Abstract
The one-bead-one-compound (OBOC) technology enables one to generate thousands to millions of chemical molecules on resin beads (100 microns diameter) such that each bead displays 1013 copies of the same chemical entity. Whole-cell binding assays have been developed to screen OBOC combinatorial libraries for ligands that bind to specific cell surface receptors. While very powerful, this screening method does not address the downstream cell signaling properties of the binding ligand. We have modified OBOC technology by introducing a fixed known cell adhesion ligand to the outer layer of each bead. This one-bead two-compound (OB2C) library configuration allows the bound cells to interact with the random immobilized chemical molecules on each bead. The bound cells can then be probed for specific cellular responses such as apoptosis and activation or inhibition of a specific cell signaling pathway. To validate this concept, an OB2C combinatorial library was created such that a random hexapeptide plus a high affinity lymphoma targeting ligand LLP2A were displayed on each bead. This LLP2A-X6 OB2C library was then screened with human T-cell leukemia cells (Molt-4) for cell death responses. After 5 days of incubation, propidium iodide was added to the bead library to stain dead cells. Beads coated by red fluorescent cells were isolated for sequence analysis. Two ligands identified by this method, when added to the lymphoid cancer cells, were able to induce cell death.
Keywords: combinatorial chemistry, one-bead-two-compound, one-bead-one-compound, high throughput screening, peptide library, immunohistochemistry, death ligands, cell signaling ligands
INTRODUCTION
One-bead-one-compound (OBOC) combinatorial libraries are prepared by “split-mix” synthesis method such that each bead displays only one chemical entity (1). There are approximately 1013 copies of the same chemical entity on each bead. Millions of compounds can be synthesized in a short period of time and screened simultaneously using on-bead cell binding assays with specific functional probes (2–4). This powerful method has been applied to discover ligands against various biological targets, such as protein kinase substrates and inhibitors (5–6), protease substrates and inhibitors (7–8), cell surface receptors (9–10), and artificial enzymes (11–12) and various ligands for the preparation of affinity column media (13–14). There are about 20 different families of receptors on the cell membrane such as the receptor tyrosine kinase, receptor tyrosine phosphatase, cytokine receptor, G-protein coupled receptors (GPCR), death receptors, selectins, and integrins (15–17). These receptors are responsible for the communication between the extracellular microenvironment and the cell interior. Synthetic molecules that target these receptors (agonists or antagonists) are useful reagents in the study of biochemical pathways involving with these receptors. Some may even become useful lead compounds for the development of pharmaceuticals. The challenge is to develop a highly efficient and economical method that enables one to rapidly discover synthetic molecules that not only interact with cell surface receptors but are also able to either stimulate or inhibit downstream cell signaling. We believe this can be achieved with the novel one-bead-two-compound (OB2C) cell based screening strategy.
Meldal et al (2002) first introduced the OB2C concept and successfully applied it to identify protease inhibitors (18–19). In their OB2C libraries, each PEGA bead displayed two compounds: a library compound (OBOC format) and a fixed fluorescence-quenched protease substrate (second compound). Upon addition of a specific protease, the peptide substrate is cleaved, and releasing the quencher from the bead, resulting in an increase in fluorescent signal. Beads that are non fluorescent may be due to the interference of the random compound with enzyme function. Inhibitors displayed on positive compound-beads may compete with the protease substrate on the same bead directly or may be binding to the enzymes such that it alters the enzyme active confirmations and therefore suppress the fluorescent signal. The OB2C concept has also been applied to the discovery of artificial enzyme in which the library compound is the random peptide (OBOC format) and the fixed second compound is a proton sensitive fluorophore for the detection of catalytic products (20).
To date, the OB2C concept has only been applied to biochemical assays. Here we introduce the application of the OB2C concept to the discovery of cell surface acting cell signaling molecules using living cells as the screening probe. In this system, the fixed second compound is a well defined cell adhesion ligand that can capture live cells on the surface of every library bead, thus exposing their cell membranes to the tethered OB2C library compounds. The bound cells can then be probed for specific cellular responses such as apoptosis, cell morphology changes, and activation or suppression of a specific cell signaling pathway. Most of the protein-protein interactions are occurring between small domains of the two protein structure. So we have selected random hexamer peptide library for the initial screening. Here we describe the design and synthesis of a simple model OB2C combinatorial hexapeptide library, and use it to illustrate how this enabling technology can be used for the discovery of “death ligands” against lymphoma cells.
RESULTS & DISCUSSION
OB2C Strategy
In OB2C combinatorial libraries, two chemical molecules are on the same bead surface. One is a fixed known cell adhesion ligand and the other molecule is a random library compound. Under this configuration, live target cells are captured by a specific cell adhesion ligand resulting in the formation of a monolayer of targeted cells on the surface of each bead. Biological responses elicited by the tethered library compounds can be detected with a number of different methods. The cartoon in Figure 1 depicts the interactions of a living cell with the two synthetic molecules on the bead surface resulting in a cell signaling response. Many cell based screening assays can be adapted to this configuration. For example, propidium iodide (PI) can be used to identify dead cells, and caspase III fluorescent substrates can be used to identify cells undergoing apoptosis. In this study, propidium iodide (PI) was used to assess the viability of Molt-4 cells.
