Abstract
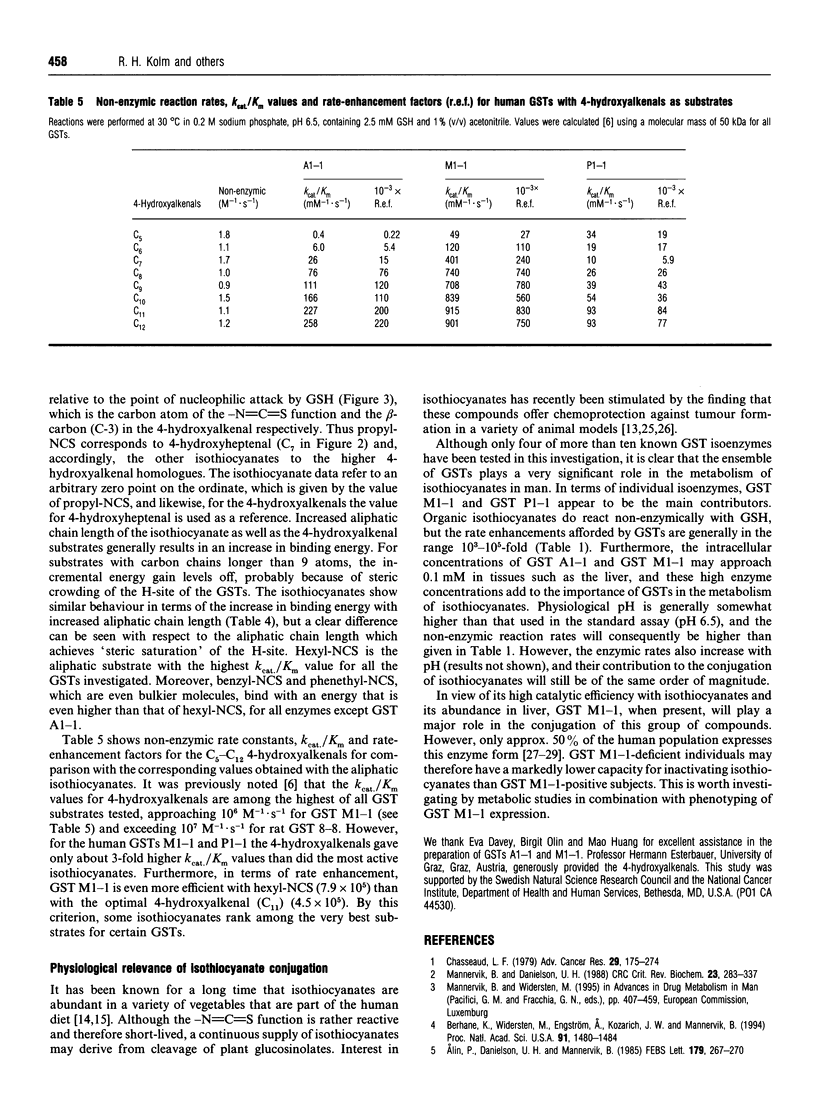
The catalytic properties of four human glutathione transferases (GSTs), A1-1, M1-1, M4-4 and P1-1, were examined with 14 isothiocyanate (R-NCS) substrates. The compounds include aliphatic and aromatic homologues, some of which are natural constituents of human food, namely sulphoraphane [1-isothiocyanato-4-(methylsulphinyl)butane], erucin [1-isothiocyanato-4-(methylthio)butane], erysolin [1-isothiocyanato-4-(methylsulphonyl)butane], benzyl-NCS, phenethyl-NCS and allyl-NCS. All isothiocyanates investigated were substrates for the four GSTs. The enzymes promote addition of the thiol group of GSH to the electrophilic central carbon of the isothiocyanate group to form dithiocarbamates [R-NH-C(=S)-SG] which have high UV absorption at 274 nm. Molar absorption coefficients and non-enzymic rate constants as well as standardized enzyme assay conditions for all compounds were established. Of the four isoenzymes investigated, GSTs M1-1 and P1-1 were generally the most efficient catalysts, whereas GST M4-4 was the least efficient. Isothiocyanates are among the GST substrates that are most rapidly conjugated. On the basis of rate-enhancement data and binding energies, the isothiocyanates were compared with 4-hydroxyalkenals, another class of natural GST substrates previously subjected to systematic kinetic analysis. The incremental transition-state stabilization attributable to an increased number of methylene groups in homologous alkyl isothiocyanates is similar to that previously noted for homologous 4-hydroxyalkenals.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alin P., Danielson U. H., Mannervik B. 4-Hydroxyalk-2-enals are substrates for glutathione transferase. FEBS Lett. 1985 Jan 7;179(2):267–270. doi: 10.1016/0014-5793(85)80532-9. [DOI] [PubMed] [Google Scholar]
- Berhane K., Widersten M., Engström A., Kozarich J. W., Mannervik B. Detoxication of base propenals and other alpha, beta-unsaturated aldehyde products of radical reactions and lipid peroxidation by human glutathione transferases. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1480–1484. doi: 10.1073/pnas.91.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Board P. G. Biochemical genetics of glutathione-S-transferase in man. Am J Hum Genet. 1981 Jan;33(1):36–43. [PMC free article] [PubMed] [Google Scholar]
- Brüsewitz G., Cameron B. D., Chasseaud L. F., Görler K., Hawkins D. R., Koch H., Mennicke W. H. The metabolism of benzyl isothiocyanate and its cysteine conjugate. Biochem J. 1977 Jan 15;162(1):99–107. doi: 10.1042/bj1620099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasseaud L. F. The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res. 1979;29:175–274. doi: 10.1016/s0065-230x(08)60848-9. [DOI] [PubMed] [Google Scholar]
- Chung F. L., Morse M. A., Eklind K. I., Lewis J. Quantitation of human uptake of the anticarcinogen phenethyl isothiocyanate after a watercress meal. Cancer Epidemiol Biomarkers Prev. 1992 Jul-Aug;1(5):383–388. [PubMed] [Google Scholar]
- Comstock K. E., Widersten M., Hao X. Y., Henner W. D., Mannervik B. A comparison of the enzymatic and physicochemical properties of human glutathione transferase M4-4 and three other human Mu class enzymes. Arch Biochem Biophys. 1994 Jun;311(2):487–495. doi: 10.1006/abbi.1994.1266. [DOI] [PubMed] [Google Scholar]
- Danielson U. H., Esterbauer H., Mannervik B. Structure-activity relationships of 4-hydroxyalkenals in the conjugation catalysed by mammalian glutathione transferases. Biochem J. 1987 Nov 1;247(3):707–713. doi: 10.1042/bj2470707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick G. R., Heaney R. K., Mullin W. J. Glucosinolates and their breakdown products in food and food plants. Crit Rev Food Sci Nutr. 1983;18(2):123–201. doi: 10.1080/10408398209527361. [DOI] [PubMed] [Google Scholar]
