Abstract
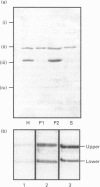
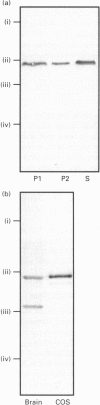
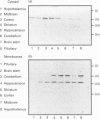
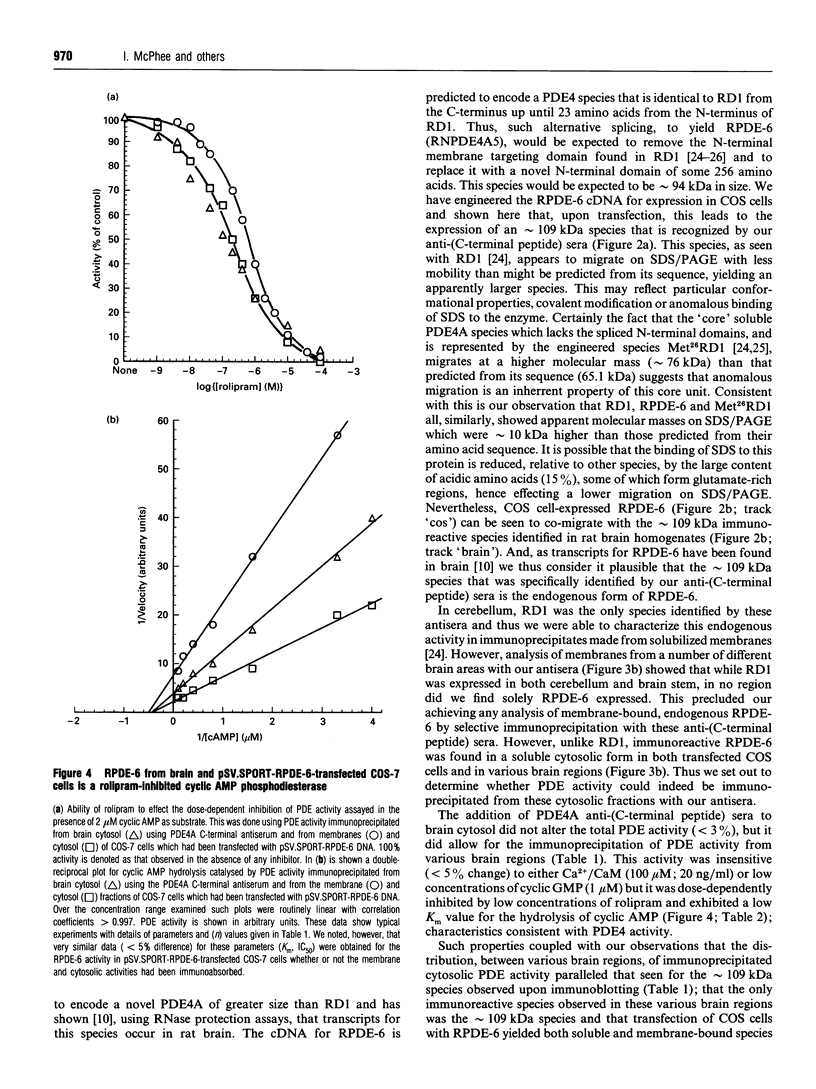
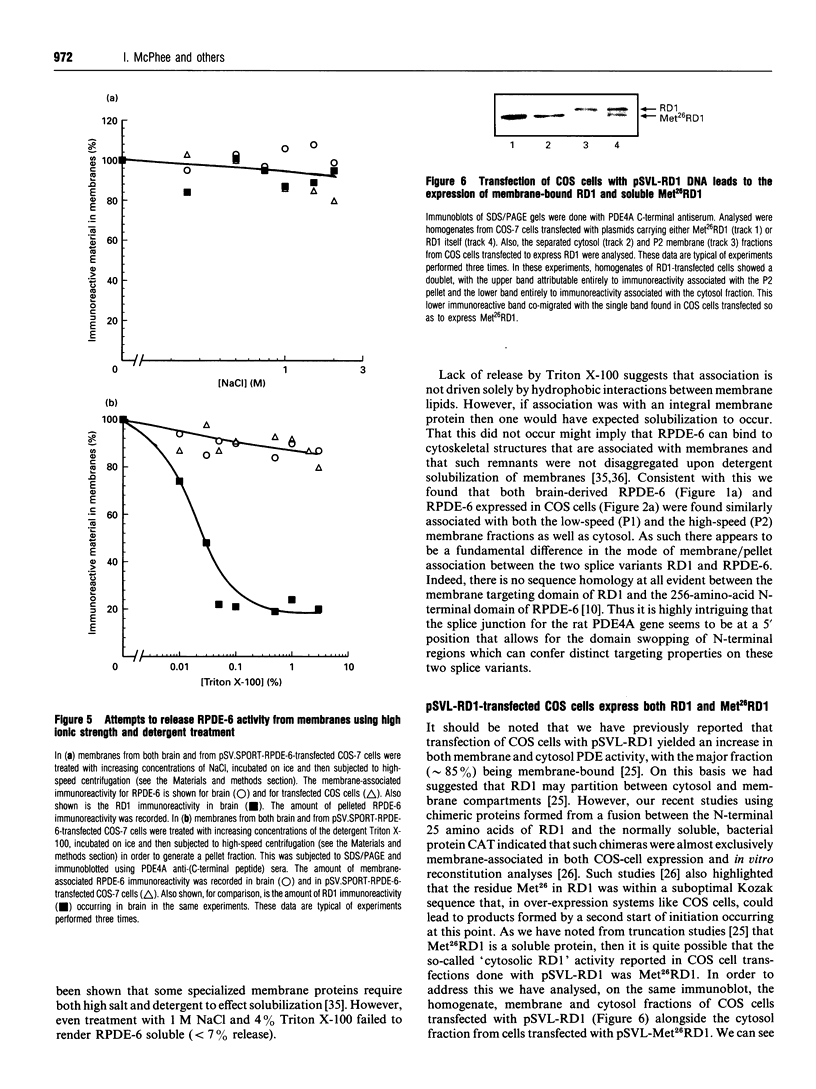
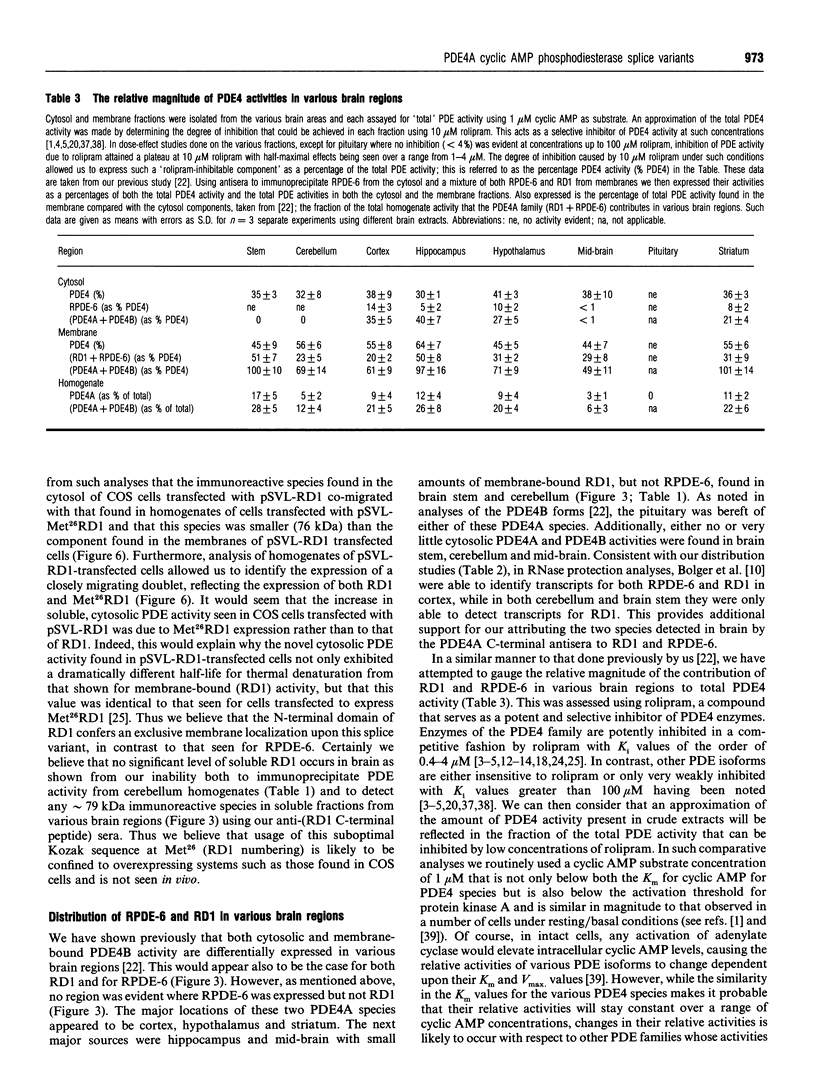
COS-7 cells were transfected with a plasmid encoding a putative splice variant of PDE4A cyclic AMP-specific phosphodiesterase, RPDE-6 (RNPDE4A5). This led to the expression of a novel, cyclic AMP-specific, rolipram-inhibited phosphodiesterase activity. In such transfected cells a novel approximately 109 kDa species was recognized by anti-peptide sera raised against a dodecapeptide whose sequence is found at the extreme C-terminus of both RPDE-6 and another PDE4A splice variant. RD1 (RNPDE4A1A). RPDE-6 activity and immunoreactivity was found distributed between both pellet (approximately 25%) and cytosol (approximately 75%) fractions of transfected COS-7 cells. Soluble and pellet RPDE-6 activities exhibited similar low Km values for cyclic AMP (approximately 2.4 microM) and were both inhibited by low concentrations of rolipram, with IC50 values for the soluble activity being lower (approximately 0.16 microM) than for the pellet activity (approximately 1.2 microM). Pellet RPDE-6 was resistant to release by either high NaCl concentrations or the detergent Triton X-100. Probing brain homogenates with the anti-(C-terminal peptide) sera identified two immunoreactive species, namely an approximately 79 kDa species reflecting RD1 and an approximately 109 kDa species that co-migrated with the immunoreactive species seen in COS cells transfected to express RPDE-6. The approximately 109 kDa species was found distributed between both the low-speed (P1) and high-speed (P2) pellet fractions as well as the cytosol fractions derived from both brain and RPDE-6-transfected COS cells. In contrast, RD1 was found exclusively in the P2 fraction. Phosphodiesterase (PDE) activity immuno-precipitated by these antisera from brain cytosol had the characteristics of COS cell-expressed RPDE-6 with KmcyclicAMP approximately 3.7 microM and IC50rolipram approximately 0.12 microM. The distribution of PDE activity immunoprecipitated from the cytosol of various brain regions paralleled that seen for the distribution of the approximately 