Abstract
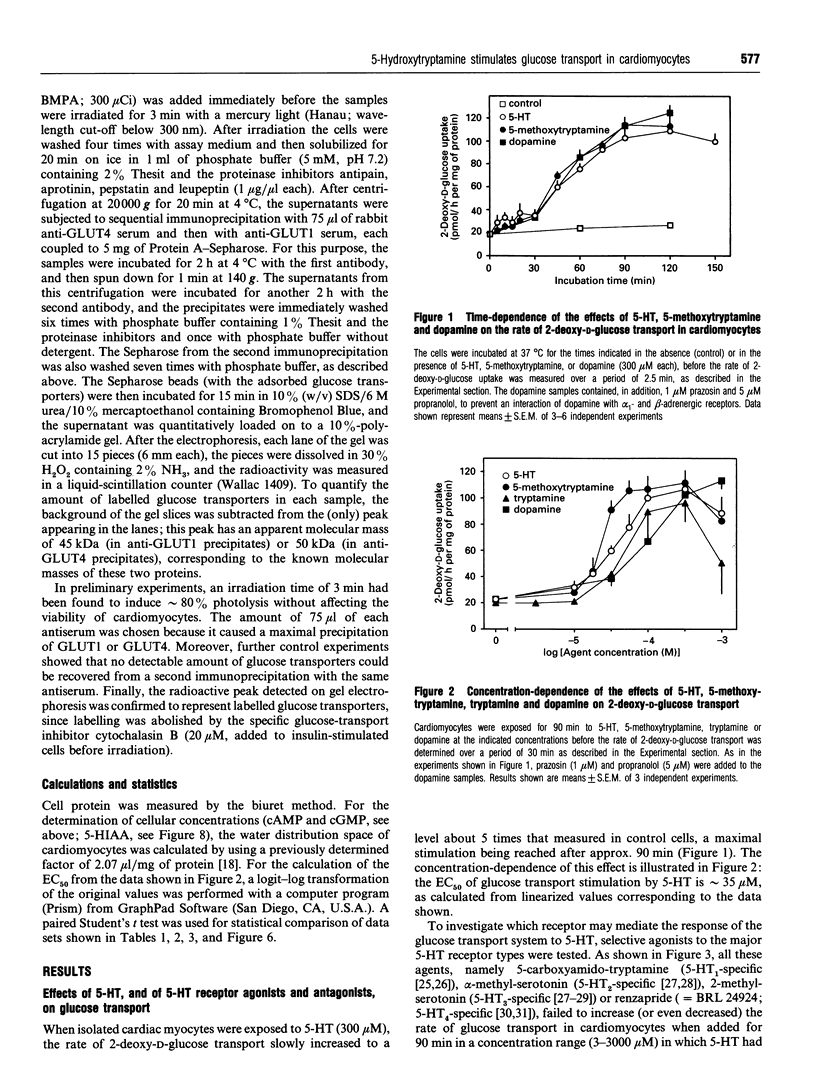
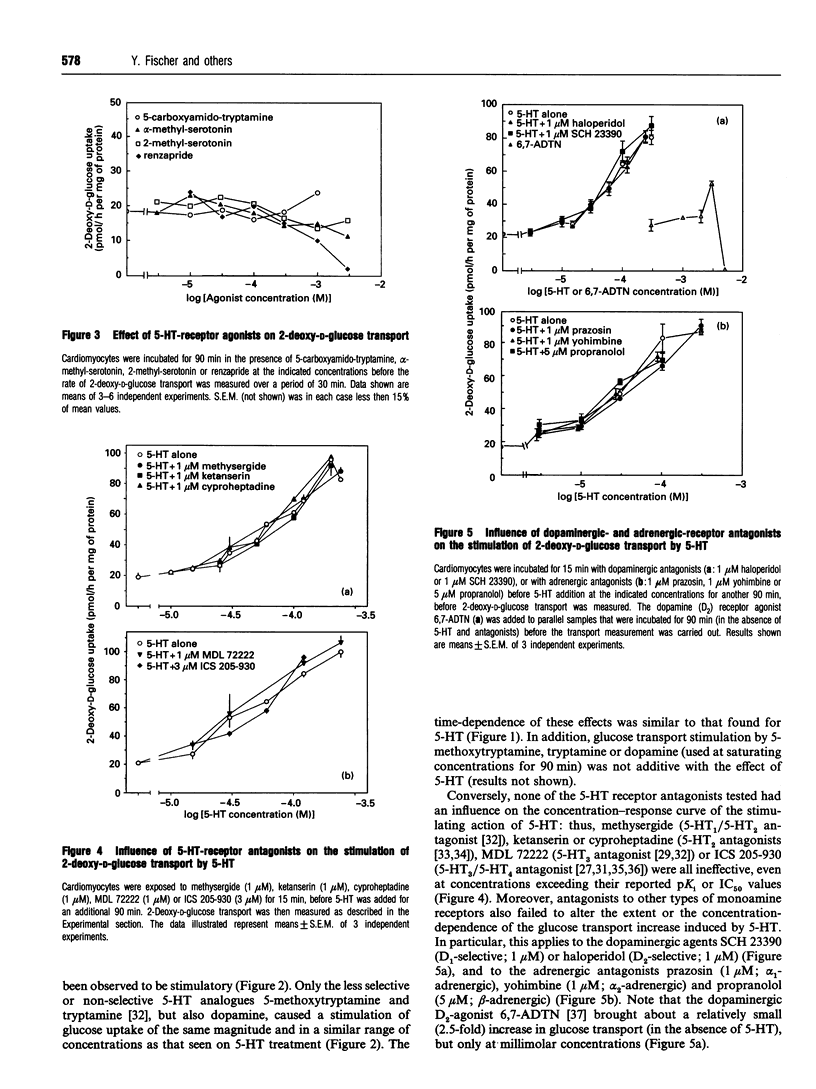
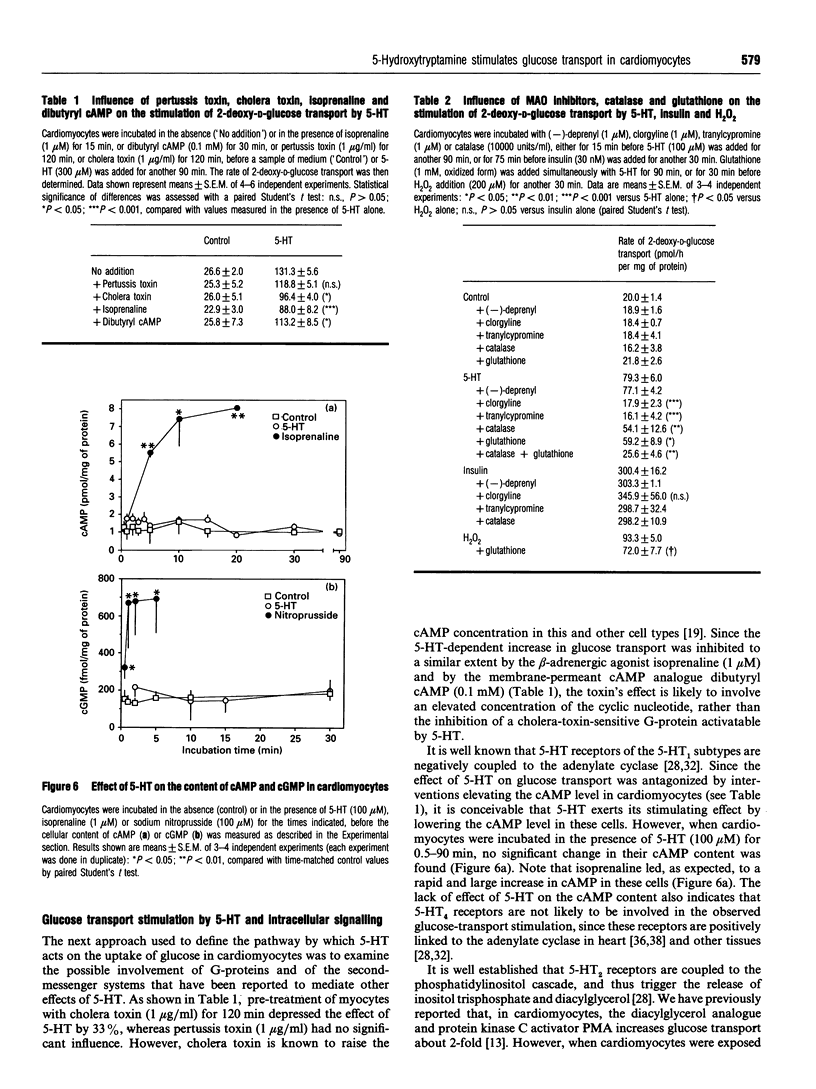
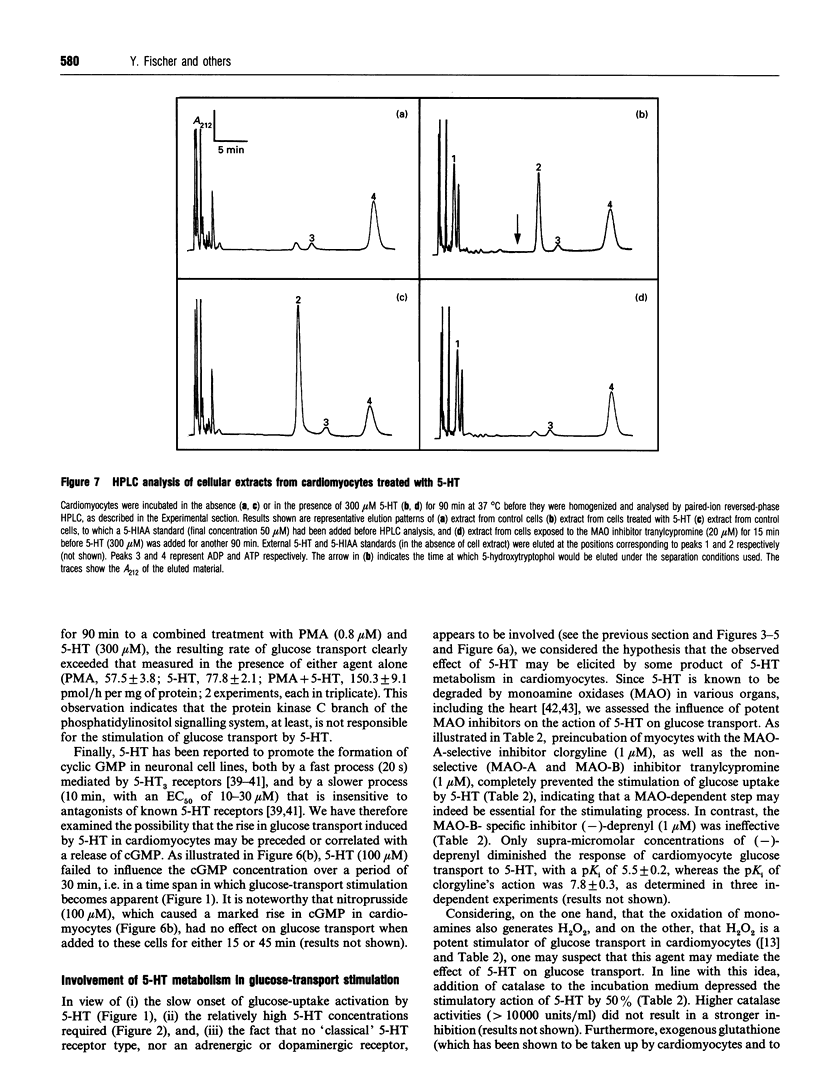
This study deals with the effect of 5-hydroxytryptamine (5-HT; serotonin) on glucose transport in isolated rat cardiac myocytes. In these cells, 5-HT (10-300 microns), as well as tryptamine, 5-methoxytryptamine and dopamine, elicited a 3-5 fold increase in glucose transport, as compared with control. This effect was maximal after 90 min, and was concomitant with a 1.8- and 1.5-fold increase in the amounts of glucose transporters GLUT1 and GLUT4 at the cell surface of the cardiomyocytes, as determined by using the photoaffinity label 3H-2-N-[4-(1-azi-2,2,2-trifluoroethyl)benzoyl]-1,3-bis-(D-manno s-4-yl) propyl-2-amine (3H-ATB-BMPA). In contrast, 3-3000 microM of the selective 5-HT receptor agonists 5-carboxyamido-tryptamine, alpha-methyl-serotonin, 2-methyl-serotonin or renzapride failed to stimulate glucose transport. The effect of 5-HT was not affected by (i) the 5-HT receptor antagonists methysergide (1 microM), ketanserin (1 microM), cyproheptadine (1 microM), MDL 72222 (1 microM) or ICS 205-930 (3 microM), nor by (ii) the adrenergic receptor antagonists prazosin (1 microM), yohimbine (1 microM) or propranolol (5 microM), nor by (iii) the dopaminergic antagonists SCH 23390 (1 microM) or haloperidol (1 microM). The monoamine oxidase inhibitors clorgyline (1 microM) and tranylcypromine (1 microM) completely suppressed the effect of 5-HT, whereas the control and insulin-stimulated rates of glucose transport were unaffected. Addition of catalase or glutathione diminished the 5-HT-dependent stimulation of glucose transport by 50%; these two factors are known to favour the degradation of H2O2 (which can be formed during the deamination of amines by monoamine oxidases). Glutathione also depressed the stimulatory action of exogenously added H2O2 (20 microM) by 30%. Furthermore, in cells treated with 5_HT, a time-dependent accumulation of 5-hydroxy-1H-indol-3-ylacetic acid (a product of 5-HT metabolism via monoamine oxidases) was observed, which paralleled the changes in glucose transport. In conclusion, the stimulation of glucose transport by 5-HT in cardiomyocytes is not mediated by a 5-HT1, 5-HT2, 5-HT3 or 5-HT4 receptor, nor by an adrenergic or dopaminergic receptor, but is likely to occur through the degradation of by a monoamine oxidase and concomitant formation of H2O2.