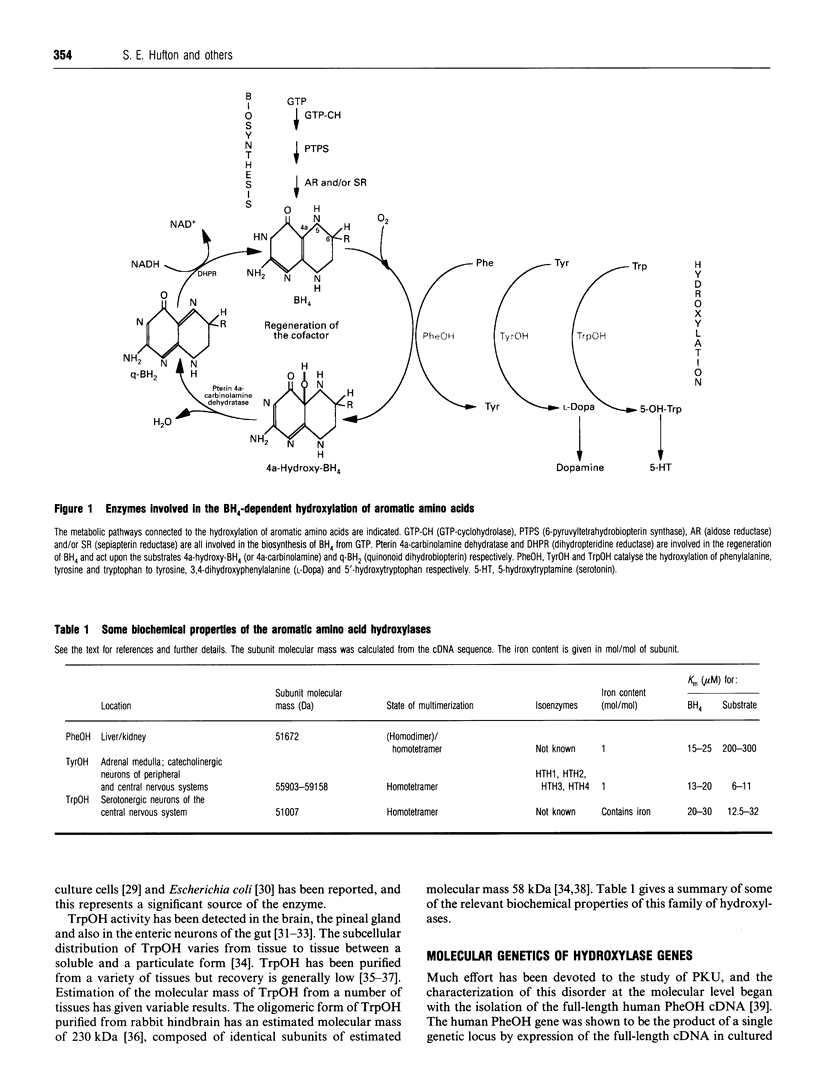
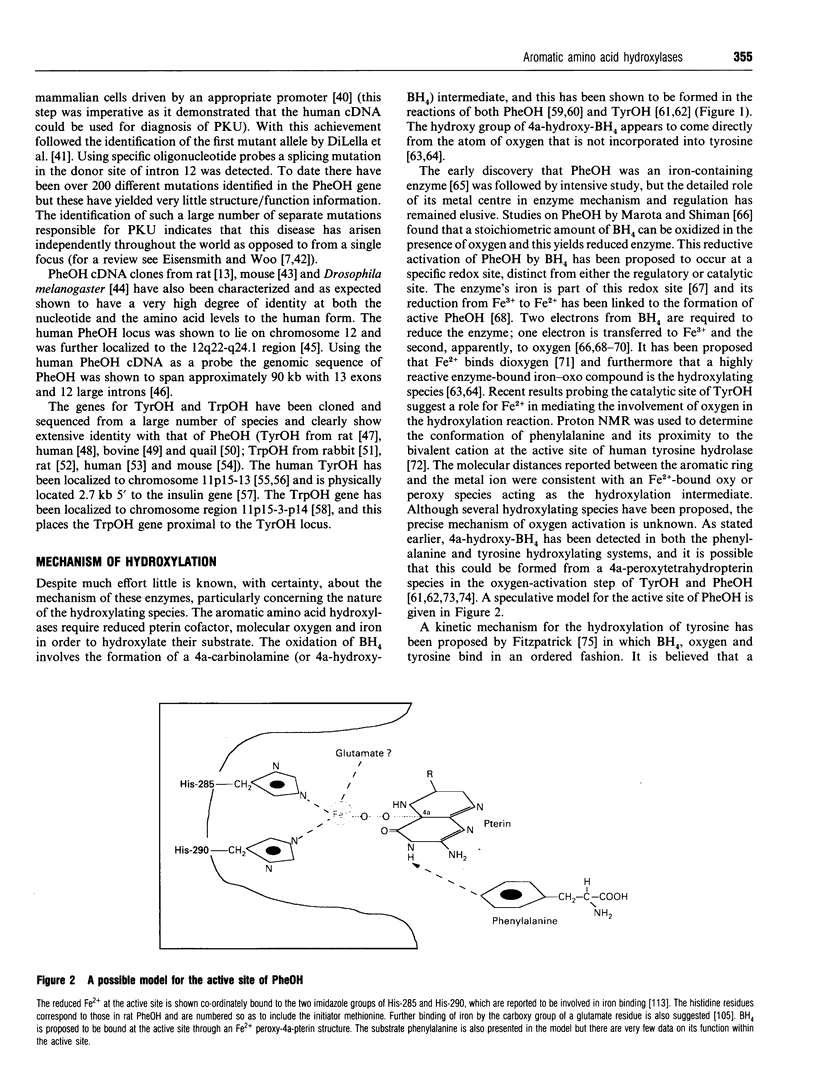
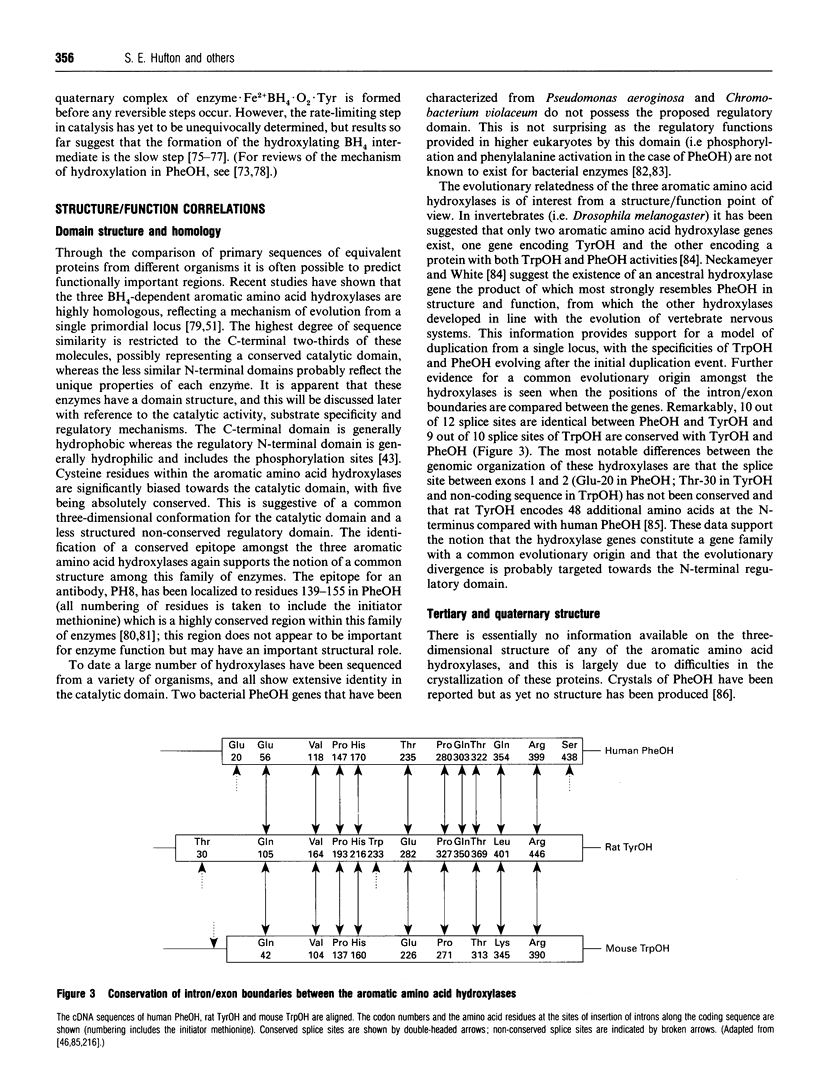
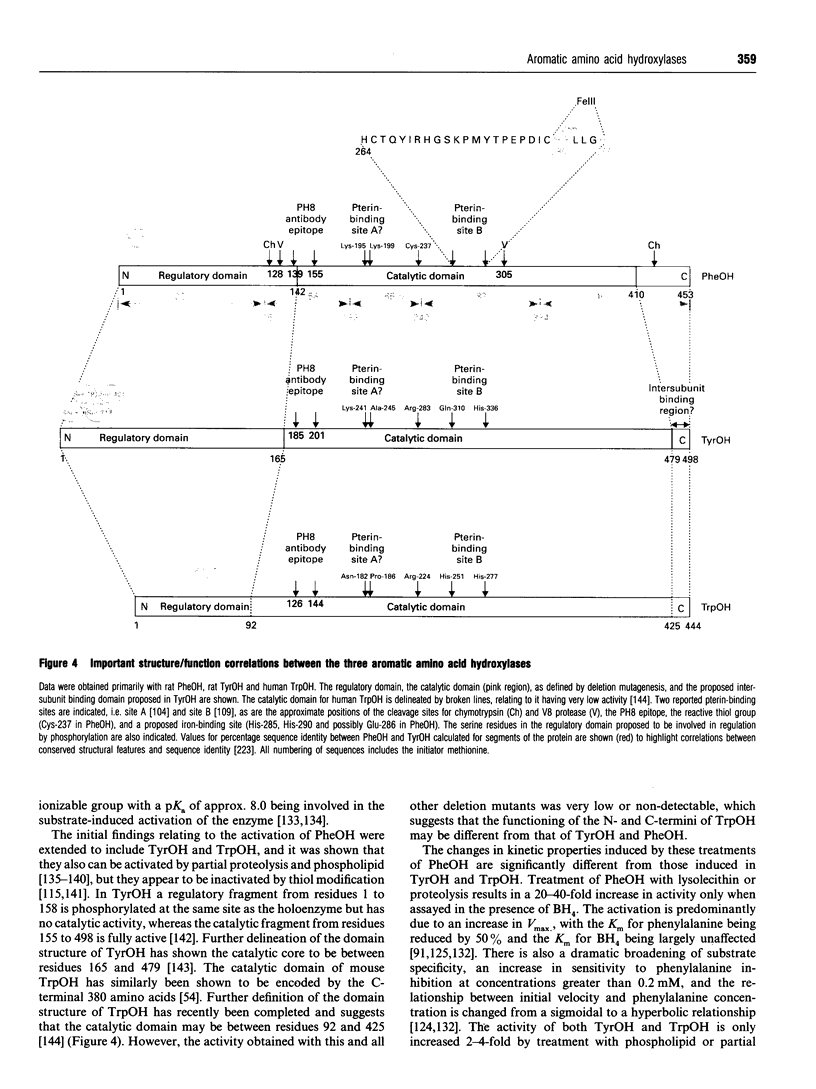
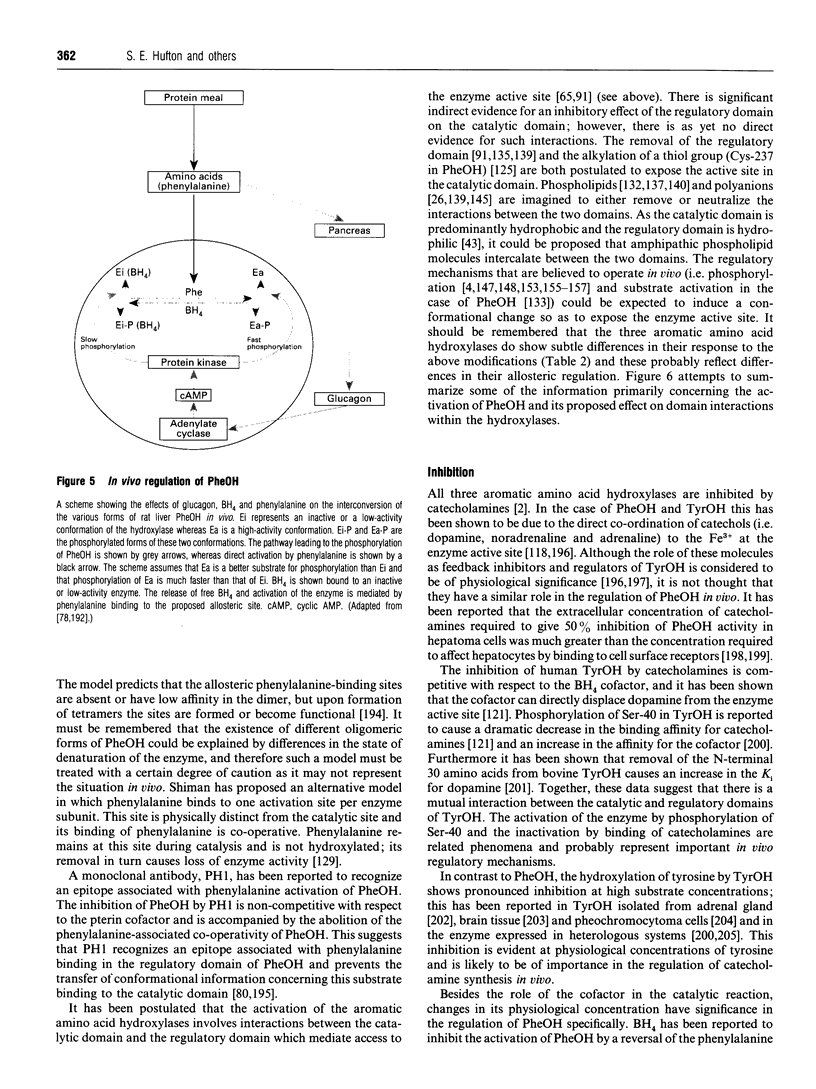
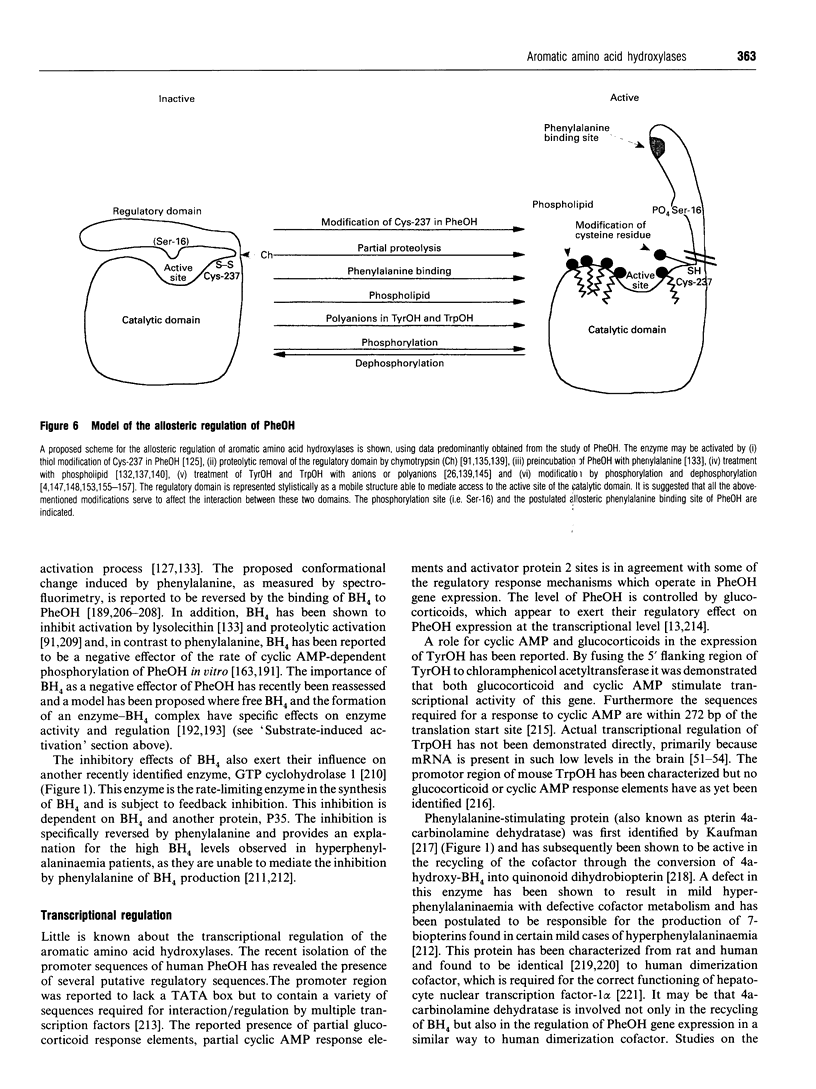
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abita J. P., Blandin-Savoja F., Rey F. Phenylalanine 4-monooxygenase from human liver. Methods Enzymol. 1987;142:27–35. doi: 10.1016/s0076-6879(87)42005-3. [DOI] [PubMed] [Google Scholar]
- Abita J. P., Milstien S., Chang N., Kaufman S. In vitro activation of rat liver phenylalanine hydroxylase by phosphorylation. J Biol Chem. 1976 Sep 10;251(17):5310–5314. [PubMed] [Google Scholar]
- Albert K. A., Helmer-Matyjek E., Nairn A. C., Müller T. H., Haycock J. W., Greene L. A., Goldstein M., Greengard P. Calcium/phospholipid-dependent protein kinase (protein kinase C) phosphorylates and activates tyrosine hydroxylase. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7713–7717. doi: 10.1073/pnas.81.24.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemany S., Tung H. Y., Shenolikar S., Pilkis S. J., Cohen P. The protein phosphatases involved in cellular regulation. Antibody to protein phosphatase-2A as a probe of phosphatase structure and function. Eur J Biochem. 1984 Nov 15;145(1):51–56. doi: 10.1111/j.1432-1033.1984.tb08520.x. [DOI] [PubMed] [Google Scholar]
- Almås B., Le Bourdelles B., Flatmark T., Mallet J., Haavik J. Regulation of recombinant human tyrosine hydroxylase isozymes by catecholamine binding and phosphorylation. Structure/activity studies and mechanistic implications. Eur J Biochem. 1992 Oct 1;209(1):249–255. doi: 10.1111/j.1432-1033.1992.tb17283.x. [DOI] [PubMed] [Google Scholar]
- Andersson K. K., Cox D. D., Que L., Jr, Flatmark T., Haavik J. Resonance Raman studies on the blue-green-colored bovine adrenal tyrosine 3-monooxygenase (tyrosine hydroxylase). Evidence that the feedback inhibitors adrenaline and noradrenaline are coordinated to iron. J Biol Chem. 1988 Dec 15;263(35):18621–18626. [PubMed] [Google Scholar]
- Andersson K. K., Vassort C., Brennan B. A., Que L., Jr, Haavik J., Flatmark T., Gros F., Thibault J. Purification and characterization of the blue-green rat phaeochromocytoma (PC12) tyrosine hydroxylase with a dopamine-Fe(III) complex. Reversal of the endogenous feedback inhibition by phosphorylation of serine-40. Biochem J. 1992 Jun 15;284(Pt 3):687–695. doi: 10.1042/bj2840687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askin D., Green A. K., Dickson A. J., Fisher M. J. Phenylalanine hydroxylation in isolated rat kidney tubules. Int J Biochem. 1990;22(1):107–114. doi: 10.1016/0020-711x(90)90085-h. [DOI] [PubMed] [Google Scholar]
- Atkinson J., Richtand N., Schworer C., Kuczenski R., Soderling T. Phosphorylation of purified rat striatal tyrosine hydroxylase by Ca2+/calmodulin-dependent protein kinase II: effect of an activator protein. J Neurochem. 1987 Oct;49(4):1241–1249. doi: 10.1111/j.1471-4159.1987.tb10016.x. [DOI] [PubMed] [Google Scholar]
- Baker R. E., Jefferson L. S., Shiman R. Immunocytochemical identification of phenylalanine hydroxylase and albumin in cultured hepatoma cells and isolated rat hepatocytes. J Cell Biol. 1981 Jul;90(1):145–152. doi: 10.1083/jcb.90.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkovic S., Wallick D., Bloom L., Gaffney B. J., Domanico P., Dix T., Pember S. On the mechanism of action of phenylalanine hydroxylase. Biochem Soc Trans. 1985 Apr;13(2):436–438. doi: 10.1042/bst0130436. [DOI] [PubMed] [Google Scholar]
- Bloom L. M., Benkovic S. J., Gaffney B. J. Characterization of phenylalanine hydroxylase. Biochemistry. 1986 Jul 29;25(15):4204–4210. doi: 10.1021/bi00363a006. [DOI] [PubMed] [Google Scholar]
- Bloom L. M., Benkovic S. J., Gaffney B. J. Characterization of phenylalanine hydroxylase. Biochemistry. 1986 Jul 29;25(15):4204–4210. doi: 10.1021/bi00363a006. [DOI] [PubMed] [Google Scholar]
- Boadle-Biber M. C. Regulation of serotonin synthesis. Prog Biophys Mol Biol. 1993;60(1):1–15. doi: 10.1016/0079-6107(93)90009-9. [DOI] [PubMed] [Google Scholar]
- Boularand S., Darmon M. C., Ganem Y., Launay J. M., Mallet J. Complete coding sequence of human tryptophan hydroxylase. Nucleic Acids Res. 1990 Jul 25;18(14):4257–4257. doi: 10.1093/nar/18.14.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. R., Coker G. T., 3rd, O'Malley K. L. Organization and evolution of the rat tyrosine hydroxylase gene. Biochemistry. 1987 Aug 11;26(16):5208–5212. doi: 10.1021/bi00390a046. [DOI] [PubMed] [Google Scholar]
- Campbell D. G., Hardie D. G., Vulliet P. R. Identification of four phosphorylation sites in the N-terminal region of tyrosine hydroxylase. J Biol Chem. 1986 Aug 15;261(23):10489–10492. [PubMed] [Google Scholar]
- Cash C. D., Vayer P., Mandel P., Maitre M. Tryptophan 5-hydroxylase. Rapid purification from whole rat brain and production of a specific antiserum. Eur J Biochem. 1985 Jun 3;149(2):239–245. doi: 10.1111/j.1432-1033.1985.tb08918.x. [DOI] [PubMed] [Google Scholar]
- Celikel R., Davis M. D., Dai X. P., Kaufman S., Xuong N. H. Crystallization and preliminary X-ray analysis of phenylalanine hydroxylase from rat liver. J Mol Biol. 1991 Apr 5;218(3):495–498. doi: 10.1016/0022-2836(91)90694-2. [DOI] [PubMed] [Google Scholar]
- Chiappelli F., Haggerty D. F., Lynch M., Popják G. Translation of phenylalanine hydroxylase-specific mRNA in vitro: evidence for pretranslational control by glucocorticoids. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2105–2109. doi: 10.1073/pnas.78.4.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron B. A., Davis M. D., Kaufman S. Electrostatic activation of rat phenylalanine hydroxylase. Biochem Biophys Res Commun. 1994 Jan 14;198(1):174–180. doi: 10.1006/bbrc.1994.1025. [DOI] [PubMed] [Google Scholar]
