Abstract
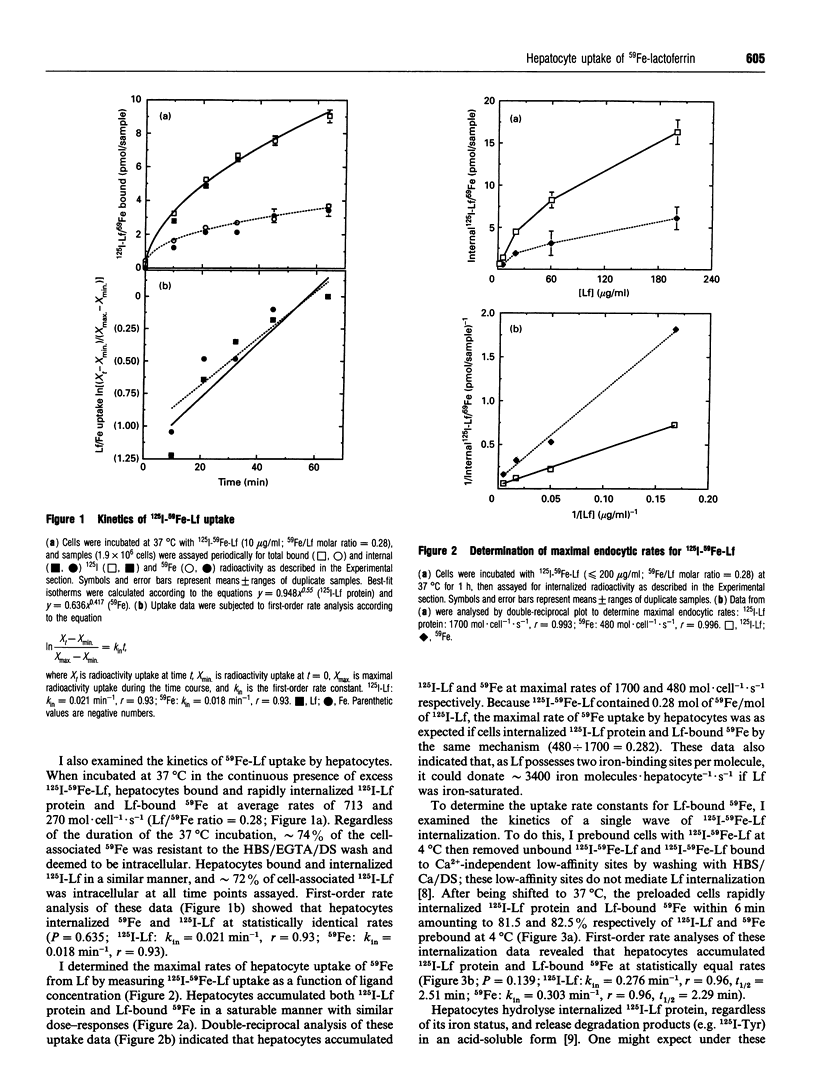
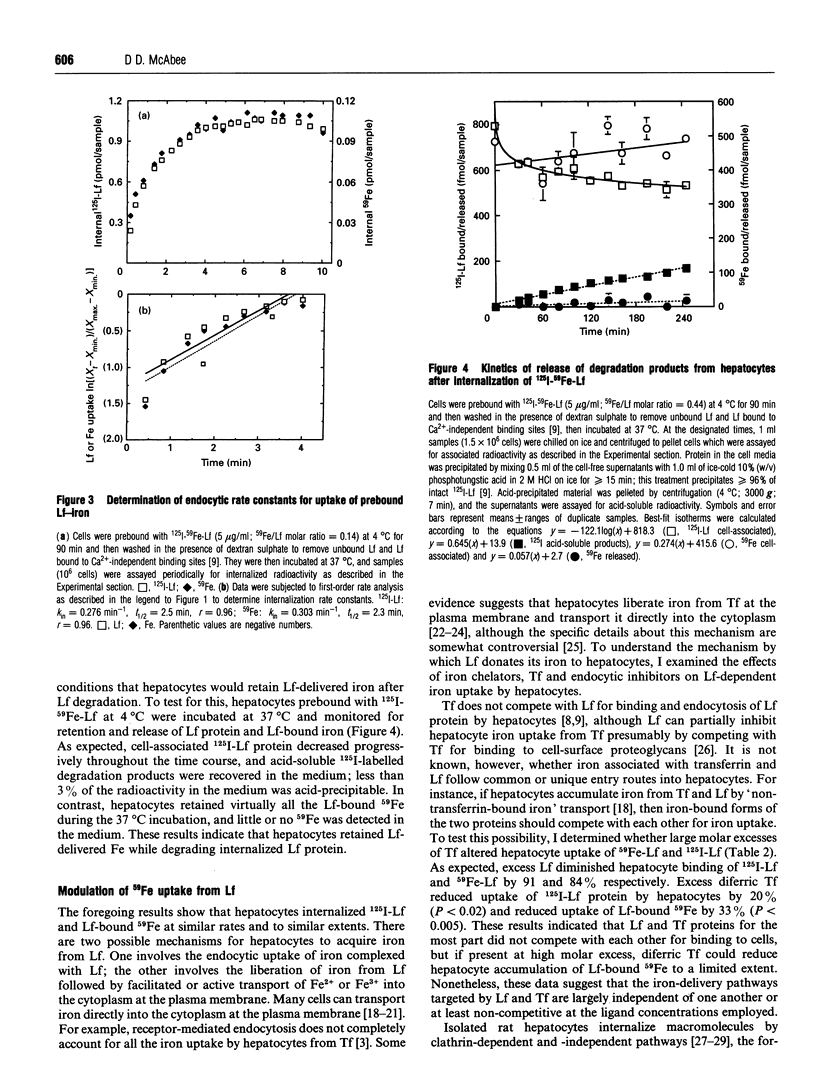
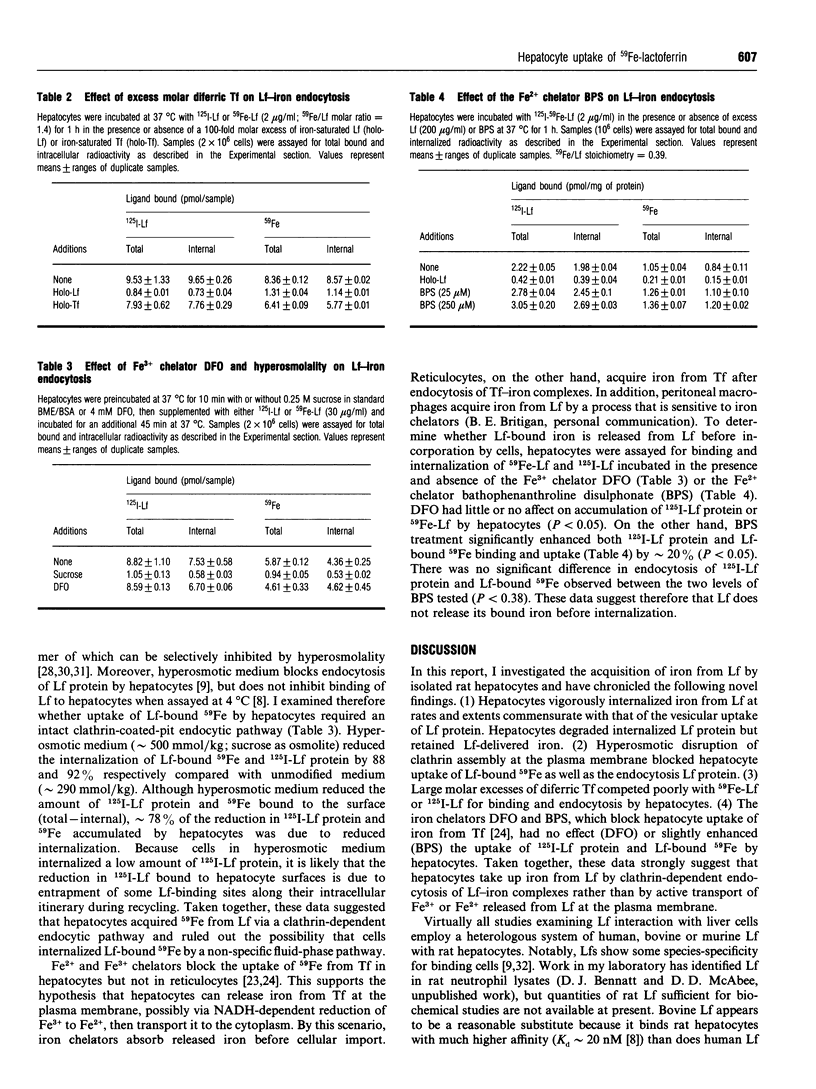
The iron-binding protein lactoferrin (Lf) present in blood is metabolized by the liver. Isolated rat hepatocytes vigorously endocytose bovine Lf via recycling Ca2(+)-dependent binding sites, but the uptake of iron from Lf by hepatocytes has not been examined. In this study, isolated rat hepatocytes were incubated with radiolabelled bovine Lf (125I-Lf, 59Fe-Lf or 125I-59Fe-Lf) at 37 degrees C, then washed at 4 degrees C in the presence of dextran sulphate with either Ca2+ or EGTA to distinguish between total bound and internal radioactivity respectively. Cells internalized 125I-Lf protein and Lf-bound 59Fe at maximal endocytic rates of 1700 and 480 mol.cell-1.s-1 respectively. When Lf was normalized for 59Fe content, these endocytic rates were equivalent and reflected an uptake potential of at least 3400 mol of iron.cell-1.s-1. Cells prebound with 125I-59Fe-Lf to Ca2+(-)dependent sites at 4 degrees C internalized more than 80% of both 125I-Lf protein and Lf-bound 59Fe approx. 6 min after warming to 37 degrees C at similar rates (125I-Lf: k(in) = 0.276 min-1, 59Fe: k(in) = 0.303 min-1). Within 4 h at 37 degrees C, cells had released 25% or less internalized Lf protein in the form of acid-soluble 125I-by-products but retained all the Lf-delivered 59Fe. Hyperosmotic disruption of clathrin-dependent endocytosis blocked the uptake of 125I-Lf and Lf-bound 59Fe. Incubation of cells with 125I-59Fe-Lf and a 100 molar excess of diferric transferrin reduced slightly the endocytosis of 125I-Lf protein and 59Fe accumulation. Treatment of cells with the ferric chelator desferrioxamine did not alter uptake of 125I-Lf protein or Lf-bound 59Fe, but the ferrous chelator bathophenanthroline disulphonate slightly elevated endocytosis of 125I-Lf protein and Lf-bound 59Fe. These findings indicate that Lf does not release its bound iron before endocytosis. It was concluded from this study that hepatocytes take up iron from Lf at high rates by a process that requires endocytosis of Lf-iron complexes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett R. M., Davis J. Lactoferrin binding to human peripheral blood cells: an interaction with a B-enriched population of lymphocytes and a subpopulation of adherent mononuclear cells. J Immunol. 1981 Sep;127(3):1211–1216. [PubMed] [Google Scholar]