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P. H., Gingrich J. A., Bates M. D., Dearry A., Falardeau P., Senogles S. E., Caron M. G. Dopamine receptor subtypes: beyond the D1/D2 classification. Trends Pharmacol Sci. 1990 Jun;11(6):231–236. doi: 10.1016/0165-6147(90)90249-8. [DOI] [PubMed] [Google Scholar]
- Blakely R. D., De Felice L. J., Hartzell H. C. Molecular physiology of norepinephrine and serotonin transporters. J Exp Biol. 1994 Nov;196:263–281. doi: 10.1242/jeb.196.1.263. [DOI] [PubMed] [Google Scholar]
- Boveris A., Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973 Jul;134(3):707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderhead D. M., Kitagawa K., Tanner L. I., Holman G. D., Lienhard G. E. Insulin regulation of the two glucose transporters in 3T3-L1 adipocytes. J Biol Chem. 1990 Aug 15;265(23):13801–13808. [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Chaouloff F., Jeanrenaud B. 5-HT1A and alpha-2 adrenergic receptors mediate the hyperglycemic and hypoinsulinemic effects of 8-hydroxy-2-(di-n-propylamino)tetralin in the conscious rat. J Pharmacol Exp Ther. 1987 Dec;243(3):1159–1166. [PubMed] [Google Scholar]
- Chaouloff F., Jeanrenaud B. Hyperinsulinemia of the genetically obese (fa/fa) rat is decreased by a low dose of the 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT). Eur J Pharmacol. 1988 Feb 16;147(1):111–118. doi: 10.1016/0014-2999(88)90639-5. [DOI] [PubMed] [Google Scholar]
- Chaouloff F., Laude D., Baudrie V. Effects of the 5-HT1C/5-5-HT2 receptor agonists DOI and alpha-methyl-5-HT on plasma glucose and insulin levels in the rat. Eur J Pharmacol. 1990 Oct 23;187(3):435–443. doi: 10.1016/0014-2999(90)90370-l. [DOI] [PubMed] [Google Scholar]