109 kDa immunoreactive species. It is suggested that the 109 kDa species identified in brain cytosol and pellet fractions is the native form of RPDE-6. The PDE4A splice variants, RD1 and RPDE-6, were shown to have distinct patterns of expression among various brain regions. PDE4A and PDE4B activities appear to provide the major source of PDE4 activity in brain membranes, whereas the cytosolic PDE4 activity is suggested to reflect predominantly the activity of the PDE4D family. Alternative splicing of the PDE4A gene confers distinct N-terminal domains on RPDE-6 and RD1, which attenuates the Vmax. of these enzymes and defines their distinct subcellular distribution pattern.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baecker P. A., Obernolte R., Bach C., Yee C., Shelton E. R. Isolation of a cDNA encoding a human rolipram-sensitive cyclic AMP phosphodiesterase (PDE IVD). Gene. 1994 Jan 28;138(1-2):253–256. doi: 10.1016/0378-1119(94)90818-4. [DOI] [PubMed] [Google Scholar]
- Beavo J. A., Conti M., Heaslip R. J. Multiple cyclic nucleotide phosphodiesterases. Mol Pharmacol. 1994 Sep;46(3):399–405. [PubMed] [Google Scholar]
- Bolger G. B. Molecular biology of the cyclic AMP-specific cyclic nucleotide phosphodiesterases: a diverse family of regulatory enzymes. Cell Signal. 1994 Nov;6(8):851–859. doi: 10.1016/0898-6568(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bolger G. B., Rodgers L., Riggs M. Differential CNS expression of alternative mRNA isoforms of the mammalian genes encoding cAMP-specific phosphodiesterases. Gene. 1994 Nov 18;149(2):237–244. doi: 10.1016/0378-1119(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Bolger G., Michaeli T., Martins T., St John T., Steiner B., Rodgers L., Riggs M., Wigler M., Ferguson K. A family of human phosphodiesterases homologous to the dunce learning and memory gene product of Drosophila melanogaster are potential targets for antidepressant drugs. Mol Cell Biol. 1993 Oct;13(10):6558–6571. doi: 10.1128/mcb.13.10.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Takayasu H., Eberwine M., Myres J. Cloning and characterization of mammalian homologs of the Drosophila dunce+ gene. Proc Natl Acad Sci U S A. 1989 May;86(10):3604–3608. doi: 10.1073/pnas.86.10.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels P., Abdel'Al S., Hulley P., Lübbert H. Brain distribution of four rat homologues of the Drosophila dunce cAMP phosphodiesterase. J Neurosci Res. 1995 Jun 1;41(2):169–178. doi: 10.1002/jnr.490410204. [DOI] [PubMed] [Google Scholar]