- Bennett R. M., Kokocinski T. Lactoferrin turnover in man. Clin Sci (Lond) 1979 Nov;57(5):453–460. doi: 10.1042/cs0570453. [DOI] [PubMed] [Google Scholar]
- Birgens H. S., Hansen N. E., Karle H., Kristensen L. O. Receptor binding of lactoferrin by human monocytes. Br J Haematol. 1983 Jul;54(3):383–391. doi: 10.1111/j.1365-2141.1983.tb02113.x. [DOI] [PubMed] [Google Scholar]
- Birgens H. S., Kristensen L. O., Borregaard N., Karle H., Hansen N. E. Lactoferrin-mediated transfer of iron to intracellular ferritin in human monocytes. Eur J Haematol. 1988 Jul;41(1):52–57. doi: 10.1111/j.1600-0609.1988.tb00868.x. [DOI] [PubMed] [Google Scholar]
- Britigan B. E., Serody J. S., Hayek M. B., Charniga L. M., Cohen M. S. Uptake of lactoferrin by mononuclear phagocytes inhibits their ability to form hydroxyl radical and protects them from membrane autoperoxidation. J Immunol. 1991 Dec 15;147(12):4271–4277. [PubMed] [Google Scholar]
- Brown E. M., Parry R. M., Jr A spectroscopic study of bovine lactoferrin. Biochemistry. 1974 Oct 22;13(22):4560–4565. doi: 10.1021/bi00719a014. [DOI] [PubMed] [Google Scholar]
- Campbell E. J. Human leukocyte elastase, cathepsin G, and lactoferrin: family of neutrophil granule glycoproteins that bind to an alveolar macrophage receptor. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6941–6945. doi: 10.1073/pnas.79.22.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daukas G., Zigmond S. H. Inhibition of receptor-mediated but not fluid-phase endocytosis in polymorphonuclear leukocytes. J Cell Biol. 1985 Nov;101(5 Pt 1):1673–1679. doi: 10.1083/jcb.101.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson L. A., Lönnerdal B. Specific binding of lactoferrin to brush-border membrane: ontogeny and effect of glycan chain. Am J Physiol. 1988 Apr;254(4 Pt 1):G580–G585. doi: 10.1152/ajpgi.1988.254.4.G580. [DOI] [PubMed] [Google Scholar]
