purified from sarcoplasmic reticulum have been studied after reconstitution into bilayers of dimyristoleoylphosphatidylcholine [di(C14:1)PC], dioleoylphosphatidylcholine[di(C18:1)PC] and dinervonylphosphatidylcholine [di(C24:1)PC]. In di(C24:1)PC the rate of phosphorylation of the ATPase by ATP was comparable with that in di(C18:1)PC (about 70 s-1), but in di(C14:1)PC the rate was much lower (21 s-1). Fluorescence responses of the ATPase suggest changes in the phosphoryl-transfer step rather than in the preceding conformational change E1Ca2ATP<-->E1'Ca2ATP. The rate of dephosphorylation of the phosphorylated ATPase was found to decrease in the order di(C24:1)PC < di(C14:1)PC < di(C18:1)PC. For the ATPase in di(C24:1)PC the rate of dephosphorylation (3.3 s-1) was slow enough to be the rate-limiting step for ATP hydrolysis; in di(C14:1)PC, it is suggested that both phosphorylation and dephosphorylation contribute to rate limitation. Phosphorylation of the ATPase in di(C24:1)PC by Pi was normal, but no phosphoenzyme could be detected in di(C14:1)PC. The rate of the Ca(2+)-transport step was normal in di(C24:1)PC, suggesting that the single Ca2+ ion bound to the ATPase in di(C24:1)PC could be transported.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker K. J., East J. M., Lee A. G. Localization of the hinge region of the Ca(2+)-ATPase of sarcoplasmic reticulum using resonance energy transfer. Biochim Biophys Acta. 1994 Jun 1;1192(1):53–60. doi: 10.1016/0005-2736(94)90142-2. [DOI] [PubMed] [Google Scholar]
- Caffrey M., Feigenson G. W. Fluorescence quenching in model membranes. 3. Relationship between calcium adenosinetriphosphatase enzyme activity and the affinity of the protein for phosphatidylcholines with different acyl chain characteristics. Biochemistry. 1981 Mar 31;20(7):1949–1961. doi: 10.1021/bi00510a034. [DOI] [PubMed] [Google Scholar]
- Cornea R. L., Thomas D. D. Effects of membrane thickness on the molecular dynamics and enzymatic activity of reconstituted Ca-ATPase. Biochemistry. 1994 Mar 15;33(10):2912–2920. doi: 10.1021/bi00176a022. [DOI] [PubMed] [Google Scholar]
- Ding J., Starling A. P., East J. M., Lee A. G. Binding sites for cholesterol on Ca(2+)-ATPase studied by using a cholesterol-containing phospholipid. Biochemistry. 1994 Apr 26;33(16):4974–4979. doi: 10.1021/bi00182a028. [DOI] [PubMed] [Google Scholar]
- East J. M., Lee A. G. Lipid selectivity of the calcium and magnesium ion dependent adenosinetriphosphatase, studied with fluorescence quenching by a brominated phospholipid. Biochemistry. 1982 Aug 17;21(17):4144–4151. doi: 10.1021/bi00260a035. [DOI] [PubMed] [Google Scholar]
- Froud R. J., East J. M., Jones O. T., Lee A. G. Effects of lipids and long-chain alkyl derivatives on the activity of (Ca2+-Mg2+)-ATPase. Biochemistry. 1986 Nov 18;25(23):7544–7552. doi: 10.1021/bi00371a043. [DOI] [PubMed] [Google Scholar]
- Gould G. W., McWhirter J. M., East J. M., Lee A. G. A fast passive Ca2+ efflux mediated by the (Ca2+ + Mg2+)-ATPase in reconstituted vesicles. Biochim Biophys Acta. 1987 Nov 2;904(1):45–54. doi: 10.1016/0005-2736(87)90085-x. [DOI] [PubMed] [Google Scholar]
- Gould G. W., McWhirter J. M., East J. M., Lee A. G. Uptake of Ca2+ mediated by the (Ca2+ + Mg2+)-ATPase in reconstituted vesicles. Biochim Biophys Acta. 1987 Nov 2;904(1):36–44. doi: 10.1016/0005-2736(87)90084-8. [DOI] [PubMed] [Google Scholar]
- Gómez-Fernández J. C., Baena M. D., Teruel J. A., Villalaín J., Vidal C. J. A fluorescence quenching study of tryptophanyl residues of (Ca2+ + Mg2+)-ATPase from sarcoplasmic reticulum. J Biol Chem. 1985 Jun 25;260(12):7168–7170. [PubMed] [Google Scholar]
