Abstract
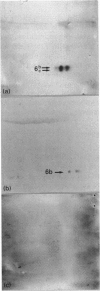
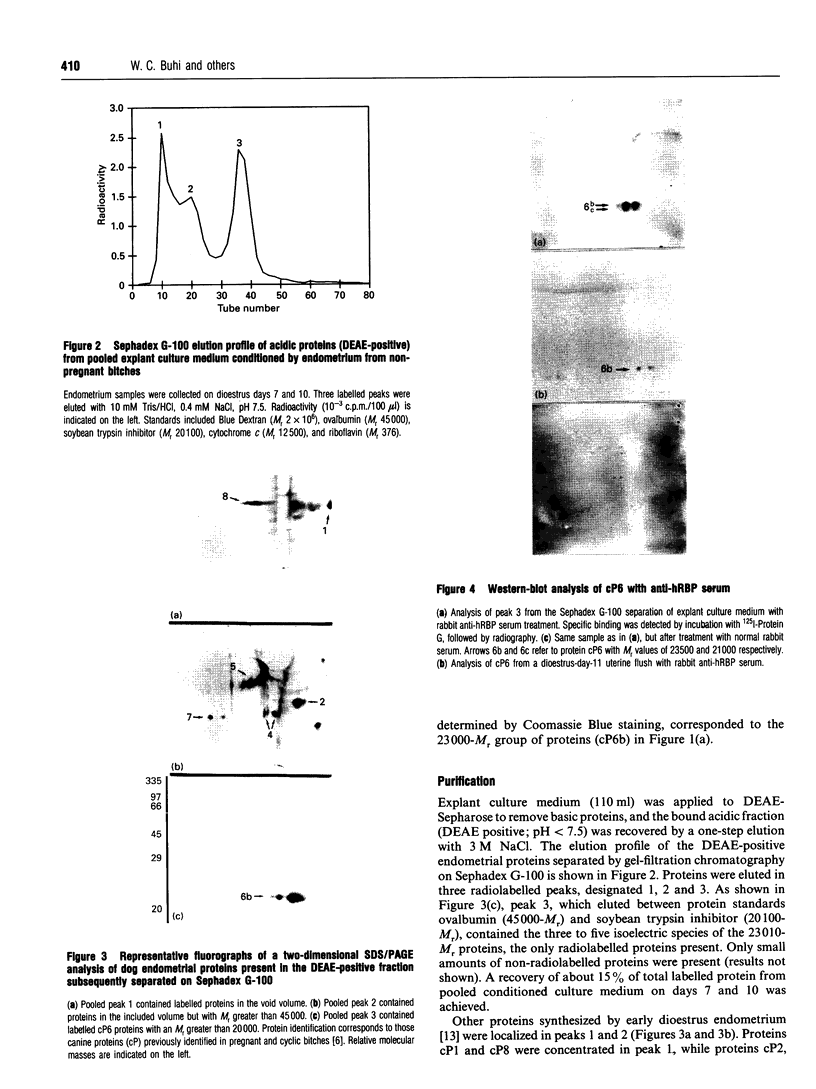
A major canine endometrial secreted protein (cP6, 23,000-M(r)) was purified by ion-exchange and gel-filtration chromatography and characterized by two-dimensional gel electrophoresis. Anti-[human retinol-binding protein (hRBP)] serum identified cP6 on immunoblot analysis and immunoprecipitated cP6 from culture medium. This major protein was also shown to bind [3H]retinol. N-terminal and internal amino acid sequences were determined and compared with previously identified protein, RNA, or DNA sequences. N-terminal analysis revealed that cP6 had high identity and similarity to serum retinol-binding proteins (RBPs), while internal sequence analysis showed a strong similarity to rat androgen-dependent epididymal protein and beta-lactoglobulins. Amino acid analysis, however, showed significant differences between these proteins and cP6 in both total amino acid content and certain selected amino acids. Immunohistochemical analysis showed staining for RBP only in the uterine luminal epithelium. These studies suggest that bitch endometrium secretes a family of proteins (cP6), some of which bind [3H]retinol, are immunologically related to the RBP family, and have N-terminal and internal sequences with a high similarity to RBP, beta-lactoglobulins and other members of the lipocalin family. This family of proteins may be important in early development for supplying retinol or derivatives to the developing embryo.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams K. L., Bazer F. W., Roberts R. M. Progesterone-induced secretion of a retinol-binding protein in the pig uterus. J Reprod Fertil. 1981 May;62(1):39–47. doi: 10.1530/jrf.0.0620039. [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bell S. C., Hales M. W., Patel S., Kirwan P. H., Drife J. O. Protein synthesis and secretion by the human endometrium and decidua during early pregnancy. Br J Obstet Gynaecol. 1985 Aug;92(8):793–803. doi: 10.1111/j.1471-0528.1985.tb03048.x. [DOI] [PubMed] [Google Scholar]
- Brief S., Chew B. P. Effects of vitamin A and beta-carotene on reproductive performance in gilts. J Anim Sci. 1985 Apr;60(4):998–1004. doi: 10.2527/jas1985.604998x. [DOI] [PubMed] [Google Scholar]
- Buhi W. C., Alvarez I. M., Sudhipong V., Dones-Smith M. M. Identification and characterization of de novo-synthesized porcine oviductal secretory proteins. Biol Reprod. 1990 Dec;43(6):929–938. doi: 10.1095/biolreprod43.6.929. [DOI] [PubMed] [Google Scholar]
- Buhi W. C., Shille V. M., Thatcher M. J., Alvarez I. M., Qiu Y. X. Identification and immunolocalization of proteins synthesized by dog endometrium and membranes. J Reprod Fertil Suppl. 1993;47:141–157. [PubMed] [Google Scholar]