- England I. G., Naess L., Blomhoff R., Berg T. Uptake, intracellular transport and release of 125I-poly(vinylpyrrolidone) and [14C]-sucrose-asialofetuin in rat liver parenchymal cells. Effects of ammonia on the intracellular transport. Biochem Pharmacol. 1986 Jan 15;35(2):201–208. doi: 10.1016/0006-2952(86)90514-9. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Hashizume S., Kuroda K., Murakami H. Cell culture assay of biological activity of lactoferrin and transferrin. Methods Enzymol. 1987;147:302–314. doi: 10.1016/0076-6879(87)47120-6. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Anderson R. G. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J Cell Biol. 1989 Feb;108(2):389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W. L., Mazurier J., Sawatzki G., Montreuil J., Spik G. Lactotransferrin receptor of mouse small-intestinal brush border. Binding characteristics of membrane-bound and triton X-100-solubilized forms. Biochem J. 1988 Jan 15;249(2):435–441. doi: 10.1042/bj2490435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W. L., Regoeczi E., Chindemi P. A., Bolyos M. Lactoferrin interferes with uptake of iron from transferrin and asialotransferrin by the rat liver. Am J Physiol. 1993 Jan;264(1 Pt 1):G112–G117. doi: 10.1152/ajpgi.1993.264.1.G112. [DOI] [PubMed] [Google Scholar]
- Imber M. J., Pizzo S. V. Clearance and binding of native and defucosylated lactoferrin. Biochem J. 1983 May 15;212(2):249–257. doi: 10.1042/bj2120249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail M., Brock J. H. Binding of lactoferrin and transferrin to the human promonocytic cell line U937. Effect on iron uptake and release. J Biol Chem. 1993 Oct 15;268(29):21618–21625. [PubMed] [Google Scholar]
- Kaplan J., Jordan I., Sturrock A. Regulation of the transferrin-independent iron transport system in cultured cells. J Biol Chem. 1991 Feb 15;266(5):2997–3004. [PubMed] [Google Scholar]
- Kaplan J., Jordan I., Sturrock A. Regulation of the transferrin-independent iron transport system in cultured cells. J Biol Chem. 1991 Feb 15;266(5):2997–3004. [PubMed] [Google Scholar]
- Kawakami H., Lönnerdal B. Isolation and function of a receptor for human lactoferrin in human fetal intestinal brush-border membranes. Am J Physiol. 1991 Nov;261(5 Pt 1):G841–G846. doi: 10.1152/ajpgi.1991.261.5.G841. [DOI] [PubMed] [Google Scholar]