Figure 1.
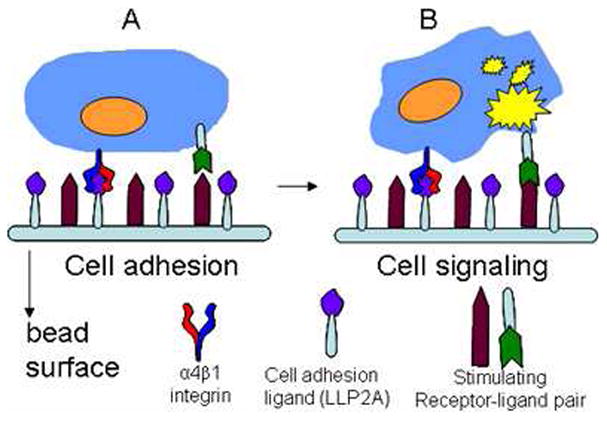
The OB2C strategy. In OB2C libraries, two chemical molecules are coated on the bead surface. One molecule is a fixed known cell adhesion ligand (e.g. LLP2A, bind to α4β1 integrin) and the other molecule is a random library compound. Cells bind to every bead due to the adhesion ligand on the bead surface (A). The interactions of the cell surface receptors with the two synthetic molecules on the bead surface may result in specific cell signaling response (B).
We have previously reported the use of OBOC combinatorial library methods to identify and optimize targeting ligands against malignant lymphoid cells (9, 21). One of these ligands, LLP2A, is able to bind to the activated α4β1 integrin of lymphoma cells with high affinity (IC50 = 2 pM) and specificity (9). In this study, we used LLP2A as the cell capturing agent for a hexapeptide library.
Library Design and Screening
Synthetic schemes for the synthesis of a OB2C hexapeptide library is shown in Figure 2. Hexapeptides are easy to make, constitute libraries of sufficiently high diversity, and are large enough to interact with receptors on the cell surface. To facilitate the preparation of a bead library with two different compounds on the bead surface for cell interaction, we first generated topologically segregated bilayer beads according to our previously published method (3). For the OB2C combinatorial library construction, multiple orthogonal protecting groups such as Fmoc (base labile), Boc (acid labile), Alloc (palladium II labile) and Dde (nucleophiles labile) were used.
Figure 2.
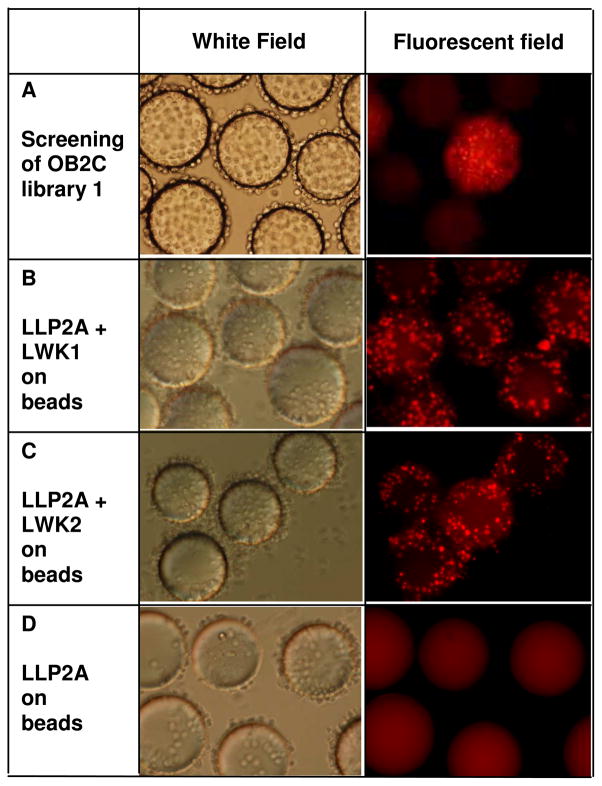
Screening OB2C linear hexapeptide library for death ligands. (A) Molt-4 cells were incubated with OB2C library beads for 5 days and then stained with propidium iodide. Under the white field, many beads were covered with cells, but only one bead could induce cell death (fluorescent field). Positive beads were sequenced and re-synthesized on beads for confirmation of cytotoxic activities. (B & C) LLP2A-LWK1 and LLP2A-LWK2 induced cell death whereas (D) LLP2A-beads did not. Most of the beads were covered with cells (White field).
After the topologically segregated bilayer beads were prepared with Fmoc on the outer layer and Boc in the inner core, the Fmoc on the outer layer was removed and then reprotected with a mixture of Fmoc-OSu and Alloc-OSu (ratio=1:1) to generate Chemset 2 (Scheme 1). For the hexapeptide library, the Fmoc protecting group on the outer layer was removed with piperidine, and a random Fmoc-amino acid was coupled to amine group of the bead by random split–mix synthesis method (22–24). This cycle was repeated for six times (Chemset 3). After the 6th cycle, Fmoc was removed and Boc was coupled as N-terminal protecting group (Chemset 4). The Alloc-protecting group was preserved until the construction of the library was complete. The Alloc group was then removed with palladium to allow attachment of the cell capturing ligand LLP2A to the remaining 50% substitution of the bead outer layer to Chemset 5 (Scheme 1). Finally all the protecting groups were removed with TFA:phenol:water:thioanisole:Tis (10:0.5:0.5:0.5:0.25, v/w/v/v/v) for 3 h. The beads were washed several times with DMF, methanol, DCM, methanol, water and with sterile PBS.