- Clark A. E., Holman G. D. Exofacial photolabelling of the human erythrocyte glucose transporter with an azitrifluoroethylbenzoyl-substituted bismannose. Biochem J. 1990 Aug 1;269(3):615–622. doi: 10.1042/bj2690615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor H. E., Feniuk W., Humphrey P. P., Perren M. J. 5-Carboxamidotryptamine is a selective agonist at 5-hydroxytryptamine receptors mediating vasodilatation and tachycardia in anaesthetized cats. Br J Pharmacol. 1986 Feb;87(2):417–426. doi: 10.1111/j.1476-5381.1986.tb10832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwish S. A., Furman B. L. Medication of the hypoglycaemic effect of 5-hydroxytryptophan by a central nervous system action. Experientia. 1974 Nov 15;30(11):1306–1307. doi: 10.1007/BF01945199. [DOI] [PubMed] [Google Scholar]
- Depré C., Hue L. Cyclic GMP in the perfused rat heart. Effect of ischaemia, anoxia and nitric oxide synthase inhibitor. FEBS Lett. 1994 May 30;345(2-3):241–245. doi: 10.1016/0014-5793(94)00459-5. [DOI] [PubMed] [Google Scholar]
- Dhasmana K. M., De Boer H. J., Banerjee A. K., Saxena P. R. Analysis of the tachycardiac response to 5-hydroxytryptamine in the spinal guinea-pig. Eur J Pharmacol. 1988 Jan 5;145(1):67–73. doi: 10.1016/0014-2999(88)90350-0. [DOI] [PubMed] [Google Scholar]
- Dobson J. G., Jr Protein kinase regulation of cardiac phosphorylase activity and contractility. Am J Physiol. 1978 May;234(5):H638–H645. doi: 10.1152/ajpheart.1978.234.5.H638. [DOI] [PubMed] [Google Scholar]
- Dumuis A., Sebben M., Bockaert J. BRL 24924: a potent agonist at a non-classical 5-HT receptor positively coupled with adenylate cyclase in colliculi neurons. Eur J Pharmacol. 1989 Mar 21;162(2):381–384. doi: 10.1016/0014-2999(89)90304-x. [DOI] [PubMed] [Google Scholar]
- Dumuis A., Sebben M., Bockaert J. The gastrointestinal prokinetic benzamide derivatives are agonists at the non-classical 5-HT receptor (5-HT4) positively coupled to adenylate cyclase in neurons. Naunyn Schmiedebergs Arch Pharmacol. 1989 Oct;340(4):403–410. doi: 10.1007/BF00167041. [DOI] [PubMed] [Google Scholar]
- Eckel J., Gerlach-Eskuchen E., Reinauer H. G-protein-mediated regulation of the insulin-responsive glucose transporter in isolated cardiac myocytes. Biochem J. 1990 Dec 15;272(3):691–696. doi: 10.1042/bj2720691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel J., Pandalis G., Reinauer H. Insulin action on the glucose transport system