- Leveugle B., Mazurier J., Legrand D., Mazurier C., Montreuil J., Spik G. Lactotransferrin binding to its platelet receptor inhibits platelet aggregation. Eur J Biochem. 1993 May 1;213(3):1205–1211. doi: 10.1111/j.1432-1033.1993.tb17871.x. [DOI] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F. Metal-combining properties of human lactoferrin (red milk protein). 1. The involvement of bicarbonate in the reaction. Eur J Biochem. 1968 Dec 5;6(4):579–584. doi: 10.1111/j.1432-1033.1968.tb00484.x. [DOI] [PubMed] [Google Scholar]
- Mazurier J., Legrand D., Hu W. L., Montreuil J., Spik G. Expression of human lactotransferrin receptors in phytohemagglutinin-stimulated human peripheral blood lymphocytes. Isolation of the receptors by antiligand-affinity chromatography. Eur J Biochem. 1989 Feb 1;179(2):481–487. doi: 10.1111/j.1432-1033.1989.tb14578.x. [DOI] [PubMed] [Google Scholar]
- McAbee D. D., Esbensen K. Binding and endocytosis of apo- and holo-lactoferrin by isolated rat hepatocytes. J Biol Chem. 1991 Dec 15;266(35):23624–23631. [PubMed] [Google Scholar]
- McAbee D. D., Nowatzke W., Oehler C., Sitaram M., Sbaschnig E., Opferman J. T., Carr J., Esbensen K. Endocytosis and degradation of bovine apo- and holo-lactoferrin by isolated rat hepatocytes are mediated by recycling calcium-dependent binding sites. Biochemistry. 1993 Dec 14;32(49):13749–13760. doi: 10.1021/bi00212a046. [DOI] [PubMed] [Google Scholar]
- Mikogami T., Heyman M., Spik G., Desjeux J. F. Apical-to-basolateral transepithelial transport of human lactoferrin in the intestinal cell line HT-29cl.19A. Am J Physiol. 1994 Aug;267(2 Pt 1):G308–G315. doi: 10.1152/ajpgi.1994.267.2.G308. [DOI] [PubMed] [Google Scholar]
- Morgan E. H., Baker E. Iron uptake and metabolism by hepatocytes. Fed Proc. 1986 Nov;45(12):2810–2816. [PubMed] [Google Scholar]
- Oka J. A., Christensen M. D., Weigel P. H. Hyperosmolarity inhibits galactosyl receptor-mediated but not fluid phase endocytosis in isolated rat hepatocytes. J Biol Chem. 1989 Jul 15;264(20):12016–12024. [PubMed] [Google Scholar]
- Oka J. A., Weigel P. H. Monensin inhibits ligand dissociation only transiently and partially and distinguishes two galactosyl receptor pathways in isolated rat hepatocytes. J Cell Physiol. 1987 Nov;133(2):243-52, 257. doi: 10.1002/jcp.1041330207. [DOI] [PubMed] [Google Scholar]