- Citron B. A., Davis M. D., Milstien S., Gutierrez J., Mendel D. B., Crabtree G. R., Kaufman S. Identity of 4a-carbinolamine dehydratase, a component of the phenylalanine hydroxylation system, and DCoH, a transregulator of homeodomain proteins. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11891–11894. doi: 10.1073/pnas.89.24.11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron B. A., Kaufman S., Milstien S., Naylor E. W., Greene C. L., Davis M. D. Mutation in the 4a-carbinolamine dehydratase gene leads to mild hyperphenylalaninemia with defective cofactor metabolism. Am J Hum Genet. 1993 Sep;53(3):768–774. [PMC free article] [PubMed] [Google Scholar]
- Cotton R. G., Grattan P. J. Phenylalanine hydroxylase of Macaca irus A simple purification by affinity chromatography. Eur J Biochem. 1975 Dec 15;60(2):427–430. doi: 10.1111/j.1432-1033.1975.tb21019.x. [DOI] [PubMed] [Google Scholar]
- Cotton R. G., Howells D. W., Saleeba J. A., Dianzani I., Smooker P. M., Jennings I. G. Structure function studies of the phenylalanine hydroxylase active site and a summary of structural features. Adv Exp Med Biol. 1993;338:55–57. doi: 10.1007/978-1-4615-2960-6_10. [DOI] [PubMed] [Google Scholar]
- Cotton R. G., Jennings I. G. Affinity chromatography of phenylalanine hydroxylase. The structure of a pteridine adsorbent. Eur J Biochem. 1978 Apr 17;85(2):357–363. doi: 10.1111/j.1432-1033.1978.tb12247.x. [DOI] [PubMed] [Google Scholar]
- Cotton R. G., McAdam W., Jennings I., Morgan F. J. A monoclonal antibody to aromatic amino acid hydroxylases. Identification of the epitope. Biochem J. 1988 Oct 1;255(1):193–196. doi: 10.1042/bj2550193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton R. G. Phenylalanine hydroxylase of Macaca irus. Purification of two components of the enzyme. Biochim Biophys Acta. 1971 Apr 14;235(1):61–72. doi: 10.1016/0005-2744(71)90033-7. [DOI] [PubMed] [Google Scholar]
- Craig S. P., Boularand S., Darmon M. C., Mallet J., Craig I. W. Localization of human tryptophan hydroxylase (TPH) to chromosome 11p15.3----p14 by in situ hybridization. Cytogenet Cell Genet. 1991;56(3-4):157–159. doi: 10.1159/000133075. [DOI] [PubMed] [Google Scholar]
- Craig S. P., Buckle V. J., Lamouroux A., Mallet J., Craig I. Localization of the human tyrosine hydroxylase gene to 11p15: gene duplication and evolution of metabolic pathways. Cytogenet Cell Genet. 1986;42(1-2):29–32. doi: 10.1159/000132246. [DOI] [PubMed] [Google Scholar]
- Dahl H. H., Mercer J. F. Isolation and sequence of a cDNA clone which contains the complete coding region of rat phenylalanine hydroxylase. Structural homology with tyrosine hydroxylase, glucocorticoid regulation, and use of alternate polyadenylation sites. J Biol Chem. 1986 Mar 25;261(9):4148–4153. [PubMed] [Google Scholar]
- Darmon M. C., Guibert B., Leviel V., Ehret M., Maitre M., Mallet J. Sequence of two mRNAs encoding active rat tryptophan hydroxylase. J Neurochem. 1988 Jul;51(1):312–316. doi: 10.1111/j.1471-4159.1988.tb04871.x. [DOI] [PubMed] [Google Scholar]
- Daubner S. C., Fitzpatrick P. F. Lysine241 of tyrosine hydroxylase is not required for binding of tetrahydrobiopterin substrate. Arch Biochem Biophys. 1993 May;302(2):455–460. doi: 10.1006/abbi.1993.1239. [DOI] [PubMed] [Google Scholar]
- Daubner S. C., Lauriano C., Haycock J. W., Fitzpatrick P. F. Site-directed mutagenesis of serine 40 of rat tyrosine hydroxylase. Effects of dopamine and cAMP-dependent phosphorylation on enzyme activity. J Biol Chem. 1992 Jun 25;267(18):12639–12646. [PubMed] [Google Scholar]
- Daubner S. C., Lauriano C., Haycock J. W., Fitzpatrick P. F. Site-directed mutagenesis of serine 40 of rat tyrosine hydroxylase. Effects of dopamine and cAMP-dependent phosphorylation on enzyme activity. J Biol Chem. 1992 Jun 25;267(18):12639–12646. [PubMed] [Google Scholar]
- Davis M. D., Kaufman S. Evidence for the formation of the 4a-carbinolamine during the tyrosine-dependent oxidation of tetrahydrobiopterin by rat liver phenylalanine hydroxylase. J Biol Chem. 1989 May 25;264(15):8585–8596. [PubMed] [Google Scholar]
- Davis M. D., Ribeiro P., Tipper J., Kaufman S. "7-tetrahydrobiopterin," a naturally occurring analogue of tetrahydrobiopterin, is a cofactor for and a potential inhibitor of the aromatic amino acid hydroxylases. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10109–10113. doi: 10.1073/pnas.89.21.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLella A. G., Kwok S. C., Ledley F. D., Marvit J., Woo S. L. Molecular structure and polymorphic map of the human phenylalanine hydroxylase gene. Biochemistry. 1986 Feb 25;25(4):743–749. doi: 10.1021/bi00352a001. [DOI] [PubMed] [Google Scholar]
- DiLella A. G., Marvit J., Lidsky A. S., Güttler F., Woo S. L. Tight linkage between a splicing mutation and a specific DNA haplotype in phenylketonuria. 1986 Aug 28-Sep 3Nature. 322(6082):799–803. doi: 10.1038/322799a0. [DOI] [PubMed] [Google Scholar]
- Dickson P. W., Jennings I. G., Cotton R. G. Delineation of the catalytic core of phenylalanine hydroxylase and identification of glutamate 286 as a critical residue for pterin function. J Biol Chem. 1994 Aug 12;269(32):20369–20375. [PubMed] [Google Scholar]
- Dix T. A., Bollag G. E., Domanico P. L., Benkovic S. J. Phenylalanine hydroxylase: absolute configuration and source of oxygen of the 4a-hydroxytetrahydropterin species. Biochemistry. 1985 Jun 4;24(12):2955–2958. doi: 10.1021/bi00333a022. [DOI] [PubMed] [Google Scholar]
- Dix T. A., Kuhn D. M., Benkovic S. J. Mechanism of oxygen activation by tyrosine hydroxylase. Biochemistry. 1987 Jun 16;26(12):3354–3361. doi: 10.1021/bi00386a016. [DOI] [PubMed] [Google Scholar]
- Dumas S., Darmon M. C., Delort J., Mallet J. Differential control of tryptophan hydroxylase expression in raphe and in pineal gland: evidence for a role of translation efficiency. J Neurosci Res. 1989 Dec;24(4):537–547. doi: 10.1002/jnr.490240412. [DOI] [PubMed] [Google Scholar]
- Døskeland A. P., Døskeland S. O., Ogreid D., Flatmark T. The effect of ligands of phenylalanine 4-monooxygenase on the cAMP-dependent phosphorylation of the enzyme. J Biol Chem. 1984 Sep 25;259(18):11242–11248. [PubMed] [Google Scholar]