Scheme 1.
Synthesis of a OB2C linear hexapeptide library. Reagents and conditions: a) 1. swelling in water for 24 h; 2. Fmoc-OSu (0.2 equiv) and DIPEA (0.4 equiv), DCM/diethyl ether (55:45), vigorously shaking 30 min; b) Boc2O (5 equiv), DIPEA (10 equiv) in DMF, 1 h; c) 20% piperidine in DMF; d) Fmoc-OSu (0.1 equiv), Alloc-OSu (0.1 equiv) and DIPEA (0.4 equiv) in DMF, 2h; e) TFA in DCM (1:1), twice (30 min each); f) “split-mix” synthetic approach using Fmoc-L-amino acids; g) Pd(PPh3)4 (0.2 equiv), PhSiH3 (20 equiv) in DCM, twice (30 min each time); h) on bead synthesis of LLP2A; i): TFA: phenol: water: thioanisole: Tis (10:0.5:0.5:0.5:0.25, v/w/v/v/v), 3 h.
Screening for Death Ligands
Apoptosis is a Type-I programmed cell death and has evolved in multicellular animals as a means of eliminating abnormal cells and older cells that are no longer needed. It can be induced by two pathways; (a) the intrinsic pathway promoted by a family of cytosolic protein (Bcl-2) to activate the inner apoptosis cascade, and (b) the extrinsic pathway induced by pro-apoptotic ligands bound to the death receptors present on the target cell surface (25–26). Examples of the latter include tumor-necrosis factor (TNF) and Apoptosis ligand 2/TNF-related apoptosis-inducing ligand (Apo2L/TRAIL). One class of agents targeting death receptors, also called “pro-apoptotic receptor agonists” (PARA) holds remarkable promise in cancer therapy (27–28). By targeting the extrinsic apoptosis pathway, these agents help to circumvent some of the most common anti-apoptotic mutations in cancer cells.
To identify potential death ligands against human T-cell leukemia, Molt-4 cells were co-cultured with approximately 2 million LLP2A-X6 OB2C library beads in RPMI 1640 medium supplemented with 10% fetal bovine serum. The library beads were pre-incubated with growth media overnight to saturate the beads with growth media as well as to block the non-specific binding of cells. The cell quantities were adjusted so that only 10% of beads were covered by cells, and most of the unbound cells in the supernatant were removed. Fresh media was added and the cells were allowed to grow on the beads. It took 5 days for the cells to cover the entire surface of most of the beads. Molt-4 cells were selected for initial validation because they express high levels of α4β1 receptors, so most of the beads in the library were covered with monolayer of cells (Figure 2A, white field). At the end of the 5th day, the cells were treated with PI (1 μg/mL) and examined under the fluorescent microscope for cell death (Figure. 2B, fluorescent field). PI was used as the indicator reagent because it stains late apoptotic cells as well as necrotic cells (29–30).
Cell bound beads which fluoresced positive for PI staining were collected and micro sequenced. Of the 2 million beads screened, 8 positive beads were detected and these death ligands were resynthesized on TentaGel beads. Their ability to induce cell death were evaluated in both Molt-4 and Jurkat (another human T-cell leukemia cell line) cells. Out of the 8 positive beads two strongly positive hexapeptide-beads LWK1 (HGSYWQ) and LWK2 (EQAHEL) were selected for further characterization (Figure. 2B and 2C). In contrast, control beads displaying LLP2A alone were not able to elicit cell death (Figure. 2D).
Characterization of Death Ligands
LWK1 and LWK2 ligands were found to induce cell death on bead. To validate and quantify their antiproliferative effects in free form as well as in immobilized form. LLP2A and the death ligands were resynthesized in biotinylated form on Rink resin with the general structure: ligand-linker-lysine(biotin)-resin (Supporting information-1). N-Fmoc protected polyoxyethylene based amino acid type hydrophilic linker (31) was added between the ligand and lysine(biotin). The structure and synthesis of the linker is shown in the supporting information (SI3). The biotinylated peptides were then purified after deprotection and TFA cleavage. To simulate the condition of the bead surface on which the ligand was discovered, LLP2A-Bio plus either LWK1-Bio or LWK2-Bio were loaded onto the streptavidin-coated 96-well plates. Irrelevant hexapeptide AQAEAR-Bio (C-X6) was used as a negative control. For peptide and LLP2A combinations, the peptides and LLP2A were premixed in 1:1 molar ratio prior to adding to the streptavidin-coated wells.