in isolated cardiocytes from adult rat. Biochem J. 1983 May 15;212(2):385–392. doi: 10.1042/bj2120385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer Y., Rose H., Kammermeier H. Highly insulin-responsive isolated rat heart muscle cells yielded by a modified isolation method. Life Sci. 1991;49(23):1679–1688. doi: 10.1016/0024-3205(91)90310-8. [DOI] [PubMed] [Google Scholar]
- Fischer Y., Rose H., Kammermeier H. Possible involvement of alanine and pyruvate in the regulation of glucose transport in heart muscle cells. FEBS Lett. 1990 Nov 12;274(1-2):127–130. doi: 10.1016/0014-5793(90)81346-p. [DOI] [PubMed] [Google Scholar]
- Fischer Y., Rose H., Thomas J., Deuticke B., Kammermeier H. Phenylarsine oxide and hydrogen peroxide stimulate glucose transport via different pathways in isolated cardiac myocytes. Biochim Biophys Acta. 1993 Nov 21;1153(1):97–104. doi: 10.1016/0005-2736(93)90280-d. [DOI] [PubMed] [Google Scholar]
- Furman B. L., Wilson G. A. Further studies on the effects of 5-hydroxytryptophan on plasma glucose and insulin in the mouse. Diabetologia. 1980 Oct;19(4):386–390. doi: 10.1007/BF00280525. [DOI] [PubMed] [Google Scholar]
- Guarnieri C., Fraticelli A., Ventura C., Vaona I., Budini R. External GSSG enhances intracellular glutathione level in isolated cardiac myocytes. Biochem Biophys Res Commun. 1987 Sep 15;147(2):658–665. doi: 10.1016/0006-291x(87)90981-8. [DOI] [PubMed] [Google Scholar]
- Humphrey P. P., Hartig P., Hoyer D. A proposed new nomenclature for 5-HT receptors. Trends Pharmacol Sci. 1993 Jun;14(6):233–236. doi: 10.1016/0165-6147(93)90016-d. [DOI] [PubMed] [Google Scholar]
- Jacob G., Bishara B., Lee S. S., Hilzenart N., Bomzon A. Cardiovascular responses to serotonin in experimental liver disease. Hepatology. 1991 Dec;14(6):1235–1242. [PubMed] [Google Scholar]
- Janssen P. A. Pharmacology of potent and selective S2-serotonergic antagonists. J Cardiovasc Pharmacol. 1985;7 (Suppl 7):S2–11. doi: 10.1097/00005344-198500077-00002. [DOI] [PubMed] [Google Scholar]
- Juengling E., Kammermeier H. Rapid assay of adenine nucleotides or creatine compounds in extracts of cardiac tissue by paired-ion reverse-phase high-performance liquid chromatography. Anal Biochem. 1980 Mar 1;102(2):358–361. doi: 10.1016/0003-2697(80)90167-0. [DOI] [PubMed] [Google Scholar]