- Døskeland A. P., Schworer C. M., Døskeland S. O., Chrisman T. D., Soderling T. R., Corbin J. D., Flatmark T. Some aspects of the phosphorylation of phenylalanine 4-monooxygenase by a calcium-dependent and calmodulin-dependent protein kinase. Eur J Biochem. 1984 Nov 15;145(1):31–37. doi: 10.1111/j.1432-1033.1984.tb08518.x. [DOI] [PubMed] [Google Scholar]
- Døskeland A. P., Vintermyr O. K., Flatmark T., Cotton R. G., Døskeland S. O. Phenylalanine positively modulates the cAMP-dependent phosphorylation and negatively modulates the vasopressin-induced and okadaic-acid-induced phosphorylation of phenylalanine 4-monooxygenase in intact rat hepatocytes. Eur J Biochem. 1992 May 15;206(1):161–170. doi: 10.1111/j.1432-1033.1992.tb16913.x. [DOI] [PubMed] [Google Scholar]
- Ehret M., Cash C. D., Hamon M., Maitre M. Formal demonstration of the phosphorylation of rat brain tryptophan hydroxylase by Ca2+/calmodulin-dependent protein kinase. J Neurochem. 1989 Jun;52(6):1886–1891. doi: 10.1111/j.1471-4159.1989.tb07272.x. [DOI] [PubMed] [Google Scholar]
- Eisensmith R. C., Woo S. L. Molecular basis of phenylketonuria and related hyperphenylalaninemias: mutations and polymorphisms in the human phenylalanine hydroxylase gene. Hum Mutat. 1992;1(1):13–23. doi: 10.1002/humu.1380010104. [DOI] [PubMed] [Google Scholar]
- Eisensmith R. C., Woo S. L. Phenylketonuria and the phenylalanine hydroxylase gene. Mol Biol Med. 1991 Feb;8(1):3–18. [PubMed] [Google Scholar]
- Fauquet M., Grima B., Lamouroux A., Mallet J. Cloning of quail tyrosine hydroxylase: amino acid homology with other hydroxylases discloses functional domains. J Neurochem. 1988 Jan;50(1):142–148. doi: 10.1111/j.1471-4159.1988.tb13241.x. [DOI] [PubMed] [Google Scholar]
- Fisher D. B., Kirkwood R., Kaufman S. Rat liver phenylalanine hydroxylase, an iron enzyme. J Biol Chem. 1972 Aug 25;247(16):5161–5167. [PubMed] [Google Scholar]
- Fitzpatrick P. F., Chlumsky L. J., Daubner S. C., O'Malley K. L. Expression of rat tyrosine hydroxylase in insect tissue culture cells and purification and characterization of the cloned enzyme. J Biol Chem. 1990 Feb 5;265(4):2042–2047. [PubMed] [Google Scholar]
- Fitzpatrick P. F., Chlumsky L. J., Daubner S. C., O'Malley K. L. Expression of rat tyrosine hydroxylase in insect tissue culture cells and purification and characterization of the cloned enzyme. J Biol Chem. 1990 Feb 5;265(4):2042–2047. [PubMed] [Google Scholar]
- Fitzpatrick P. F. Mechanistic studies of tyrosine hydroxylase. Adv Exp Med Biol. 1993;338:81–86. doi: 10.1007/978-1-4615-2960-6_16. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick P. F. Steady-state kinetic mechanism of rat tyrosine hydroxylase. Biochemistry. 1991 Apr 16;30(15):3658–3662. doi: 10.1021/bi00229a010. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick P. F. Studies of the rate-limiting step in the tyrosine hydroxylase reaction: alternate substrates, solvent isotope effects, and transition-state analogues. Biochemistry. 1991 Jul 2;30(26):6386–6391. doi: 10.1021/bi00240a006. [DOI] [PubMed] [Google Scholar]
- Fujisawa H., Nakata H. Tryptophan 5-monooxygenase from rat brain stem. Methods Enzymol. 1987;142:83–87. doi: 10.1016/s0076-6879(87)42012-0. [DOI] [PubMed] [Google Scholar]
- Gershon M. D. The enteric nervous system. Annu Rev Neurosci. 1981;4:227–272. doi: 10.1146/annurev.ne.04.030181.001303. [DOI] [PubMed] [Google Scholar]
- Gibbs B. S., Benkovic S. J. Affinity labeling of the active site and the reactive sulfhydryl associated with activation of rat liver phenylalanine hydroxylase. Biochemistry. 1991 Jul 9;30(27):6795–6802. doi: 10.1021/bi00241a024. [DOI] [PubMed] [Google Scholar]
- Gibbs B. S., Wojchowski D., Benkovic S. J. Expression of rat liver phenylalanine hydroxylase in insect cells and site-directed mutagenesis of putative non-heme iron-binding sites. J Biol Chem. 1993 Apr 15;268(11):8046–8052. [PubMed] [Google Scholar]
- Gillam S. S., Woo S. L., Woolf L. I. The isolation and properties of phenylalanine hydroxylase from rat liver. Biochem J. 1974 Jun;139(3):731–739. doi: 10.1042/bj1390731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschall D. W., Dietrich R. F., Benkovic S. J., Shiman R. Phenylalanine hydroxylase. Correlation of the iron content with activity and the preparation and reconstitution of the apoenzyme. J Biol Chem. 1982 Jan 25;257(2):845–849. [PubMed] [Google Scholar]
- Green A. K., Cotton R. G., Jennings I., Fisher M. J. Experimental determination of the phosphorylation state of phenylalanine hydroxylase. Biochem J. 1990 Jan 15;265(2):563–568. doi: 10.1042/bj2650563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenett H. E., Ledley F. D., Reed L. L., Woo S. L. Full-length cDNA for rabbit tryptophan hydroxylase: functional domains and evolution of aromatic amino acid hydroxylases. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5530–5534. doi: 10.1073/pnas.84.16.5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima B., Lamouroux A., Blanot F., Biguet N. F., Mallet J. Complete coding sequence of rat tyrosine hydroxylase mRNA. Proc Natl Acad Sci U S A. 1985 Jan;82(2):617–621. doi: 10.1073/pnas.82.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima B., Lamouroux A., Boni C., Julien J. F., Javoy-Agid F., Mallet J. A single human gene encoding multiple tyrosine hydroxylases with different predicted functional characteristics. Nature. 1987 Apr 16;326(6114):707–711. doi: 10.1038/326707a0. [DOI] [PubMed] [Google Scholar]
- Haavik J., Andersson K. K., Petersson L., Flatmark T. Soluble tyrosine hydroxylase (tyrosine 3-monooxygenase) from bovine adrenal medulla: large-scale purification and physicochemical properties. Biochim Biophys Acta. 1988 Mar 23;953(2):142–156. doi: 10.1016/0167-4838(88)90019-2. [DOI] [PubMed] [Google Scholar]