- Gordeladze J. O. The pharmacodynamic action of the cyclic AMP phosphodiesterase inhibitor rolipram on prolactin producing rat pituitary adenoma (GH4C1) cells. Biosci Rep. 1990 Aug;10(4):375–388. doi: 10.1007/BF01117237. [DOI] [PubMed] [Google Scholar]
- Horton Y. M., Sullivan M., Houslay M. D. Molecular cloning of a novel splice variant of human type IVA (PDE-IVA) cyclic AMP phosphodiesterase and localization of the gene to the p13.2-q12 region of human chromosome 19 [corrected]. Biochem J. 1995 Jun 1;308(Pt 2):683–691. doi: 10.1042/bj3080683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay M. D. The use of selective inhibitors and computer modelling to evaluate the role of specific high affinity cyclic AMP phosphodiesterases in the hormonal regulation of hepatocyte intracellular cyclic AMP concentrations. Cell Signal. 1990;2(1):85–98. doi: 10.1016/0898-6568(90)90036-a. [DOI] [PubMed] [Google Scholar]
- Jackson S. P., Schoenwaelder S. M., Yuan Y., Rabinowitz I., Salem H. H., Mitchell C. A. Adhesion receptor activation of phosphatidylinositol 3-kinase. von Willebrand factor stimulates the cytoskeletal association and activation of phosphatidylinositol 3-kinase and pp60c-src in human platelets. J Biol Chem. 1994 Oct 28;269(43):27093–27099. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Livi G. P., Kmetz P., McHale M. M., Cieslinski L. B., Sathe G. M., Taylor D. P., Davis R. L., Torphy T. J., Balcarek J. M. Cloning and expression of cDNA for a human low-Km, rolipram-sensitive cyclic AMP phosphodiesterase. Mol Cell Biol. 1990 Jun;10(6):2678–2686. doi: 10.1128/mcb.10.6.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobban M., Shakur Y., Beattie J., Houslay M. D. Identification of two splice variant forms of type-IVB cyclic AMP phosphodiesterase, DPD (rPDE-IVB1) and PDE-4 (rPDE-IVB2) in brain: selective localization in membrane and cytosolic compartments and differential expression in various brain regions. Biochem J. 1994 Dec 1;304(Pt 2):399–406. doi: 10.1042/bj3040399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe M. E. Site-specific mutations in the COOH-terminus of placental alkaline phosphatase: a single amino acid change converts a phosphatidylinositol-glycan-anchored protein to a secreted protein. J Cell Biol. 1992 Feb;116(3):799–807. doi: 10.1083/jcb.116.