- Hardwicke P. M., Green N. M. The effect of delipidation on the adenosine triphosphatase of sarcoplasmic reticulum. Electron microscopy and physical properties. Eur J Biochem. 1974 Feb 15;42(1):183–193. doi: 10.1111/j.1432-1033.1974.tb03328.x. [DOI] [PubMed] [Google Scholar]
- Henao F., de Foresta B., Orlowski S., Cuenda A., Gutiérrez-Merino C., Champeil P. Kinetic characterization of the normal and procaine-perturbed reaction cycles of the sarcoplasmic reticulum calcium pump. Eur J Biochem. 1991 Dec 5;202(2):559–567. doi: 10.1111/j.1432-1033.1991.tb16408.x. [DOI] [PubMed] [Google Scholar]
- Hidalgo C., Thomas D. D., Ikemoto N. Effect of the lipid environment on protein motion and enzymatic activity of sarcoplasmic reticulum calcium ATPase. J Biol Chem. 1978 Oct 10;253(19):6879–6887. [PubMed] [Google Scholar]
- Johannsson A., Keightley C. A., Smith G. A., Richards C. D., Hesketh T. R., Metcalfe J. C. The effect of bilayer thickness and n-alkanes on the activity of the (Ca2+ + Mg2+)-dependent ATPase of sarcoplasmic reticulum. J Biol Chem. 1981 Feb 25;256(4):1643–1650. [PubMed] [Google Scholar]
- Kubo K., Suzuki H., Kanazawa T. Characterization of the substrate-induced conformational change of N-iodoacetyl-N'-(5-sulfo-1-naphthyl)ethylenediamine-labeled sarcoplasmic reticulum Ca2(+)-ATPase by using different kinds of substrate. Biochim Biophys Acta. 1990 Sep 3;1040(2):251–259. doi: 10.1016/0167-4838(90)90084-s. [DOI] [PubMed] [Google Scholar]
- Mata A. M., Stefanova H. I., Gore M. G., Khan Y. M., East J. M., Lee A. G. Localization of Cys-344 on the (Ca(2+)-Mg(2+)-ATPase of sarcoplasmic reticulum using resonance energy transfer. Biochim Biophys Acta. 1993 Apr 8;1147(1):6–12. doi: 10.1016/0005-2736(93)90309-n. [DOI] [PubMed] [Google Scholar]
- Michelangeli F., Grimes E. A., East J. M., Lee A. G. Effects of phospholipids on the function of (Ca2(+)-Mg2+)-ATPase. Biochemistry. 1991 Jan 15;30(2):342–351. doi: 10.1021/bi00216a006. [DOI] [PubMed] [Google Scholar]
- Michelangeli F., Orlowski S., Champeil P., Grimes E. A., East J. M., Lee A. G. Effects of phospholipids on binding of calcium to (Ca2(+)-Mg2(+)-ATPase. Biochemistry. 1990 Sep 11;29(36):8307–8312. doi: 10.1021/bi00488a015. [DOI] [PubMed] [Google Scholar]
- Nakamura H., Jilka R. L., Boland R., Martonosi A. N. Mechanism of ATP hydrolysis by sarcoplasmic reticulum and the role of phospholipids. J Biol Chem. 1976 Sep 10;251(17):5414–5423. [PubMed] [Google Scholar]
- Nakamura S., Suzuki H., Kanazawa T. The ATP-induced change of tryptophan fluorescence reflects a conformational change upon formation of ADP-sensitive phosphoenzyme in the sarcoplasmic reticulum Ca(2+)-ATPase. Stopped-flow spectrofluorometry and continuous flow-rapid quenching method. J Biol Chem. 1994 Jun 10;269(23):16015–16019. [PubMed] [Google Scholar]
- Obara M., Suzuki H., Kanazawa T. Conformational changes in the vicinity of the N-iodoacetyl-N'-(5-sulfo-1-naphthyl)ethylenediamine attached to the specific thiol of sarcoplasmic reticulum Ca2+-ATPase throughout the catalytic cycle. J Biol Chem. 1988 Mar 15;263(8):3690–3697. [PubMed] [Google Scholar]
- Orlowski S., Champeil P. The two calcium ions initially bound to nonphosphorylated sarcoplasmic reticulum Ca(2+)-ATPase can no longer be kinetically distinguished when they dissociate from phosphorylated ATPase toward the lumen. Biochemistry. 1991 Nov 26;30(47):11331–11342. doi: 10.1021/bi00111a020. [DOI] [PubMed] [Google Scholar]