- Haavik J., Døskeland A. P., Flatmark T. Stereoselective effects in the interactions of pterin cofactors with rat-liver phenylalanine 4-monooxygenase. Eur J Biochem. 1986 Oct 1;160(1):1–8. doi: 10.1111/j.1432-1033.1986.tb09932.x. [DOI] [PubMed] [Google Scholar]
- Haavik J., Flatmark T. Isolation and characterization of tetrahydropterin oxidation products generated in the tyrosine 3-monooxygenase (tyrosine hydroxylase) reaction. Eur J Biochem. 1987 Oct 1;168(1):21–26. doi: 10.1111/j.1432-1033.1987.tb13381.x. [DOI] [PubMed] [Google Scholar]
- Haavik J., Le Bourdelles B., Martinez A., Flatmark T., Mallet J. Recombinant human tyrosine hydroxylase isozymes. Reconstitution with iron and inhibitory effect of other metal ions. Eur J Biochem. 1991 Jul 15;199(2):371–378. doi: 10.1111/j.1432-1033.1991.tb16133.x. [DOI] [PubMed] [Google Scholar]
- Haavik J., Martínez A., Flatmark T. pH-dependent release of catecholamines from tyrosine hydroxylase and the effect of phosphorylation of Ser-40. FEBS Lett. 1990 Mar 26;262(2):363–365. doi: 10.1016/0014-5793(90)80230-g. [DOI] [PubMed] [Google Scholar]
- Haavik J., Martínez A., Flatmark T. pH-dependent release of catecholamines from tyrosine hydroxylase and the effect of phosphorylation of Ser-40. FEBS Lett. 1990 Mar 26;262(2):363–365. doi: 10.1016/0014-5793(90)80230-g. [DOI] [PubMed] [Google Scholar]
- Haavik J., Schelling D. L., Campbell D. G., Andersson K. K., Flatmark T., Cohen P. Identification of protein phosphatase 2A as the major tyrosine hydroxylase phosphatase in adrenal medulla and corpus striatum: evidence from the effects of okadaic acid. FEBS Lett. 1989 Jul 17;251(1-2):36–42. doi: 10.1016/0014-5793(89)81424-3. [DOI] [PubMed] [Google Scholar]
- Hamon M., Bourgoin S., Artaud F., Héry F. Rat brain stem tryptophan hydroxylase: mechanism of activation by calcium. J Neurochem. 1977 Apr;28(4):811–818. doi: 10.1111/j.1471-4159.1977.tb10632.x. [DOI] [PubMed] [Google Scholar]
- Hamon M., Bourgoin S., Hery F., Simmonet G. Phospholipid-induced activation of tryptophan hydroxylase from the rat brainstem. Biochem Pharmacol. 1978 Mar 15;27(6):915–922. doi: 10.1016/0006-2952(78)90419-7. [DOI] [PubMed] [Google Scholar]
- Hamon M., Bourgoin S., Hery F., Ternaux J. P., Glowinski J. In vivo and in vitro activation of soluble tryptophan hydroxylase from rat brainstem. Nature. 1976 Mar 4;260(5546):61–63. doi: 10.1038/260061a0. [DOI] [PubMed] [Google Scholar]
- Harada T., Kagamiyama H., Hatakeyama K. Feedback regulation mechanisms for the control of GTP cyclohydrolase I activity. Science. 1993 Jun 4;260(5113):1507–1510. doi: 10.1126/science.8502995. [DOI] [PubMed] [Google Scholar]
- Hauer C. R., Rebrin I., Thöny B., Neuheiser F., Curtius H. C., Hunziker P., Blau N., Ghisla S., Heizmann C. W. Phenylalanine hydroxylase-stimulating protein/pterin-4 alpha-carbinolamine dehydratase from rat and human liver. Purification, characterization, and complete amino acid sequence. J Biol Chem. 1993 Mar 5;268(7):4828–4831. [PubMed] [Google Scholar]
- Haycock J. W., Ahn N. G., Cobb M. H., Krebs E. G. ERK1 and ERK2, two microtubule-associated protein 2 kinases, mediate the phosphorylation of tyrosine hydroxylase at serine-31 in situ. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2365–2369. doi: 10.1073/pnas.89.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock J. W., Haycock D. A. Tyrosine hydroxylase in rat brain dopaminergic nerve terminals. Multiple-site phosphorylation in vivo and in synaptosomes. J Biol Chem. 1991 Mar 25;266(9):5650–5657. [PubMed] [Google Scholar]
- Haycock J. W. Multiple signaling pathways in bovine chromaffin cells regulate tyrosine hydroxylase phosphorylation at Ser19, Ser31, and Ser40. Neurochem Res. 1993 Jan;18(1):15–26. doi: 10.1007/BF00966919. [DOI] [PubMed] [Google Scholar]
- Haycock J. W. Phosphorylation of tyrosine hydroxylase in situ at serine 8, 19, 31, and 40. J Biol Chem. 1990 Jul 15;265(20):11682–11691. [PubMed] [Google Scholar]
- Haycock J. W. Quantitation of tyrosine hydroxylase, protein levels: spot immunolabeling with an affinity-purified antibody. Anal Biochem. 1989 Sep;181(2):259–266. doi: 10.1016/0003-2697(89)90240-6. [DOI] [PubMed] [Google Scholar]
- Hill M. A., Marota J. J., Shiman R. Reaction of rat liver phenylalanine hydroxylase with fatty acid hydroperoxides. Characterization and mechanism. J Biol Chem. 1988 Apr 25;263(12):5646–5655. [PubMed] [Google Scholar]
- Håkanson R., Lombard des Gouttes M. N., Owman C. Activities of tryptophan hydroxylase, dopa decarboxylase, and monoamine oxidase as correlated with the appearance of monoamines in developing rat pineal gland. Life Sci. 1967 Dec 15;6(24):2577–2585. doi: 10.1016/0024-3205(67)90107-5. [DOI] [PubMed] [Google Scholar]
- Ichimura T., Isobe T., Okuyama T., Takahashi N., Araki K., Kuwano R., Takahashi Y. Molecular cloning of cDNA coding for brain-specific 14-3-3 protein, a protein kinase-dependent activator of tyrosine and tryptophan hydroxylases. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7084–7088. doi: 10.1073/pnas.85.19.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe T., Ichimura T., Sunaya T., Okuyama T., Takahashi N., Kuwano R., Takahashi Y. Distinct forms of the protein kinase-dependent activator of tyrosine and tryptophan hydroxylases. J Mol Biol. 1991 Jan 5;217(1):125–132. doi: 10.1016/0022-2836(91)90616-e. [DOI] [PubMed] [Google Scholar]
- Iwaki M., Phillips R. S., Kaufman S. Proteolytic modification of the amino-terminal and carboxyl-terminal regions of rat hepatic phenylalanine hydroxylase. J Biol Chem. 1986 Feb 15;261(5):2051–2056. [PubMed] [Google Scholar]
- Jennings I. G., Kemp B. E., Cotton R. G. Localization of cofactor binding sites with monoclonal anti-idiotype antibodies: phenylalanine hydroxylase. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5734–5738. doi: 10.1073/pnas.88.13.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings I. G., Russell R. G., Armarego W. L., Cotton R. G. Functional analysis of the effect of monoclonal antibodies on monkey liver phenylalanine hydroxylase. Biochem J. 1986 Apr 1;235(1):133–138. doi: 10.1042/bj2350133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings I., Cotton R. Structural similarities among enzyme pterin binding sites as demonstrated by a monoclonal anti-idiotypic antibody. J Biol Chem. 1990 Feb 5;265(4):1885–1889. [PubMed] [Google Scholar]