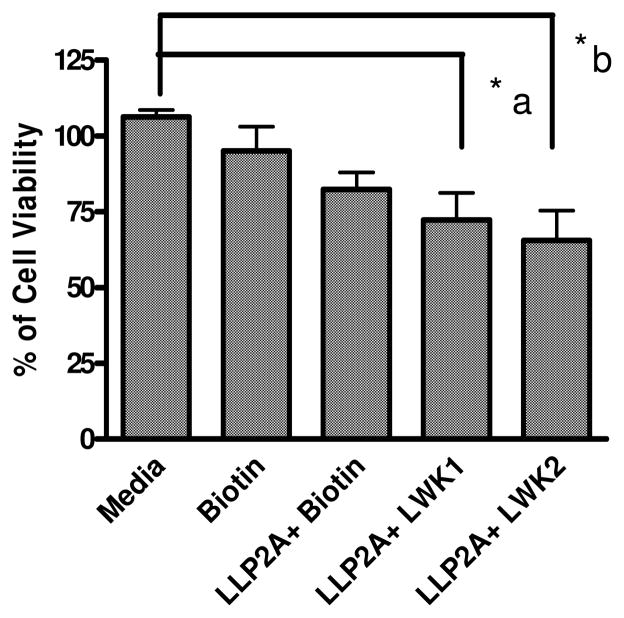
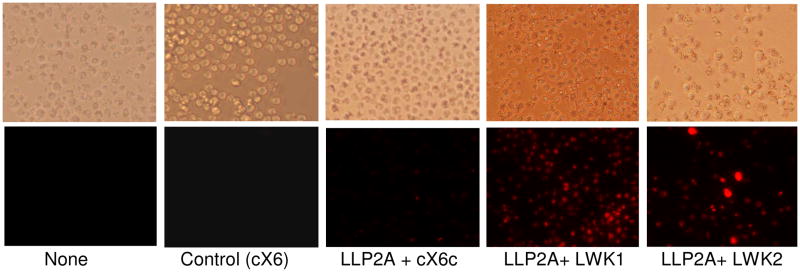
Molt-4 cells were then seeded onto the peptide-coated plate, and incubated for 3 days. The residual viable cells were determined by MTS assay, which measures the mitochondrial reductase enzyme activity of the viable cells. The result of this study is shown in Figure 3. The combination of LLP2A and LWK1 or LLP2A and LWK2 showed the highest anti-proliferative effects with 62–65% cell viability (p<0.05%) when compared to the control (100%). In contrast, immobilized LLP2A alone showed 20% antiproliferative activity only. The MTS assay was found to correlate well with the PI staining assay which stains only dead cells. As shown in Figure 4, PI staining was prominent in the wells coated with the combination peptides (LLP2A and LWK1 or LLP2A and LWK2), but not LLP2A alone nor irrelevant hexapeptide C-X6 alone.
Figure 3.
Effects of immobilized peptides on Molt-4 cells. Streptavidin-coated plates were loaded with biotin-linker (denoted as Biotin) alone or with biotinylated ligands. Molt-4 cells were then cultured in these peptide-coated plates for 3 days. At the end of the 3rd day MTS assay was performed to evaluate for cell viability. Approximately 30–40% reduction in cell activity (*a,b p < 0.05%) was observed in LLP2A+LWK1 and LLP2A+LWK2 ligand coated wells when compared to uncoated wells. Only 20% reduction was noticed in LLP2A coated wells.
Figure 4.
Propidium Iodide (PI) staining for dead cells: A similar experiment was set up like in Fig 3, with the exception that the cells were stained with PI at the end of the 3rd day. The fluorescent photomicrographs clearly shows the presence of dead cells in the wells coated with biotinylated LWK1 and LWK2 ligands, but not in uncoated wells or wells coated with biotin-linker or biotin-LLP2A.
To test the function of the peptides in solution, the above MTS experiment was repeated with biotinylated LWK1 and biotinylated LWK2 in concentrations ranging from 10 μM to 100 μM. Biotinylated LLP2A and biotinylated-linker were used as controls. Biotin-LWK1 alone in free form could elicit a 30% reduction in cell growth (30%) only at high concentration (100 μM), and biotin-LWK2 was inactive in free form (Fig 5). However, when the biotinylated peptides were complexed with streptavidin, their cytotoxic effects increased dramatically. (Figure 3 and 4).
Figure 5.
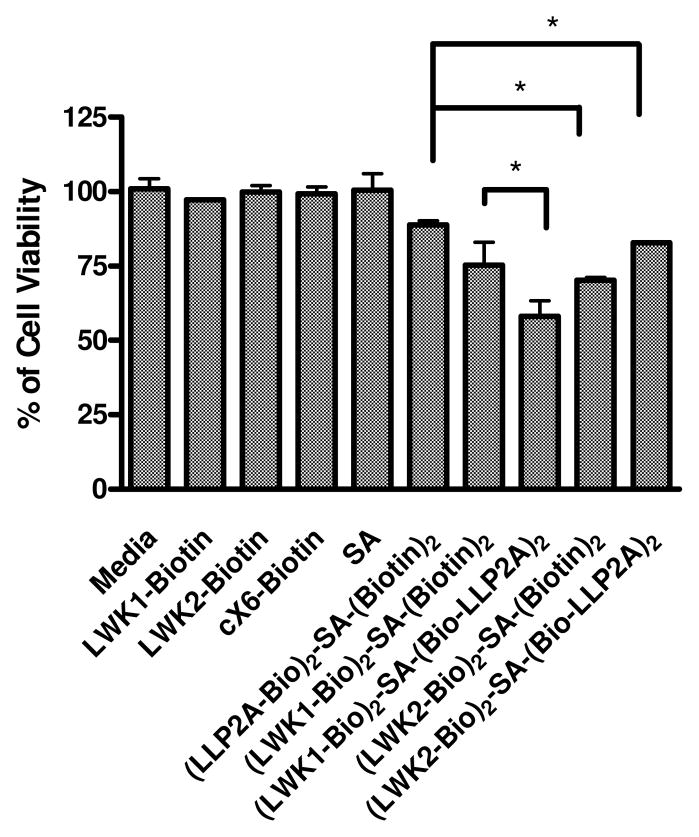
Cellular effects of death ligand-streptavidin complex on Molt-4 cells. The SA-ligand complexes were incubated at 1 μM (based on SA) for 3 days with the target cells. At the end of 3rd day cell viability assay was performed using MTS assay. (LLP2A-Bio)2-SA-(Bio-LWK1)2 showed significantly more killing than (LWK1-Bio)2-SA-(Biotin)2 or (LLP2A-Bio)2-SA-(Biotin)2 (*a,b,c P<0.05%). Unexpectedly, (LLP2A-Bio)2-SA-(Bio-LWK2)2 showed no significant killing. LWK1-Biotin and LWK2-Biotin did not show any killing in their free form at the same concentration.