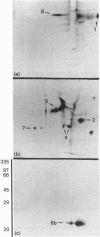
- Buhi W. C., Thatcher M. J., Shille V. M., Alvarez I. M., Lannon A. P., Johnson J. Synthesis of uterine endometrial proteins during early diestrus in the cyclic and pregnant dog, and after estrogen and progesterone treatment. Biol Reprod. 1992 Sep;47(3):326–336. doi: 10.1095/biolreprod47.3.326. [DOI] [PubMed] [Google Scholar]
- Chiocca E. A., Davies P. J., Stein J. P. Regulation of tissue transglutaminase gene expression as a molecular model for retinoid effects on proliferation and differentiation. J Cell Biochem. 1989 Mar;39(3):293–304. doi: 10.1002/jcb.240390309. [DOI] [PubMed] [Google Scholar]
- Clawitter J., Trout W. E., Burke M. G., Araghi S., Roberts R. M. A novel family of progesterone-induced, retinol-binding proteins from uterine secretions of the pig. J Biol Chem. 1990 Feb 25;265(6):3248–3255. [PubMed] [Google Scholar]
- Concannon P. W., Hansel W., Visek W. J. The ovarian cycle of the bitch: plasma estrogen, LH and progesterone. Biol Reprod. 1975 Aug;13(1):112–121. doi: 10.1095/biolreprod13.1.112. [DOI] [PubMed] [Google Scholar]
- Cowan S. W., Newcomer M. E., Jones T. A. Crystallographic refinement of human serum retinol binding protein at 2A resolution. Proteins. 1990;8(1):44–61. doi: 10.1002/prot.340080108. [DOI] [PubMed] [Google Scholar]
- Fazleabas A. T., Verhage H. G. Synthesis and release of polypeptides by the baboon (Papio anubis) uterine endometrium in culture. Biol Reprod. 1987 Nov;37(4):979–988. doi: 10.1095/biolreprod37.4.979. [DOI] [PubMed] [Google Scholar]
- Godkin J. D., Bazer F. W., Roberts R. M. Ovine trophoblast protein 1, an early secreted blastocyst protein, binds specifically to uterine endometrium and affects protein synthesis. Endocrinology. 1984 Jan;114(1):120–130. doi: 10.1210/endo-114-1-120. [DOI] [PubMed] [Google Scholar]
- Godovac-Zimmermann J. The structural motif of beta-lactoglobulin and retinol-binding protein: a basic framework for binding and transport of small hydrophobic molecules? Trends Biochem Sci. 1988 Feb;13(2):64–66. doi: 10.1016/0968-0004(88)90031-x. [DOI] [PubMed] [Google Scholar]
- Gross T. S., Plante C., Thatcher W. W., Hansen P. J., Helmer S. D., Putney D. J. Secretory proteins of the bovine conceptus alter endometrial prostaglandin and protein secretion in vitro. Biol Reprod. 1988 Nov;39(4):977–987. doi: 10.1095/biolreprod39.4.977. [DOI] [PubMed] [Google Scholar]
- Halliday J. A., Bell K., Shaw D. C. The complete amino acid sequence of feline beta-lactoglobulin II and a partial revision of the equine beta-lactoglobulin II sequence. Biochim Biophys Acta. 1991 Mar 8;1077(1):25–30. doi: 10.1016/0167-4838(91)90521-z. [DOI] [PubMed] [Google Scholar]