- Kaneda N., Kobayashi K., Ichinose H., Kishi F., Nakazawa A., Kurosawa Y., Fujita K., Nagatsu T. Isolation of a novel cDNA clone for human tyrosine hydroxylase: alternative RNA splicing produces four kinds of mRNA from a single gene. Biochem Biophys Res Commun. 1987 Aug 14;146(3):971–975. doi: 10.1016/0006-291x(87)90742-x. [DOI] [PubMed] [Google Scholar]
- Kataoka K., Takada H., Yoshimura T., Furuyoshi S., Esaki N., Ohshima T., Soda K. Site-directed mutagenesis of a hexapeptide segment involved in substrate recognition of phenylalanine dehydrogenase from Thermoactinomyces intermedius. J Biochem. 1993 Jul;114(1):69–75. doi: 10.1093/oxfordjournals.jbchem.a124142. [DOI] [PubMed] [Google Scholar]
- Katz I. R., Yamauchi T., Kaufman S. Activation of tyrosine hydroxylase by polyanions and salts. An electrostatic effect. Biochim Biophys Acta. 1976 Mar 11;429(1):84–95. doi: 10.1016/0005-2744(76)90032-2. [DOI] [PubMed] [Google Scholar]
- Katz I., Lloyd T., Kaufman S. Studies on phenylalanine and tyrosine hydroxylation by rat brain tyrosine hydroxylase. Biochim Biophys Acta. 1976 Oct 11;445(3):567–578. doi: 10.1016/0005-2744(76)90111-x. [DOI] [PubMed] [Google Scholar]
- Kaufman S. A protein that stimulates rat liver phenylalanine hydroxylase. J Biol Chem. 1970 Sep 25;245(18):4751–4759. [PubMed] [Google Scholar]
- Kaufman S. A protein that stimulates rat liver phenylalanine hydroxylase. J Biol Chem. 1970 Sep 25;245(18):4751–4759. [PubMed] [Google Scholar]
- Kaufman S., Fisher D. B. Purification and some physical properties of phenylalanine hydroxylase from rat liver. J Biol Chem. 1970 Sep 25;245(18):4745–4750. [PubMed] [Google Scholar]
- Kaufman S., Mason K. Specificity of amino acids as activators and substrates for phenylalanine hydroxylase. J Biol Chem. 1982 Dec 25;257(24):14667–14678. [PubMed] [Google Scholar]
- Kaufman S. The phenylalanine hydroxylating system from mammalian liver. Adv Enzymol Relat Areas Mol Biol. 1971;35:245–319. doi: 10.1002/9780470122808.ch6. [DOI] [PubMed] [Google Scholar]
- Kaufman S. The phenylalanine hydroxylating system. Adv Enzymol Relat Areas Mol Biol. 1993;67:77–264. doi: 10.1002/9780470123133.ch2. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B. Protein kinase recognition sequence motifs. Trends Biochem Sci. 1990 Sep;15(9):342–346. doi: 10.1016/0968-0004(90)90073-k. [DOI] [PubMed] [Google Scholar]
- Kemp B. E. Phosphorylation of synthetic peptide analogs of rabbit cardiac troponin inhibitory subunit by the cyclic AMP-dependent protein kinase. J Biol Chem. 1979 Apr 25;254(8):2638–2642. [PubMed] [Google Scholar]
- Kim K. S., Wessel T. C., Stone D. M., Carver C. H., Joh T. H., Park D. H. Molecular cloning and characterization of cDNA encoding tryptophan hydroxylase from rat central serotonergic neurons. Brain Res Mol Brain Res. 1991 Mar;9(4):277–283. doi: 10.1016/0169-328x(91)90073-7. [DOI] [PubMed] [Google Scholar]
- Kiuchi K., Kiuchi K., Titani K., Fujita K., Suzuki K., Nagatsu T. Limited proteolysis of tyrosine hydroxylase by Ca(2+)-activated neutral protease (calpain). Biochemistry. 1991 Oct 29;30(43):10416–10419. doi: 10.1021/bi00107a008. [DOI] [PubMed] [Google Scholar]
- Koizumi S., Tanaka F., Kaneda N., Kano K., Nagatsu T. Nanosecond pulse fluorometry of conformational change in phenylalanine hydroxylase associated with activation. Biochemistry. 1988 Jan 26;27(2):640–646. doi: 10.1021/bi00402a022. [DOI] [PubMed] [Google Scholar]
- Konecki D. S., Wang Y., Trefz F. K., Lichter-Konecki U., Woo S. L. Structural characterization of the 5' regions of the human phenylalanine hydroxylase gene. Biochemistry. 1992 Sep 8;31(35):8363–8368. doi: 10.1021/bi00150a033. [DOI] [PubMed] [Google Scholar]
- Kuczenski R. T., Mandell A. J. Regulatory properties of soluble and particulate rat brain tyrosine hydroxylase. J Biol Chem. 1972 May 25;247(10):3114–3122. [PubMed] [Google Scholar]
- Kuhn D. M., O'Callaghan J. P., Juskevich J., Lovenberg W. Activation of brain tryptophan hydroxylase by ATP-MG2+: dependence on calmodulin. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4688–4691. doi: 10.1073/pnas.77.8.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn D. M., Ruskin B., Lovenberg W. Tryptophan hydroxylase. The role of oxygen, iron, and sulfhydryl groups as determinants of stability and catalytic activity. J Biol Chem. 1980 May 10;255(9):4137–4143. [PubMed] [Google Scholar]
- Kwok S. C., Ledley F. D., DiLella A. G., Robson K. J., Woo S. L. Nucleotide sequence of a full-length complementary DNA clone and amino acid sequence of human phenylalanine hydroxylase. Biochemistry. 1985 Jan 29;24(3):556–561. doi: 10.1021/bi00324a002. [DOI] [PubMed] [Google Scholar]
- Lazarus R. A., Dietrich R. F., Wallick D. E., Benkovic S. J. On the mechanism of action of phenylalanine hydroxylase. Biochemistry. 1981 Nov 24;20(24):6834–6841. doi: 10.1021/bi00527a015. [DOI] [PubMed] [Google Scholar]
- Lazarus R. A., Wallick D. E., Dietrich R. F., Gottschall D. W., Benkovic S. J., Gaffney B. J., Shiman R. The mechanism of phenylalanine hydroxylase. Fed Proc. 1982 Jul;41(9):2605–2607. [PubMed] [Google Scholar]
- Ledley F. D., DiLella A. G., Kwok S. C., Woo S. L. Homology between phenylalanine and tyrosine hydroxylases reveals common structural and functional domains. Biochemistry. 1985 Jul 2;24(14):3389–3394. doi: 10.1021/bi00335a001. [DOI] [PubMed] [Google Scholar]
- Ledley F. D., Grenett H. E., Bartos D. P., van Tuinen P., Ledbetter D. H., Woo S. L. Assignment of human tryptophan hydroxylase locus to chromosome 11: gene duplication and translocation in evolution of aromatic amino acid hydroxylases. Somat Cell Mol Genet. 1987 Sep;13(5):575–580. doi: 10.1007/BF01534499. [DOI] [PubMed] [Google Scholar]