- Kolm R. H., Sroga G. E., Mannervik B. Participation of the phenolic hydroxyl group of Tyr-8 in the catalytic mechanism of human glutathione transferase P1-1. Biochem J. 1992 Jul 15;285(Pt 2):537–540. doi: 10.1042/bj2850537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolm R. H., Stenberg G., Widersten M., Mannervik B. High-level bacterial expression of human glutathione transferase P1-1 encoded by semisynthetic DNA. Protein Expr Purif. 1995 Jun;6(3):265–271. doi: 10.1006/prep.1995.1034. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Danielson U. H. Glutathione transferases--structure and catalytic activity. CRC Crit Rev Biochem. 1988;23(3):283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Guthenberg C. Glutathione transferase (human placenta). Methods Enzymol. 1981;77:231–235. doi: 10.1016/s0076-6879(81)77030-7. [DOI] [PubMed] [Google Scholar]
- Mennicke W. H., Görler K., Krumbiegel G., Lorenz D., Rittmann N. Studies on the metabolism and excretion of benzyl isothiocyanate in man. Xenobiotica. 1988 Apr;18(4):441–447. doi: 10.3109/00498258809041680. [DOI] [PubMed] [Google Scholar]
- Mennicke W. H., Görler K., Krumbiegel G. Metabolism of some naturally occurring isothiocyanates in the rat. Xenobiotica. 1983 Apr;13(4):203–207. doi: 10.3109/00498258309052256. [DOI] [PubMed] [Google Scholar]
- Meyer D. J., Crease D. J., Ketterer B. Forward and reverse catalysis and product sequestration by human glutathione S-transferases in the reaction of GSH with dietary aralkyl isothiocyanates. Biochem J. 1995 Mar 1;306(Pt 2):565–569. doi: 10.1042/bj3060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg G., Björnestedt R., Mannervik B. Heterologous expression of recombinant human glutathione transferase A1-1 from a hepatoma cell line. Protein Expr Purif. 1992 Feb;3(1):80–84. doi: 10.1016/1046-5928(92)90060-a. [DOI] [PubMed] [Google Scholar]
- Warholm M., Guthenberg C., Mannervik B., von Bahr C., Glaumann H. Identification of a new glutathione S-transferase in human liver. Acta Chem Scand B. 1980;34(8):607–621. doi: 10.3891/acta.chem.scand.34b-0607. [DOI] [PubMed] [Google Scholar]
- Warholm M., Guthenberg C., Mannervik B., von Bahr C. Purification of a new glutathione S-transferase (transferase mu) from human liver having high activity with benzo(alpha)pyrene-4,5-oxide. Biochem Biophys Res Commun. 1981 Jan 30;98(2):512–519. doi: 10.1016/0006-291x(81)90870-6. [DOI] [PubMed] [Google Scholar]
- Widersten M., Pearson W. R., Engström A., Mannervik B. Heterologous expression of the allelic variant mu-class glutathione transferases mu and psi. Biochem J. 1991 Jun 1;276(Pt 2):519–524. doi: 10.1042/bj2760519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Kensler T. W., Cho C. G., Posner G. H., Talalay P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3147–3150. doi: 10.1073/pnas.91.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Kolm R. H., Mannervik B., Talalay P. Reversible conjugation of isothiocyanates with glutathione catalyzed by human glutathione transferases. Biochem Biophys Res Commun. 1995 Jan 17;206(2):748–755. doi: 10.1006/bbrc.1995.1106. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Talalay P. Anticarcinogenic activities of organic isothiocyanates: chemistry and mechanisms. Cancer Res. 1994 Apr 1;54(7 Suppl):1976s–1981s. [PubMed] [Google Scholar]
- Zhang Y., Talalay P., Cho C. G., Posner G. H. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]