Streptavidin (SA) is a tetrameric protein with four biotin-binding sites. It can be used as a convenient protein scaffold to construct homo- or hetero- dimeric, trimeric or tetrameric ligands by simply mixing the appropriate ratio of biotinylated peptide(s) and biotinylated linker prior to mixing with one-quarter molar ratio of streptavidin. Although the final streptavidin complex will not be homogenous, the intended hybrid ligand will be the predominant complex in the mixture. These SA-ligand complexes were incubated with the target cells at 1 μM concentration (based on SA concentration) for 3 days, at which time the MTS assay was performed (Figure 5). The (LLP2A-Bio)2-SA-(Bio-LWK1)2 hetero-complex showed significantly higher anti-proliferative activity than (LWK1-Bio)2-SA-(Biotin)2 or (LLP2A-Bio)2-SA-(Biotin)2 complexes (p>0.05%). This can be easily explained by the fact that LLP2A, with its high affinity against activated α4β1 integrin, was able to direct LWK1 to the cell surface of the target cells, causing apoptosis. The anti-proliferative activity of (LLP2A-Bio)2-SA-(Bio-LWK2)2 hetero-complex, on the other hand, was unexpectedly low. The reason for this result is not so clear, although one plausible explanation is that LWK2 may require polyvalent ligands to elicit cellular effects, which is the case on the bead surface but cannot be duplicated in using soluble streptavidin-biotin ligand complex.
In principle, LWK1 can be further optimized through screening a focused OBOC library (analogues of LWK1) without the second capturing molecule LLP2A. Such a library can then be screened for beads that not only bind strongly to the target cells but also cause apoptosis. However, unless the death ligand receptor is unique to the target cancer cells, cytotoxic specificity is a concern. In that case, there may be an advantage to exploiting the existing cancer cell surface targeting ligands (32–35), by using them as high-affinity and high-specificity cancer targeting agents to deliver the optimized death ligand to the tumor cells.
In summary, we have demonstrated an enabling ultra-high throughput OB2C cell based library screening method (Figure 1). OB2C is highly versatile, efficient and economical. This technology can in principle be utilized for the discovery of chemical modulators against cell surface receptors with the appropriate gene reporter systems (GFP, luciferase) or antibodies against the specific signaling proteins (e.g. phospho-specific, methylation-specific, sulfo-specific). With an appropriate reporter system, one should be able to rapidly detect beads that can elicit a specific biochemical or cellular response (agonists). Similarly, if the cells are stimulated by an exogenous agonist, molecules that suppress specific biochemical or cellular response (antagonists) can also be discovered with this approach.
EXPERIMENTAL SECTION
Materials
TentaGel S NH2 resin (90 μm diameter) and rink amide MBHA resin were purchased from Rapp Polymere GmbH (Tübingen, Germany). HOBt, Alloc-OSu, and Fmoc-OSu were obtained from GL Biochem (Shanghai, China). Fmoc-protected amino acids were purchased from Anaspec (Fremont, CA). DIC, DIPEA, phenylsilane, tetrakisphenylphosphine palladium, all organic solvents, and other chemical reagents were purchased from Sigma-Aldrich (Milwaukee, WI). All solvents were directly used in the library synthesis without any purification unless otherwise noted.
General methods
Coupling completeness and Fmoc deprotection were monitored by Kaiser test. For Fmoc deprotection, beads were incubated with 20% piperidine solution in DMF twice (5 min, 15 min) and then thoroughly washed with DMF, MeOH and DMF three times each, respectively. For Boc deprotection, beads were incubated with 55% TFA/DCM for 30 min, twice, and then washed with 2% DIPEA/DMF once and DMF (five times). For Alloc deprotection, the resulting beads were incubated with (Pd(PPh3)4) (0.2 equiv) and PhSiH3 (20 equiv) in DCM for 30 min (twice) and washed with 0.5% diethyldithiocarbamic acid sodium salt in DMF (3 times) and DMF (10 times). For library synthesis, a typical synthetic cycle utilizing the “split-mix” approach is described as follows: (1) beads were split into aliquots as desired; (2) each aliquot of beads was coupled with a specific Fmoc-protected amino acid in the presence of HOBt and DIC for 2 h; (3) after coupling all aliquots of beads were mixed together and washed with DMF five times.