- Ledley F. D., Grenett H. E., DiLella A. G., Kwok S. C., Woo S. L. Gene transfer and expression of human phenylalanine hydroxylase. Science. 1985 Apr 5;228(4695):77–79. doi: 10.1126/science.3856322. [DOI] [PubMed] [Google Scholar]
- Ledley F. D., Grenett H. E., Dunbar B. S., Woo S. L. Mouse phenylalanine hydroxylase. Homology and divergence from human phenylalanine hydroxylase. Biochem J. 1990 Apr 15;267(2):399–405. doi: 10.1042/bj2670399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis E. J., Harrington C. A., Chikaraishi D. M. Transcriptional regulation of the tyrosine hydroxylase gene by glucocorticoid and cyclic AMP. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3550–3554. doi: 10.1073/pnas.84.11.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidsky A. S., Law M. L., Morse H. G., Kao F. T., Rabin M., Ruddle F. H., Woo S. L. Regional mapping of the phenylalanine hydroxylase gene and the phenylketonuria locus in the human genome. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6221–6225. doi: 10.1073/pnas.82.18.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd T., Kaufman S. The stimulation of partially purified bovine caudate tyrosine hydroxylase by phosphatidyl-L-serine. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1262–1270. doi: 10.1016/0006-291x(74)90450-1. [DOI] [PubMed] [Google Scholar]
- Lovenberg W., Jequier E., Sjoerdsma A. Tryptophan hydroxylation: measurement in pineal gland, brainstem, and carcinoid tumor. Science. 1967 Jan 13;155(3759):217–219. doi: 10.1126/science.155.3759.217. [DOI] [PubMed] [Google Scholar]
- Markey K. A., Kondo H., Shenkman L., Goldstein M. Purification and characterization of tyrosine hydroxylase from a clonal pheochromocytoma cell line. Mol Pharmacol. 1980 Jan;17(1):79–85. [PubMed] [Google Scholar]
- Marota J. J., Shiman R. Stoichiometric reduction of phenylalanine hydroxylase by its cofactor: a requirement for enzymatic activity. Biochemistry. 1984 Mar 13;23(6):1303–1311. doi: 10.1021/bi00301a044. [DOI] [PubMed] [Google Scholar]
- Martínez A., Abeygunawardana C., Haavik J., Flatmark T., Mildvan A. S. Conformation and interaction of phenylalanine with the divalent cation at the active site of human recombinant tyrosine hydroxylase as determined by proton NMR. Biochemistry. 1993 Jun 29;32(25):6381–6390. doi: 10.1021/bi00076a011. [DOI] [PubMed] [Google Scholar]
- Martínez A., Andersson K. K., Haavik J., Flatmark T. EPR and 1H-NMR spectroscopic studies on the paramagnetic iron at the active site of phenylalanine hydroxylase and its interaction with substrates and inhibitors. Eur J Biochem. 1991 Jun 15;198(3):675–682. doi: 10.1111/j.1432-1033.1991.tb16066.x. [DOI] [PubMed] [Google Scholar]
- Martínez A., Haavik J., Flatmark T. Cooperative homotropic interaction of L-noradrenaline with the catalytic site of phenylalanine 4-monooxygenase. Eur J Biochem. 1990 Oct 5;193(1):211–219. doi: 10.1111/j.1432-1033.1990.tb19325.x. [DOI] [PubMed] [Google Scholar]
- Martínez A., Olafsdottir S., Flatmark T. The cooperative binding of phenylalanine to phenylalanine 4-monooxygenase studied by 1H-NMR paramagnetic relaxation. Changes in water accessibility to the iron at the active site upon substrate binding. Eur J Biochem. 1993 Jan 15;211(1-2):259–266. doi: 10.1111/j.1432-1033.1993.tb19894.x. [DOI] [PubMed] [Google Scholar]
- Meek J. L., Neff N. H. Tryptophan 5-hydroxylase: approximation of half-life and rate of axonal transport. J Neurochem. 1972 Jun;19(6):1519–1525. doi: 10.1111/j.1471-4159.1972.tb05096.x. [DOI] [PubMed] [Google Scholar]
- Mendel D. B., Khavari P. A., Conley P. B., Graves M. K., Hansen L. P., Admon A., Crabtree G. R. Characterization of a cofactor that regulates dimerization of a mammalian homeodomain protein. Science. 1991 Dec 20;254(5039):1762–1767. doi: 10.1126/science.1763325. [DOI] [PubMed] [Google Scholar]
- Mercer J. F., McAdam W., Chambers G. W., Walker I. D. The W and L allelic forms of phenylalanine hydroxylase in the rat differ by a threonine to isoleucine substitution. Biochem J. 1986 Jun 15;236(3):679–683. doi: 10.1042/bj2360679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. R., Shiman R. Reversible inactivation of phenylalanine hydroxylase by catecholamines in cultured hepatoma cells. J Biol Chem. 1976 Jun 25;251(12):3671–3676. [PubMed] [Google Scholar]
- Milstien S., Abita J. P., Chang N., Kaufman S. Hepatic phenylalanine 4-monooxygenase is a phosphoprotein. Proc Natl Acad Sci U S A. 1976 May;73(5):1591–1593. doi: 10.1073/pnas.73.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales G., Requena J. M., Jimenez-Ruiz A., Lopez M. C., Ugarte M., Alonso C. Sequence and expression of the Drosophila phenylalanine hydroxylase mRNA. Gene. 1990 Sep 14;93(2):213–219. doi: 10.1016/0378-1119(90)90227-i. [DOI] [PubMed] [Google Scholar]
- Nagatsu T., Ichinose H. Comparative studies on the structure of human tyrosine hydroxylase with those of the enzyme of various mammals. Comp Biochem Physiol C. 1991;98(1):203–210. [PubMed] [Google Scholar]
- Nairn A. C., Hemmings H. C., Jr, Greengard P. Protein kinases in the brain. Annu Rev Biochem. 1985;54:931–976. doi: 10.1146/annurev.bi.54.070185.004435. [DOI] [PubMed] [Google Scholar]
- Nakata H., Fujisawa H. Purification and characterization of phenylalanine 4-monooxygenase from rat liver. Biochim Biophys Acta. 1980 Aug 7;614(2):313–327. doi: 10.1016/0005-2744(80)90221-1. [DOI] [PubMed] [Google Scholar]
- Nakata H., Fujisawa H. Tryptophan 5-monooxygenase from mouse mastocytoma P815. A simple purification and general properties. Eur J Biochem. 1982 Jun;124(3):595–601. doi: 10.1111/j.1432-1033.1982.tb06636.x. [DOI] [PubMed] [Google Scholar]
- Neckameyer W. S., White K. A single locus encodes both phenylalanine hydroxylase and tryptophan hydroxylase activities in Drosophila. J Biol Chem. 1992 Feb 25;267(6):4199–4206. [PubMed] [Google Scholar]