- Simmonds A. C., East J. M., Jones O. T., Rooney E. K., McWhirter J., Lee A. G. Annular and non-annular binding sites on the (Ca2+ + Mg2+)-ATPase. Biochim Biophys Acta. 1982 Dec 22;693(2):398–406. doi: 10.1016/0005-2736(82)90447-3. [DOI] [PubMed] [Google Scholar]
- Simmonds A. C., Rooney E. K., Lee A. G. Interactions of cholesterol hemisuccinate with phospholipids and (Ca2+-Mg2+)-ATPase. Biochemistry. 1984 Mar 27;23(7):1432–1441. doi: 10.1021/bi00302a015. [DOI] [PubMed] [Google Scholar]
- Stahl N., Jencks W. P. Reactions of the sarcoplasmic reticulum calcium adenosinetriphosphatase with adenosine 5'-triphosphate and Ca2+ that are not satisfactorily described by an E1-E2 model. Biochemistry. 1987 Dec 1;26(24):7654–7667. doi: 10.1021/bi00398a019. [DOI] [PubMed] [Google Scholar]
- Starling A. P., East J. M., Lee A. G. Effects of gel phase phospholipid on the Ca(2+)-ATPase. Biochemistry. 1995 Mar 7;34(9):3084–3091. doi: 10.1021/bi00009a040. [DOI] [PubMed] [Google Scholar]
- Starling A. P., East J. M., Lee A. G. Effects of phosphatidylcholine fatty acyl chain length on calcium binding and other functions of the (Ca(2+)-Mg2+)-ATPase. Biochemistry. 1993 Feb 16;32(6):1593–1600. doi: 10.1021/bi00057a025. [DOI] [PubMed] [Google Scholar]
- Starling A. P., East J. M., Lee A. G. Evidence that the effects of phospholipids on the activity of the Ca(2+)-ATPase do not involve aggregation. Biochem J. 1995 May 15;308(Pt 1):343–346. doi: 10.1042/bj3080343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starling A. P., Hughes G., East J. M., Lee A. G. Mechanism of stimulation of the calcium adenosinetriphosphatase by jasmone. Biochemistry. 1994 Mar 15;33(10):3023–3031. doi: 10.1021/bi00176a035. [DOI] [PubMed] [Google Scholar]
- Starling A. P., Khan Y. M., East J. M., Lee A. G. Characterization of the single Ca(2+)-binding site on the Ca(2+)-ATPase reconstituted with short- or long-chain phosphatidylcholines. Biochem J. 1994 Dec 1;304(Pt 2):569–575. doi: 10.1042/bj3040569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova H. I., Mata A. M., Gore M. G., East J. M., Lee A. G. Labeling the (Ca(2+)-Mg2+)-ATPase of sarcoplasmic reticulum at Glu-439 with 5-(bromomethyl)fluorescein. Biochemistry. 1993 Jun 15;32(23):6095–6103. doi: 10.1021/bi00074a022. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Nakamura S., Kanazawa T. Effects of divalent cations bound to the catalytic site on ATP-induced conformational changes in the sarcoplasmic reticulum Ca(2+)-ATPase: stopped-flow analysis of the fluorescence of N-acetyl-N'-(5-sulfo-1-naphthyl)ethylenediamine attached to cysteine-674. Biochemistry. 1994 Jul 12;33(27):8240–8246. doi: 10.1021/bi00193a010. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Obara M., Kuwayama H., Kanazawa T. A conformational change of N-iodoacetyl-N'-(5-sulfo-1-naphthyl)ethylenediamine-labeled sarcoplasmic reticulum Ca2+-ATPase upon ATP binding to the catalytic site. J Biol Chem. 1987 Nov 15;262(32):15448–15456. [PubMed] [Google Scholar]
- Warren G. B., Toon P. A., Birdsall N. J., Lee A. G., Metcalfe J. C. Complete control of the lipid environment of membrane-bound proteins: application to a calcium transport system. FEBS Lett. 1974 Apr 15;41(1):122–124. doi: 10.1016/0014-5793(74)80969-5. [DOI] [PubMed] [Google Scholar]
- Warren G. B., Toon P. A., Birdsall N. J., Lee A. G., Metcalfe J. C. Reversible lipid titrations of the activity of pure adenosine triphosphatase-lipid complexes. Biochemistry. 1974 Dec 31;13(27):5501–5507. doi: 10.1021/bi00724a008. [DOI] [PubMed] [Google Scholar]