A Perkin-Elmer/Applied Biosystems Protein Sequencer (ABI Procise 494) was used for library bead decoding. Analytical HPLC analysis (Vydac column, 4.6 mm × 250 mm, 5 μm, 300Å, C18, 1.0 ml/min, 20 min gradient from 100% aqueous media (0.1% TFA) to 100% acetonitrile (0.1% TFA), 214, 220, 254 and 280 nm) and preparative HPLC purification (Vadac column, 20 mm × 250 mm, 5 μm, 300 Å, C18, 7.0 ml/min, 45 min gradient from 100% aqueous media (0.1% TFA) to 100% acetonitrile (0.1% TFA), 214 nm) were performed on a Beckman System Gold HPLC system (Fullerton, CA). Mass spectra were acquired on a Thermo Fisher LCQ (San Jose, CA) fitted with an electrospray source in the positive ion mode. Loop injections were made with standard electrospray conditions using a methanol and 0.1% formic acid/water solvent system.
Synthesis of OB2C hexapeptide library
TentaGel resin (2 g, loading: 0.30 mmol/g) was swollen in water for 24 h and was then protected with Fmoc-OSu (0.2 equiv, 0.12 mmol)/DIPEA (0.4 equiv, 0.24 mmol) in DCM/diethyl ether (55: 45) for 30 min under vigorous shaking followed by treatment with (Boc)2O (5 equiv, 3 mmol)/DIPEA (10 equiv, 6 mmol) for 2 h. After Fmoc-deprotection (see the general method), a solution of Fmoc-OSu (0.1 equiv, 0.06 mmole), Alloc-OSu (0.1 equiv, 0.06 mmol) and DIPEA (0.4 equiv, 0.24 mmol) in DMF was used to protect the exposed N-terminus. Upon Fmoc deprotection and Boc deprotection (see general methods); the beads were assembled as a hexapeptide library using 19 Fmoc-protected L-amino acids (cysteine excluded) according to the standard “split-mix” approach. The combined beads were Fmoc-deprotected and protected by (Boc)2O (1.44 mmole)/DIPEA (1.44 mmole) in DMF. After Alloc deprotection (see the general methods), LLP2A was synthesized on the beads (9). The beads were then dried under vacuum before adding a TFA-based cleavage cocktail (TFA: phenol: water: thioanisole: Tis, 10:0.5:0.5:0.5:0.25, v/w/v/v/v) for 3 h. After neutralization with 10% DIPEA/DMF (twice), the resin was washed sequentially with DMF, MeOH, DCM, DMF, DMF/H2O, and H2O, three times each. Then the beads were stored in 70% ethanol.
Screening OB2C library for death ligands
Approximately 2 miilion library beads were washed sequentially with ethanol, double-distilled water, PBS, and incubated with growth medium (RPMI 1640 supplemented with 10% fetal bovine serum and 100 IU/mL of penicillin and 100 μg/mL streptomycin) for 3 h. The cell based binding assay was performed by incubating beads with Molt-4, cells in Petri dishes in a 37°C humidified incubator (5% CO2) with shaking (40 rpm). Due to the high binding affinity of LLP2A to the α4β1 integrin expressed on the leukemic cell lines, most of the beads were bound with cells within 10 min of incubation. After covering 10% of the bead surface, the excess cells were removed by washing one time with growth media. At this point it took 5 days for cells to cover the beads. Change the media after two days gently. The cell-bound beads were kept in growth medium and monitored for 5 days. After 5 days, cells were treated with 1 μg/mL PI for 30 min. The cell-bound beads were washed gently to remove excess PI and observed under inverted Olympus fluorescence microscope. The positive beads were isolated and treated with 8 M guanidine/HCl to remove all cells from the beads. The beads were further washed with PBS and water three times each and decoded using an automated protein microsequencer.
Cell proliferation (MTS) assay
Cell proliferation studies were conducted on 96 well plates coated with either biotinylated LLP2A (LLP2A-bio) and biotin-linker or a mixture of LLP2A-bio and biotinylated discovered ligand (Ligand-bio) (1:1 molar ratio) in 10–100 μM range concentration. Sterile streptavidin coated plates (Sigma, St. Louis, MO) were used. Equimolar concentration of biotin was substituted in place of ligand for control experiments. LLP2A-bio and ligand-bio were incubated with plates for 2 h at room temperature, washed 3 times with PBS, and blocked with sterile 1% BSA in PBS for 1h. After 1h blocking, plates were washed. Molt-4 cells (1 X 104 cells per well) were seeded. After 3 days, the MTS/PMS solution was added and incubated for 4h. The absorbance was measured at 490 nm on a microplate reader (CellTiter 96® Aqueous Non-Radioactive Cell Proliferation Assay, Promega, Madison, WI).
Propidium iodide (PI) staining
PI staining was performed in Molt-4 cells with experimental conditions as detailed in cell proliferation assay. At the end of the 3rd day in the streptavidin coated plates PI (1 μg/mL final concentration) was added and incubated for 10 min. After one wash with PBS cells were photographed using fluorescence microscope.