- Kaumann A. J. Do human atrial 5-HT4 receptors mediate arrhythmias? Trends Pharmacol Sci. 1994 Dec;15(12):451–455. doi: 10.1016/0165-6147(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Kaumann A. J., Sanders L., Brown A. M., Murray K. J., Brown M. J. A 5-hydroxytryptamine receptor in human atrium. Br J Pharmacol. 1990 Aug;100(4):879–885. doi: 10.1111/j.1476-5381.1990.tb14108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolter T., Uphues I., Wichelhaus A., Reinauer H., Eckel J. Contraction-induced translocation of the glucose transporter Glut4 in isolated ventricular cardiomyocytes. Biochem Biophys Res Commun. 1992 Dec 15;189(2):1207–1214. doi: 10.1016/0006-291x(92)92333-s. [DOI] [PubMed] [Google Scholar]
- Kozka I. J., Gould M. K. Inhibitory effects of N-ethylmaleimide on insulin- and oxidant-stimulated sugar transport and on 125I-labelled insulin binding by rat soleus muscle. Biochim Biophys Acta. 1984 Feb 14;797(2):212–220. doi: 10.1016/0304-4165(84)90124-7. [DOI] [PubMed] [Google Scholar]
- Laude D., Baudrie V., Martin G. R., Chaouloff F. Effects of the 5-HT1 receptor agonists DP-5-CT, CGS 12066B, and RU 24969 on plasma adrenaline and glucose levels in the rat. Naunyn Schmiedebergs Arch Pharmacol. 1990 Oct;342(4):378–381. doi: 10.1007/BF00169452. [DOI] [PubMed] [Google Scholar]
- Legan E. Effects of streptozotocin-induced hyperglycemia on agonist-stimulated phosphatidylinositol turnover in rat aorta. Life Sci. 1989;45(5):371–378. doi: 10.1016/0024-3205(89)90622-x. [DOI] [PubMed] [Google Scholar]
- Leysen J. E., Niemegeers C. J., Van Nueten J. M., Laduron P. M. [3H]Ketanserin (R 41 468), a selective 3H-ligand for serotonin2 receptor binding sites. Binding properties, brain distribution, and functional role. Mol Pharmacol. 1982 Mar;21(2):301–314. [PubMed] [Google Scholar]
- Lundquist I., Ekholm R., Ericson L. E. Monoamines in the pancreatic islets of the mouse. 5-hydroxytryptamine as an intracellular modifier of insulin secretion, and the hypoglycaemic action of monoamine oxidase inhibitors. Diabetologia. 1971 Dec;7(6):414–422. doi: 10.1007/BF01212056. [DOI] [PubMed] [Google Scholar]