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchmont R. J., Houslay M. D. A peripheral and an intrinsic enzyme constitute the cyclic AMP phosphodiesterase activity of rat liver plasma membranes. Biochem J. 1980 May 1;187(2):381–392. doi: 10.1042/bj1870381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin M. M., Cieslinski L. B., Burman M., Torphy T. J., Livi G. P. A low-Km, rolipram-sensitive, cAMP-specific phosphodiesterase from human brain. Cloning and expression of cDNA, biochemical characterization of recombinant protein, and tissue distribution of mRNA. J Biol Chem. 1993 Mar 25;268(9):6470–6476. [PubMed] [Google Scholar]
- Milatovich A., Bolger G., Michaeli T., Francke U. Chromosome localizations of genes for five cAMP-specific phosphodiesterases in man and mouse. Somat Cell Mol Genet. 1994 Mar;20(2):75–86. doi: 10.1007/BF02290677. [DOI] [PubMed] [Google Scholar]
- Monaco L., Vicini E., Conti M. Structure of two rat genes coding for closely related rolipram-sensitive cAMP phosphodiesterases. Multiple mRNA variants originate from alternative splicing and multiple start sites. J Biol Chem. 1994 Jan 7;269(1):347–357. [PubMed] [Google Scholar]
- Nicholson C. D., Challiss R. A., Shahid M. Differential modulation of tissue function and therapeutic potential of selective inhibitors of cyclic nucleotide phosphodiesterase isoenzymes. Trends Pharmacol Sci. 1991 Jan;12(1):19–27. doi: 10.1016/0165-6147(91)90484-a. [DOI] [PubMed] [Google Scholar]
- Scotland G., Houslay M. D. Chimeric constructs show that the unique N-terminal domain of the cyclic AMP phosphodiesterase RD1 (RNPDE4A1A; rPDE-IVA1) can confer membrane association upon the normally cytosolic protein chloramphenicol acetyltransferase. Biochem J. 1995 Jun 1;308(Pt 2):673–681. doi: 10.1042/bj3080673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette C., Vicini E., Conti M. The ratPDE3/IVd phosphodiesterase gene codes for multiple proteins differentially activated by cAMP-dependent protein kinase. J Biol Chem. 1994 Jul 15;269(28):18271–18274. [PubMed] [Google Scholar]