Supplementary Material
Acknowledgments
This study is supported by funds from NIH-RO3CA129788, DOD-CML064046, NCI-U19CA113298 and Children’s Miracle Network of UC Davis Medical Center. We would like to thank Mr. David Olivos for editorial assistance.
Footnotes
Supporting Information Available: Details of the OB2C library synthesis (LLP2A and random hexa peptide), synthesis and characterization LWK1 and LWK2 peptides and structure and synthesis of Fmoc-hydrophilic linker are shown in the supporting information. This material is free via the Internet at http://pubs.acs.org.
References
- 1.Lam KS, Salmon SE, Hersh EM, Hruby VJ, Kazmierski WM, Knapp RJ. A new type of synthetic peptide library for identifying ligand-binding activity. Nature. 1991;354:82–84. doi: 10.1038/354082a0. [DOI] [PubMed] [Google Scholar]
- 2.Aina OH, Marik J, Gandour-Edwards R, Lam KS. Near-infrared optical imaging of ovarian cancer xenografts with novel alpha 3-integrin binding peptide “OA02”. Mol Imaging. 2005;4:439–447. doi: 10.2310/7290.2005.05169. [DOI] [PubMed] [Google Scholar]
- 3.Liu R, Wang X, Song A, Bao T, Lam KS. Development and applications of topologically segregated bilayer beads in one-bead one-compound combinatorial libraries. QSAR Comb Sci. 2005;24:1127–1140. doi: 10.1111/j.1399-3011.2005.00192.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Gambhir SS. Significance of one-bead-one-compound combinational chemistry. Nat Chem Biol. 2006;2:351–352. doi: 10.1038/nchembio0706-351. [DOI] [PubMed] [Google Scholar]
- 5.Lam KS, Lebl M, Krchnak V. The “One-Bead-One-Compound” Combinatorial Library Method. Chem Rev. 1997;97:411–448. doi: 10.1021/cr9600114. [DOI] [PubMed] [Google Scholar]
- 6.Lam KS, Liu R, Miyamoto S, Lehman AL, Tuscano JM. Applications of one-bead one-compound combinatorial libraries and chemical microarrays in signal transduction research. Acc Chem Res. 2003;36:370–377. doi: 10.1021/ar0201299. [DOI] [PubMed] [Google Scholar]
- 7.Meldal M, Svendsen I, Breddam K, Auzanneau FI. Portion-mixing peptide libraries of quenched fluorogenic substrates for complete subsite mapping of endoprotease specificity. Proc Natl Acad Sci U S A. 1994;91:3314–3318. doi: 10.1073/pnas.91.8.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olsen JA, Jensen KJ, Nielsen J. Combinatorial solid-phase synthesis of hapalosin mimetics. J Comb Chem. 2000;2:143–150. doi: 10.1021/cc990062p. [DOI] [PubMed] [Google Scholar]
- 9.Peng L, Liu R, Marik J, Wang X, Takada Y, Lam KS. Combinatorial chemistry identifies high-affinity peptidomimetics against alpha4beta1 integrin for in vivo tumor imaging. Nature chemical biology. 2006;2:381–389. doi: 10.1038/nchembio798. [DOI] [PubMed] [Google Scholar]
- 10.Yao N, Xiao W, Wang X, Marik J, Park SH, Takada Y, Lam KS. Discovery of targeting ligands for breast cancer cells using the one-bead one-compound combinatorial method. J Med Chem. 2009;52:126–133. doi: 10.1021/jm801062d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Copeland GT, Miller SJ. Selection of enantioselective acyl transfer catalysts from a pooled peptide library through a fluorescence-based activity assay: an approach to kinetic resolution of secondary alcohols of broad structural scope. J Am Chem Soc. 2001;123:6496–6502. doi: 10.1021/ja0108584. [DOI] [PubMed] [Google Scholar]
- 12.Evans CA, Miller SJ. Proton-activated fluorescence as a tool for simultaneous screening of combinatorial chemical reactions. Curr Opin Chem Biol. 2002;6:333–338. doi: 10.1016/s1367-5931(02)00324-1. [DOI] [PubMed] [Google Scholar]
- 13.Tozzi C, Anfossi L, Baggiani C, Giovannoli C, Giraudi G. A combinatorial approach to obtain affinity media with binding properties towards the aflatoxins. Analytical and bioanalytical chemistry. 2003;375:994–999. doi: 10.1007/s00216-003-1754-z. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman DB, Hentsch ME, Baumbach GA, Buettner JA, Dadd CA, Huang PY, Hammond DJ, Carbonell RG. Affinity purification of fibrinogen using a ligand from a peptide library. Biotechnology and Bioengineering. 2002;77:278–289. doi: 10.1002/bit.10120. [DOI] [PubMed] [Google Scholar]
- 15.Deller MC, Jones EY. Cell surface receptors. Current Opinion in Structural Biology. 2000;10:213–219. doi: 10.1016/s0959-440x(00)00072-5. [DOI] [PubMed] [Google Scholar]