- Manchester J., Kong X., Nerbonne J., Lowry O. H., Lawrence J. C., Jr Glucose transport and phosphorylation in single cardiac myocytes: rate-limiting steps in glucose metabolism. Am J Physiol. 1994 Mar;266(3 Pt 1):E326–E333. doi: 10.1152/ajpendo.1994.266.3.E326. [DOI] [PubMed] [Google Scholar]
- Morgan H. E., Neely J. R., Wood R. E., Liébecq C., Liebermeister H., Park C. R. Factors affecting glucose transport in heart muscle and erythrocytes. Fed Proc. 1965 Sep-Oct;24(5):1040–1045. [PubMed] [Google Scholar]
- Neely J. R., Liebermeister H., Morgan H. E. Effect of pressure development on membrane transport of glucose in isolated rat heart. Am J Physiol. 1967 Apr;212(4):815–822. doi: 10.1152/ajplegacy.1967.212.4.815. [DOI] [PubMed] [Google Scholar]
- Reiser G., Hamprecht B. Serotonin raises the cyclic GMP level in a neuronal cell line via 5-HT3 receptors. Eur J Pharmacol. 1989 May 11;172(2):195–198. doi: 10.1016/0922-4106(89)90010-2. [DOI] [PubMed] [Google Scholar]
- Reviriego J., Marín J. Effects of 5-hydroxytryptamine on human isolated placental chorionic arteries and veins. Br J Pharmacol. 1989 Apr;96(4):961–969. doi: 10.1111/j.1476-5381.1989.tb11908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson B. P., Engel G., Donatsch P., Stadler P. A. Identification of serotonin M-receptor subtypes and their specific blockade by a new class of drugs. Nature. 1985 Jul 11;316(6024):126–131. doi: 10.1038/316126a0. [DOI] [PubMed] [Google Scholar]
- Saxena P. R., Lawang A. A comparison of cardiovascular and smooth muscle effects of 5-hydroxytryptamine and 5-carboxamidotryptamine, a selective agonist of 5-HT1 receptors. Arch Int Pharmacodyn Ther. 1985 Oct;277(2):235–252. [PubMed] [Google Scholar]
- Shah A. M., Andries L. J., Meulemans A. L., Brutsaert D. L. Endocardium modulates myocardial inotropic response to 5-hydroxytryptamine. Am J Physiol. 1989 Dec;257(6 Pt 2):H1790–H1797. doi: 10.1152/ajpheart.1989.257.6.H1790. [DOI] [PubMed] [Google Scholar]
- Smith S. A., Pogson C. L. Tryptophan and the control of plasma glucose concentrations in the rat. Biochem J. 1977 Dec 15;168(3):495–506. doi: 10.1042/bj1680495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strolin Benedetti M., Dostert P., Tipton K. F. Developmental aspects of the monoamine-degrading enzyme monoamine oxidase. Dev Pharmacol Ther. 1992;18(3-4):191–200. [PubMed] [Google Scholar]