- O'Malley K. L., Anhalt M. J., Martin B. M., Kelsoe J. R., Winfield S. L., Ginns E. I. Isolation and characterization of the human tyrosine hydroxylase gene: identification of 5' alternative splice sites responsible for multiple mRNAs. Biochemistry. 1987 Nov 3;26(22):6910–6914. doi: 10.1021/bi00396a007. [DOI] [PubMed] [Google Scholar]
- O'Malley K. L., Rotwein P. Human tyrosine hydroxylase and insulin genes are contiguous on chromosome 11. Nucleic Acids Res. 1988 May 25;16(10):4437–4446. [PMC free article] [PubMed] [Google Scholar]
- Onishi A., Liotta L. J., Benkovic S. J. Cloning and expression of Chromobacterium violaceum phenylalanine hydroxylase in Escherichia coli and comparison of amino acid sequence with mammalian aromatic amino acid hydroxylases. J Biol Chem. 1991 Oct 5;266(28):18454–18459. [PubMed] [Google Scholar]
- POTTER L. T., COOPER T., WILLMAN V. L., WOLFE D. E. SYNTHESIS, BINDING, RELEASE, AND METABOLISM OF NOREPINEPHRINE IN NORMAL AND TRANSPLANTED DOG HEARTS. Circ Res. 1965 May;16:468–481. doi: 10.1161/01.res.16.5.468. [DOI] [PubMed] [Google Scholar]
- Parniak M. A., Davis M. D., Kaufman S. Effect of alkaline pH on the activity of rat liver phenylalanine hydroxylase. J Biol Chem. 1988 Jan 25;263(3):1223–1230. [PubMed] [Google Scholar]
- Parniak M. A., Jennings I. G., Cotton R. G. Interaction with a monoclonal antibody alters the expression of co-operativity by phenylalanine hydroxylase from rat liver. Biochem J. 1989 Jan 15;257(2):383–388. doi: 10.1042/bj2570383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelech S., Cohen P., Fisher M. J., Pogson C. I., El-Maghrabi M. R., Pilkis S. J. The protein phosphatases involved in cellular regulation. Glycolysis, gluconeogenesis and aromatic amino acid breakdown in rat liver. Eur J Biochem. 1984 Nov 15;145(1):39–49. doi: 10.1111/j.1432-1033.1984.tb08519.x. [DOI] [PubMed] [Google Scholar]
- Pember S. O., Villafranca J. J., Benkovic S. J. Phenylalanine hydroxylase from Chromobacterium violaceum is a copper-containing monooxygenase. Kinetics of the reductive activation of the enzyme. Biochemistry. 1986 Oct 21;25(21):6611–6619. doi: 10.1021/bi00369a042. [DOI] [PubMed] [Google Scholar]
- Petrack B., Sheppy F., Fetzer V. Studies on tyrosine hydroxylase from bovine adrenal medulla. J Biol Chem. 1968 Feb 25;243(4):743–748. [PubMed] [Google Scholar]
- Phillips R. S., Iwaki M., Kaufman S. Ligand effects on the limited proteolysis of phenylalanine hydroxylase: evidence for multiple conformational states. Biochem Biophys Res Commun. 1983 Feb 10;110(3):919–925. doi: 10.1016/0006-291x(83)91050-1. [DOI] [PubMed] [Google Scholar]
- Phillips R. S., Kaufman S. Ligand effects on the phosphorylation state of hepatic phenylalanine hydroxylase. J Biol Chem. 1984 Feb 25;259(4):2474–2479. [PubMed] [Google Scholar]
- Phillips R. S., Parniak M. A., Kaufman S. Spectroscopic investigation of ligand interaction with hepatic phenylalanine hydroxylase: evidence for a conformational change associated with activation. Biochemistry. 1984 Aug 14;23(17):3836–3842. doi: 10.1021/bi00312a007. [DOI] [PubMed] [Google Scholar]
- Pollock R. J., Kapatos G., Kaufman S. Effect of cyclic AMP-dependent protein phosphorylating conditions on the pH-dependent activity of tyrosine hydroxylase from beef and rat striata. J Neurochem. 1981 Oct;37(4):855–860. doi: 10.1111/j.1471-4159.1981.tb04471.x. [DOI] [PubMed] [Google Scholar]
- Raese J., Patrick R. L., Barchas J. D. Phospholipid-induced activation of tyrosine hydroxylase from rat brain striatal synaptosomes. Biochem Pharmacol. 1976 Oct 15;25(20):2245–2250. doi: 10.1016/0006-2952(76)90005-8. [DOI] [PubMed] [Google Scholar]
- Rao D. N., Kaufman S. Purification and state of activation of rat kidney phenylalanine hydroxylase. J Biol Chem. 1986 Jul 5;261(19):8866–8876. [PubMed] [Google Scholar]
- Ribeiro P., Pigeon D., Kaufman S. The hydroxylation of phenylalanine and tyrosine by tyrosine hydroxylase from cultured pheochromocytoma cells. J Biol Chem. 1991 Aug 25;266(24):16207–16211. [PubMed] [Google Scholar]
- Richardson S. C., Aspbury R. A., Fisher M. J. The role of reversible phosphorylation in the hormonal control of phenylalanine hydroxylase in isolated rat proximal kidney tubules. Biochem J. 1993 Jun 1;292(Pt 2):419–424. doi: 10.1042/bj2920419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S. C., Fisher M. J. Characterization of phenylalanine hydroxylase from rat kidney. Int J Biochem. 1993 Apr;25(4):581–588. doi: 10.1016/0020-711x(93)90667-4. [DOI] [PubMed] [Google Scholar]
- Richtand N. M., Inagami T., Misono K., Kuczenski R. Purification and characterization of rat striatal tyrosine hydroxylase. Comparison of the activation by cyclic AMP-dependent phosphorylation and by other effectors. J Biol Chem. 1985 Jul 15;260(14):8465–8473. [PubMed] [Google Scholar]
- Saadat S., Stehle A. D., Lamouroux A., Mallet J., Thoenen H. Predicted amino acid sequence of bovine tyrosine hydroxylase and its similarity to tyrosine hydroxylases from other species. J Neurochem. 1988 Aug;51(2):572–578. doi: 10.1111/j.1471-4159.1988.tb01077.x. [DOI] [PubMed] [Google Scholar]
- Scriver C. R., Kaufman S., Woo S. L. Mendelian hyperphenylalaninemia. Annu Rev Genet. 1988;22:301–321. doi: 10.1146/annurev.ge.22.120188.001505. [DOI] [PubMed] [Google Scholar]
- Shiman R., Akino M., Kaufman S. Solubilization and partial purification of tyrosine hydroxylase from bovine adrenal medulla. J Biol Chem. 1971 Mar 10;246(5):1330–1340. [PubMed] [Google Scholar]
- Shiman R., Gray D. W., Hill M. A. Regulation of rat liver phenylalanine hydroxylase. I. Kinetic properties of the enzyme's iron and enzyme reduction site. J Biol Chem. 1994 Oct 7;269(40):24637–24646. [PubMed] [Google Scholar]
- Shiman R., Gray D. W., Pater A. A simple purification of phenylalanine hydroxylase by substrate-induced hydrophobic chromatography. J Biol Chem. 1979 Nov 25;254(22):11300–11306. [PubMed] [Google Scholar]