- Oria R., Alvarez-Hernández X., Licéaga J., Brock J. H. Uptake and handling of iron from transferrin, lactoferrin and immune complexes by a macrophage cell line. Biochem J. 1988 May 15;252(1):221–225. doi: 10.1042/bj2520221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterloh K., Aisen P. Pathways in the binding and uptake of ferritin by hepatocytes. Biochim Biophys Acta. 1989 Mar 28;1011(1):40–45. doi: 10.1016/0167-4889(89)90075-x. [DOI] [PubMed] [Google Scholar]
- Quail E. A., Morgan E. H. Role of membrane surface potential and other factors in the uptake of non-transferrin-bound iron by reticulocytes. J Cell Physiol. 1994 May;159(2):238–244. doi: 10.1002/jcp.1041590207. [DOI] [PubMed] [Google Scholar]
- Randell E. W., Parkes J. G., Olivieri N. F., Templeton D. M. Uptake of non-transferrin-bound iron by both reductive and nonreductive processes is modulated by intracellular iron. J Biol Chem. 1994 Jun 10;269(23):16046–16053. [PubMed] [Google Scholar]
- Schryvers A. B., Morris L. J. Identification and characterization of the human lactoferrin-binding protein from Neisseria meningitidis. Infect Immun. 1988 May;56(5):1144–1149. doi: 10.1128/iai.56.5.1144-1149.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of rat liver cells. 3. Enzymatic requirements for tissue dispersion. Exp Cell Res. 1973 Dec;82(2):391–398. doi: 10.1016/0014-4827(73)90357-1. [DOI] [PubMed] [Google Scholar]
- Sibille J. C., Kondo H., Aisen P. Interactions between isolated hepatocytes and Kupffer cells in iron metabolism: a possible role for ferritin as an iron carrier protein. Hepatology. 1988 Mar-Apr;8(2):296–301. doi: 10.1002/hep.1840080218. [DOI] [PubMed] [Google Scholar]
- Sibille J. C., Octave J. N., Schneider Y. J., Trouet A., Crichton R. Subcellular localization of transferrin protein and iron in the perfused rat liver. Effect of Triton WR 1339, digitonin and temperature. Eur J Biochem. 1986 Feb 17;155(1):47–55. doi: 10.1111/j.1432-1033.1986.tb09457.x. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sturrock A., Alexander J., Lamb J., Craven C. M., Kaplan J. Characterization of a transferrin-independent uptake system for iron in HeLa cells. J Biol Chem. 1990 Feb 25;265(6):3139–3145. [PubMed] [Google Scholar]