- 16.Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nature Cell Biology. 2002;4:E83–E90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- 17.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nature Reviews Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 18.Meldal M. The one-bead two-compound assay for solid phase screening of combinatorial libraries. Biopolymers. 2002;66:93–100. doi: 10.1002/bip.10229. [DOI] [PubMed] [Google Scholar]
- 19.Meldal M. ‘One bead two compound libraries’ for detecting chemical and biochemical conversions. Current Opinion in Chemical Biology. 2004;8:238–244. doi: 10.1016/j.cbpa.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Copeland G, Miller S. A chemosensor-based approach to catalyst discovery in solution and on solid support. J Am Chem Soc. 1999;121:4306–4307. [Google Scholar]
- 21.Peng L, Liu R, Andrei M, Xiao W, Lam KS. In vivo optical imaging of human lymphoma xenograft using a library-derived peptidomimetic against alpha4beta1 integrin. Mol Cancer Ther. 2008;7:432–437. doi: 10.1158/1535-7163.MCT-07-0575. [DOI] [PubMed] [Google Scholar]
- 22.Houghten RA, Pinilla C, Blondelle SE, Appel JR, Dooley CT, Cuervo JH. Generation and use of synthetic peptide combinatorial libraries for basic research and drug discovery. Nature. 1991;354:84–86. doi: 10.1038/354084a0. [DOI] [PubMed] [Google Scholar]
- 23.Hruby VJ, Lam KS, Alobeidi F, Kazmierski WM, Knapp R, Hersh EM, Salmon SE. The Design and Synthesis of Large Peptide Libraries Suitable for Discovery of Peptide-Macromolecular Interactions. Abstracts of Papers of the American Chemical Society. 1991;202:93-Medi. [Google Scholar]
- 24.Lam KS, Salmon SE, Hersh EM, Hruby VJ, Kazmierski WM, Knapp RJ. A new type of synthetic peptide library for identifying ligand-binding activity. Nature. 1991;354:82–84. doi: 10.1038/354082a0. [DOI] [PubMed] [Google Scholar]
- 25.Chowdhury I, Tharakan B, Bhat GK. Current concepts in apoptosis: The physiological suicide program revisited. Cellular & Molecular Biology Letters. 2006;11:506–525. doi: 10.2478/s11658-006-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elmore S. Apoptosis: A review of programmed cell death. Toxicologic Pathology. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moretto P, Hotte SJ. Targeting apoptosis: preclinical and early clinical experience with mapatumumab, an agonist monoclonal antibody targeting TRAIL-R1. Expert Opinion on Investigational Drugs. 2009;18:311–325. doi: 10.1517/13543780902752463. [DOI] [PubMed] [Google Scholar]
- 28.Ashkenazi A. Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nature Reviews Drug Discovery. 2008;7:1001–1012. doi: 10.1038/nrd2637. [DOI] [PubMed] [Google Scholar]
- 29.Hug H, Los M, Hirt W, Debatin KM. Rhodamine 110-linked amino acids and peptides as substrates to measure caspase activity upon apoptosis induction in intact cells. Biochemistry. 1999;38:13906–13911. doi: 10.1021/bi9913395. [DOI] [PubMed] [Google Scholar]
- 30.Darzynkiewicz Z, Li X, Gong J. Assays of cell viability: discrimination of cells dying by apoptosis. Methods in cell biology. 1994;41:15–38. doi: 10.1016/s0091-679x(08)61707-0. [DOI] [PubMed] [Google Scholar]
- 31.Song A, Wang X, Zhang J, Marik J, Lebrilla CB, Lam KS. Synthesis of hydrophilic and flexible linkers for peptide derivatization in solid phase. Bioorg Med Chem Lett. 2004;14:161–165. doi: 10.1016/j.bmcl.2003.09.067. [DOI] [PubMed] [Google Scholar]
- 32.Aina OH, Liu R, Sutcliffe JL, Marik J, Pan CX, Lam KS. From Combinatorial Chemistry to Cancer-Targeting Peptides. Molecular pharmaceutics. 2007;4:631–651. doi: 10.1021/mp700073y. [DOI] [PubMed] [Google Scholar]
- 33.Kumaresan PR, Ahir V, Liu R, Wang Y, Maitra A, Lam KS. Identification of cancer specific high affinity targeting ligands by screening One-bead-one-compound and small molecule. Molecular Cancer Therapeutics. 2007;6:3408s–3408s. [Google Scholar]
- 34.Peng L, Liu RW, Marik J, Wang XB, Takada Y, Lam KS. Combinatorial chemistry identifies high-affinity peptidomimetics against alpha(4)beta(1) integrin for in vivo tumor imaging. Nature chemical biology. 2006;2:381–389. doi: 10.1038/nchembio798. [DOI] [PubMed] [Google Scholar]
- 35.Yao N, Xiao W, Wang X, Marik J, Park SH, Takada Y, Lam KS. Discovery of Targeting Ligands for Breast Cancer Cells Using the One-Bead One-Compound Combinatorial Method. Journal of medicinal chemistry. 2008 doi: 10.1021/jm801062d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao K, Luo JT, Fowler WL, Li YP, Lee JS, Xing L, Cheng RH, Wang L, Lam KS. A self-assembling nanoparticle for paclitaxel delivery in ovarian cancer. Biomaterials. 2009;30:6006–6016. doi: 10.1016/j.biomaterials.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.