- Harney J. P., Ali M., Vedeckis W. V., Bazer F. W. Porcine conceptus and endometrial retinoid-binding proteins. Reprod Fertil Dev. 1994;6(2):211–219. doi: 10.1071/rd9940211. [DOI] [PubMed] [Google Scholar]
- Harney J. P., Mirando M. A., Smith L. C., Bazer F. W. Retinol-binding protein: a major secretory product of the pig conceptus. Biol Reprod. 1990 Mar;42(3):523–532. doi: 10.1095/biolreprod42.3.523. [DOI] [PubMed] [Google Scholar]
- Helmer S. D., Hansen P. J., Anthony R. V., Thatcher W. W., Bazer F. W., Roberts R. M. Identification of bovine trophoblast protein-1, a secretory protein immunologically related to ovine trophoblast protein-1. J Reprod Fertil. 1987 Jan;79(1):83–91. doi: 10.1530/jrf.0.0790083. [DOI] [PubMed] [Google Scholar]
- Holst P. A., Phemister R. D. The prenatal development of the dog: preimplantation events. Biol Reprod. 1971 Oct;5(2):194–206. doi: 10.1093/biolreprod/5.2.194. [DOI] [PubMed] [Google Scholar]
- Liu K. H., Baumbach G. A., Gillevet P. M., Godkin J. D. Purification and characterization of bovine placental retinol-binding protein. Endocrinology. 1990 Dec;127(6):2696–2704. doi: 10.1210/endo-127-6-2696. [DOI] [PubMed] [Google Scholar]
- Liu K. H., Godkin J. D. Characterization and immunolocalization of bovine uterine retinol-binding protein. Biol Reprod. 1992 Dec;47(6):1099–1104. doi: 10.1095/biolreprod47.6.1099. [DOI] [PubMed] [Google Scholar]
- Murray M. K., Verhage H. G., Buhi W. C., Jaffe R. C. The detection and purification of a cat uterine secretory protein that is estrogen dependent (CUPED). Biol Reprod. 1985 Jun;32(5):1219–1227. doi: 10.1095/biolreprod32.5.1219. [DOI] [PubMed] [Google Scholar]
- Nieder G. L., Macon G. R. Uterine and oviducal protein secretion during early pregnancy in the mouse. J Reprod Fertil. 1987 Sep;81(1):287–294. doi: 10.1530/jrf.0.0810287. [DOI] [PubMed] [Google Scholar]
- North A. C. Three-dimensional arrangement of conserved amino acid residues in a superfamily of specific ligand-binding proteins. Int J Biol Macromol. 1989 Feb;11(1):56–58. doi: 10.1016/0141-8130(89)90041-x. [DOI] [PubMed] [Google Scholar]
- Ogilvie S., Kvello-Stenstrom A. G., Hammond G., Buhi W. C., Larkin L. H., Shiverick K. T. Identification of proteins immunochemically related to human pregnancy-specific beta 1-glycoprotein in the rat placenta. Endocrinology. 1989 Jul;125(1):287–294. doi: 10.1210/endo-125-1-287. [DOI] [PubMed] [Google Scholar]
- Olson P. N., Bowen R. A., Behrendt M. D., Olson J. D., Nett T. M. Concentrations of reproductive hormones in canine serum throughout late anestrus, proestrus and estrus. Biol Reprod. 1982 Dec;27(5):1196–1206. doi: 10.1095/biolreprod27.5.1196. [DOI] [PubMed] [Google Scholar]