- Shiman R., Gray D. W. Substrate activation of phenylalanine hydroxylase. A kinetic characterization. J Biol Chem. 1980 May 25;255(10):4793–4800. [PubMed] [Google Scholar]
- Shiman R., Mortimore G. E., Schworer C. M., Gray D. W. Regulation of phenylalanine hydroxylase activity by phenylalanine in vivo, in vitro, and in perfused rat liver. J Biol Chem. 1982 Oct 10;257(19):11213–11216. [PubMed] [Google Scholar]
- Shiman R., Xia T., Hill M. A., Gray D. W. Regulation of rat liver phenylalanine hydroxylase. II. Substrate binding and the role of activation in the control of enzymatic activity. J Biol Chem. 1994 Oct 7;269(40):24647–24656. [PubMed] [Google Scholar]
- Siegmund H. U., Kaufman S. Hydroxylation of 4-methylphenylalanine by rat liver phenylalanine hydroxylase. J Biol Chem. 1991 Feb 15;266(5):2903–2910. [PubMed] [Google Scholar]
- Smith S. C., Kemp B. E., McAdam W. J., Mercer J. F., Cotton R. G. Two apparent molecular weight forms of human and monkey phenylalanine hydroxylase are due to phosphorylation. J Biol Chem. 1984 Sep 25;259(18):11284–11289. [PubMed] [Google Scholar]
- Smith S. C., McAdam W. J., Kemp B. E., Morgan F. J., Cotton R. G. A monoclonal antibody to the phosphorylated form of phenylalanine hydroxylase. Definition of the phosphopeptide epitope. Biochem J. 1987 Jun 15;244(3):625–631. doi: 10.1042/bj2440625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll J., Goldman D. Isolation and structural characterization of the murine tryptophan hydroxylase gene. J Neurosci Res. 1991 Apr;28(4):457–465. doi: 10.1002/jnr.490280402. [DOI] [PubMed] [Google Scholar]
- Stoll J., Kozak C. A., Goldman D. Characterization and chromosomal mapping of a cDNA encoding tryptophan hydroxylase from a mouse mastocytoma cell line. Genomics. 1990 May;7(1):88–96. doi: 10.1016/0888-7543(90)90522-v. [DOI] [PubMed] [Google Scholar]
- Tipper J., Kaufman S. Phenylalanine-induced phosphorylation and activation of rat hepatic phenylalanine hydroxylase in vivo. J Biol Chem. 1992 Jan 15;267(2):889–896. [PubMed] [Google Scholar]
- Tong J. H., Kaufman S. Tryptophan hydroxylase. Purification and some properties of the enzyme from rabbit hindbrain. J Biol Chem. 1975 Jun 10;250(11):4152–4158. [PubMed] [Google Scholar]
- Tourian A. Activation of phenylalanine hydroxylase by phenylalanine. Biochim Biophys Acta. 1971 Aug 20;242(2):345–354. doi: 10.1016/0005-2744(71)90226-9. [DOI] [PubMed] [Google Scholar]
- Vulliet P. R., Woodgett J. R., Cohen P. Phosphorylation of tyrosine hydroxylase by calmodulin-dependent multiprotein kinase. J Biol Chem. 1984 Nov 25;259(22):13680–13683. [PubMed] [Google Scholar]
- Vulliet P. R., Woodgett J. R., Ferrari S., Hardie D. G. Characterization of the sites phosphorylated on tyrosine hydroxylase by Ca2+ and phospholipid-dependent protein kinase, calmodulin-dependent multiprotein kinase and cyclic AMP-dependent protein kinase. FEBS Lett. 1985 Mar 25;182(2):335–339. doi: 10.1016/0014-5793(85)80328-8. [DOI] [PubMed] [Google Scholar]
- Walker S. J., Liu X., Roskoski R., Vrana K. E. Catalytic core of rat tyrosine hydroxylase: terminal deletion analysis of bacterially expressed enzyme. Biochim Biophys Acta. 1994 May 18;1206(1):113–119. doi: 10.1016/0167-4838(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Wallick D. E., Bloom L. M., Gaffney B. J., Benkovic S. J. Reductive activation of phenylalanine hydroxylase and its effect on the redox state of the non-heme iron. Biochemistry. 1984 Mar 13;23(6):1295–1302. doi: 10.1021/bi00301a043. [DOI] [PubMed] [Google Scholar]
- Wang Y. H., Citron B. A., Ribeiro P., Kaufman S. High-level expression of rat PC12 tyrosine hydroxylase cDNA in Escherichia coli: purification and characterization of the cloned enzyme. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8779–8783. doi: 10.1073/pnas.88.19.8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S. L., Gillam S. S., Woolf L. I. The isolation and properties of phenylalanine hydroxylase from human liver. Biochem J. 1974 Jun;139(3):741–749. doi: 10.1042/bj1390741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wretborn M., Humble E., Ragnarsson U., Engström L. Amino acid sequence at the phosphorylated site of rat liver phenylalanine hydroxylase and phosphorylation of a corresponding synthetic peptide. Biochem Biophys Res Commun. 1980 Mar 28;93(2):403–408. doi: 10.1016/0006-291x(80)91091-8. [DOI] [PubMed] [Google Scholar]
- Wu J., Filer D., Friedhoff A. J., Goldstein M. Site-directed mutagenesis of tyrosine hydroxylase. Role of serine 40 in catalysis. J Biol Chem. 1992 Dec 25;267(36):25754–25758. [PubMed] [Google Scholar]
- Xia T., Gray D. W., Shiman R. Regulation of rat liver phenylalanine hydroxylase. III. Control of catalysis by (6R)-tetrahydrobiopterin and phenylalanine. J Biol Chem. 1994 Oct 7;269(40):24657–24665. [PubMed] [Google Scholar]
- Yamauchi T., Fujisawa H. Regulation of rat brainstem tryptophan 5-monooxygenase. Calcium-dependent reversible activation by ATP and magnesium. Arch Biochem Biophys. 1979 Nov;198(1):219–226. doi: 10.1016/0003-9861(79)90413-2. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., Nakata H., Fujisawa H. A new activator protein that activates tryptophan 5-monooxygenase and tyrosine 3-monooxygenase in the presence of Ca2+-, calmodulin-dependent protein kinase. Purification and characterization. J Biol Chem. 1981 Jun 10;256(11):5404–5409. [PubMed] [Google Scholar]
- Zhao G., Xia T., Song J., Jensen R. A. Pseudomonas aeruginosa possesses homologues of mammalian phenylalanine hydroxylase and 4 alpha-carbinolamine dehydratase/DCoH as part of a three-component gene cluster. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1366–1370. doi: 10.1073/pnas.91.4.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond R. E., Schwarzschild M. A., Rittenhouse A. R. Acute regulation of tyrosine hydroxylase by nerve activity and by neurotransmitters via phosphorylation. Annu Rev Neurosci. 1989;12:415–461. doi: 10.1146/annurev.ne.12.030189.002215. [DOI] [PubMed] [Google Scholar]