- Sugimoto Y., Kimura I., Yamada J., Watanabe Y., Takeuchi N., Horisaka K. Effects of serotonin on blood glucose and insulin levels of glucose- and streptozotocin-treated mice. Jpn J Pharmacol. 1990 Sep;54(1):93–96. doi: 10.1254/jjp.54.93. [DOI] [PubMed] [Google Scholar]
- Tohda M., Nomura Y. Serotonin stimulates both cytosolic and membrane-bound guanylate cyclase in NG108-15 cells. J Neurochem. 1990 Nov;55(5):1800–1805. doi: 10.1111/j.1471-4159.1990.tb04971.x. [DOI] [PubMed] [Google Scholar]
- Tohda M., Sakuma I., Nomura Y. The slow cyclic GMP increase caused by serotonin in NG108-15 cells is not inhibited by antagonists of known serotonin receptors: possible existence of a new receptor subtype coupled with membrane-bound guanylate cyclase. J Neurochem. 1991 Aug;57(2):714–717. doi: 10.1111/j.1471-4159.1991.tb03804.x. [DOI] [PubMed] [Google Scholar]
- Villalón C. M., den Boer M. O., Heiligers J. P., Saxena P. R. Mediation of 5-hydroxytryptamine-induced tachycardia in the pig by the putative 5-HT4 receptor. Br J Pharmacol. 1990 Aug;100(4):665–667. doi: 10.1111/j.1476-5381.1990.tb14073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallberg-Henriksson H. Glucose transport into skeletal muscle. Influence of contractile activity, insulin, catecholamines and diabetes mellitus. Acta Physiol Scand Suppl. 1987;564:1–80. [PubMed] [Google Scholar]
- Wozniak K. M., Linnoila M. Hyperglycemic properties of serotonin receptor antagonists. Life Sci. 1991;49(2):101–109. doi: 10.1016/0024-3205(91)90023-5. [DOI] [PubMed] [Google Scholar]
- Yamada J., Sugimoto Y., Kimura I., Takeuchi N., Horisaka K. Serotonin-induced hypoglycemia and increased serum insulin levels in mice. Life Sci. 1989;45(20):1931–1936. doi: 10.1016/0024-3205(89)90547-x. [DOI] [PubMed] [Google Scholar]
- Zhao M., Muntz K. H. Differential downregulation of beta 2-adrenergic receptors in tissue compartments of rat heart is not altered by sympathetic denervation. Circ Res. 1993 Nov;73(5):943–951. doi: 10.1161/01.res.73.5.943. [DOI] [PubMed] [Google Scholar]