- Thirion J., Wattiaux R. Effect of monensin on fluid phase and receptor mediated endocytosis by rat hepatocyte monolayers. Biochem Biophys Res Commun. 1988 May 16;152(3):1275–1281. doi: 10.1016/s0006-291x(88)80423-6. [DOI] [PubMed] [Google Scholar]
- Thorstensen K., Aisen P. Release of iron from diferric transferrin in the presence of rat liver plasma membranes: no evidence of a plasma membrane diferric transferrin reductase. Biochim Biophys Acta. 1990 Apr 9;1052(1):29–35. doi: 10.1016/0167-4889(90)90053-g. [DOI] [PubMed] [Google Scholar]
- Thorstensen K. Hepatocytes and reticulocytes have different mechanisms for the uptake of iron from transferrin. J Biol Chem. 1988 Nov 15;263(32):16837–16841. [PubMed] [Google Scholar]
- Thorstensen K., Romslo I. The role of transferrin in the mechanism of cellular iron uptake. Biochem J. 1990 Oct 1;271(1):1–9. doi: 10.1042/bj2710001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorstensen K., Romslo I. Uptake of iron from transferrin by isolated rat hepatocytes. A redox-mediated plasma membrane process? J Biol Chem. 1988 Jun 25;263(18):8844–8850. [PubMed] [Google Scholar]
- Van Snick J. L., Masson P. L. The binding of human lactoferrin to mouse peritoneal cells. J Exp Med. 1976 Dec 1;144(6):1568–1580. doi: 10.1084/jem.144.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright T. L., Fitz J. G., Weisiger R. A. Non-transferrin-bound iron uptake by rat liver. Role of membrane potential difference. J Biol Chem. 1988 Feb 5;263(4):1842–1847. [PubMed] [Google Scholar]
- Ziere G. J., van Dijk M. C., Bijsterbosch M. K., van Berkel T. J. Lactoferrin uptake by the rat liver. Characterization of the recognition site and effect of selective modification of arginine residues. J Biol Chem. 1992 Jun 5;267(16):11229–11235. [PubMed] [Google Scholar]
- van Snick J. L., Markowetz B., Masson P. L. The ingestion and digestion of human lactoferrin by mouse peritoneal macrophages and the transfer of its iron into ferritin. J Exp Med. 1977 Sep 1;146(3):817–827. doi: 10.1084/jem.146.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]