- Shahid M., Nicholson C. D. Comparison of cyclic nucleotide phosphodiesterase isoenzymes in rat and rabbit ventricular myocardium: positive inotropic and phosphodiesterase inhibitory effects of Org 30029, milrinone and rolipram. Naunyn Schmiedebergs Arch Pharmacol. 1990 Dec;342(6):698–705. doi: 10.1007/BF00175715. [DOI] [PubMed] [Google Scholar]
- Shakur Y., Pryde J. G., Houslay M. D. Engineered deletion of the unique N-terminal domain of the cyclic AMP-specific phosphodiesterase RD1 prevents plasma membrane association and the attainment of enhanced thermostability without altering its sensitivity to inhibition by rolipram. Biochem J. 1993 Jun 15;292(Pt 3):677–686. doi: 10.1042/bj2920677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakur Y., Wilson M., Pooley L., Lobban M., Griffiths S. L., Campbell A. M., Beattie J., Daly C., Houslay M. D. Identification and characterization of the type-IVA cyclic AMP-specific phosphodiesterase RD1 as a membrane-bound protein expressed in cerebellum. Biochem J. 1995 Mar 15;306(Pt 3):801–809. doi: 10.1042/bj3060801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusarewicz P., Nilsson T., Hui N., Watson R., Warren G. Isolation of a matrix that binds medial Golgi enzymes. J Cell Biol. 1994 Feb;124(4):405–413. doi: 10.1083/jcb.124.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souness J. E., Scott L. C. Stereospecificity of rolipram actions on eosinophil cyclic AMP-specific phosphodiesterase. Biochem J. 1993 Apr 15;291(Pt 2):389–395. doi: 10.1042/bj2910389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan M., Egerton M., Shakur Y., Marquardsen A., Houslay M. D. Molecular cloning and expression, in both COS-1 cells and S. cerevisiae, of a human cytosolic type-IVA, cyclic AMP specific phosphodiesterase (hPDE-IVA-h6.1). Cell Signal. 1994 Sep;6(7):793–812. doi: 10.1016/0898-6568(94)00039-5. [DOI] [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]
- Wilson M., Sullivan M., Brown N., Houslay M. D. Purification, characterization and analysis of rolipram inhibition of a human type-IVA cyclic AMP-specific phosphodiesterase expressed in yeast. Biochem J. 1994 Dec 1;304(Pt 2):407–415. doi: 10.1042/bj3040407. [DOI] [PMC free article] [PubMed] [Google Scholar]