- Papiz M. Z., Sawyer L., Eliopoulos E. E., North A. C., Findlay J. B., Sivaprasadarao R., Jones T. A., Newcomer M. E., Kraulis P. J. The structure of beta-lactoglobulin and its similarity to plasma retinol-binding protein. 1986 Nov 27-Dec 3Nature. 324(6095):383–385. doi: 10.1038/324383a0. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervaiz S., Brew K. Homology and structure-function correlations between alpha 1-acid glycoprotein and serum retinol-binding protein and its relatives. FASEB J. 1987 Sep;1(3):209–214. doi: 10.1096/fasebj.1.3.3622999. [DOI] [PubMed] [Google Scholar]
- Pervaiz S., Brew K. Homology of beta-lactoglobulin, serum retinol-binding protein, and protein HC. Science. 1985 Apr 19;228(4697):335–337. doi: 10.1126/science.2580349. [DOI] [PubMed] [Google Scholar]
- Pervaiz S., Brew K. Purification and characterization of the major whey proteins from the milks of the bottlenose dolphin (Tursiops truncatus), the Florida manatee (Trichechus manatus latirostris), and the beagle (Canis familiaris). Arch Biochem Biophys. 1986 May 1;246(2):846–854. doi: 10.1016/0003-9861(86)90341-3. [DOI] [PubMed] [Google Scholar]
- Poulik M. D., Farrah D., Malek G. H., Shinnick C. J., Smithies O. Low molecular weight urinary proteins. I. Partial amino acid sequences of the retinol-binding proteins of man and dog. Biochim Biophys Acta. 1975 Dec 15;412(2):326–334. [PubMed] [Google Scholar]
- Roberts R. M., Bazer F. W. The functions of uterine secretions. J Reprod Fertil. 1988 Mar;82(2):875–892. doi: 10.1530/jrf.0.0820875. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Shille V. M., Thatcher M. J., Simmons K. J. Efforts to induce estrus in the bitch, using pituitary gonadotropins. J Am Vet Med Assoc. 1984 Jun 15;184(12):1469–1473. [PubMed] [Google Scholar]
- Smith S. M., Pang K., Sundin O., Wedden S. E., Thaller C., Eichele G. Molecular approaches to vertebrate limb morphogenesis. Development. 1989;107 (Suppl):121–131. doi: 10.1242/dev.107.Supplement.121. [DOI] [PubMed] [Google Scholar]
- Stallings-Mann M., Trout W. E., Roberts R. M. A novel family of progesterone-induced, retinol-binding proteins from uterine secretions of the pig. J Biol Chem. 1991 Dec 5;266(34):23515–23515. [PubMed] [Google Scholar]
- Thatcher M. D., Shille V. M., Fliss M. F., Bazer F. W., Sisum W., Randal S. Characterization of feline conceptus proteins during pregnancy. Biol Reprod. 1991 Jan;44(1):108–120. doi: 10.1095/biolreprod44.1.108. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trout W. E., Hall J. A., Stallings-Mann M. L., Galvin J. M., Anthony R. V., Roberts R. M. Steroid regulation of the synthesis and secretion of retinol-binding protein by the uterus of the pig. Endocrinology. 1992 May;130(5):2557–2564. doi: 10.1210/endo.130.5.1572282. [DOI] [PubMed] [Google Scholar]
- Wolf G. Multiple functions of vitamin A. Physiol Rev. 1984 Jul;64(3):873–937. doi: 10.1152/physrev.1984.64.3.873. [DOI] [PubMed] [Google Scholar]